Heat Stress Increases Mammary Epithelial Cells and Reduces Viable Immune Cells in Milk of Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experiment
2.2. Milk Sampling and Processing
2.3. Flow Cytometry Analysis
2.4. Statistical Analyses
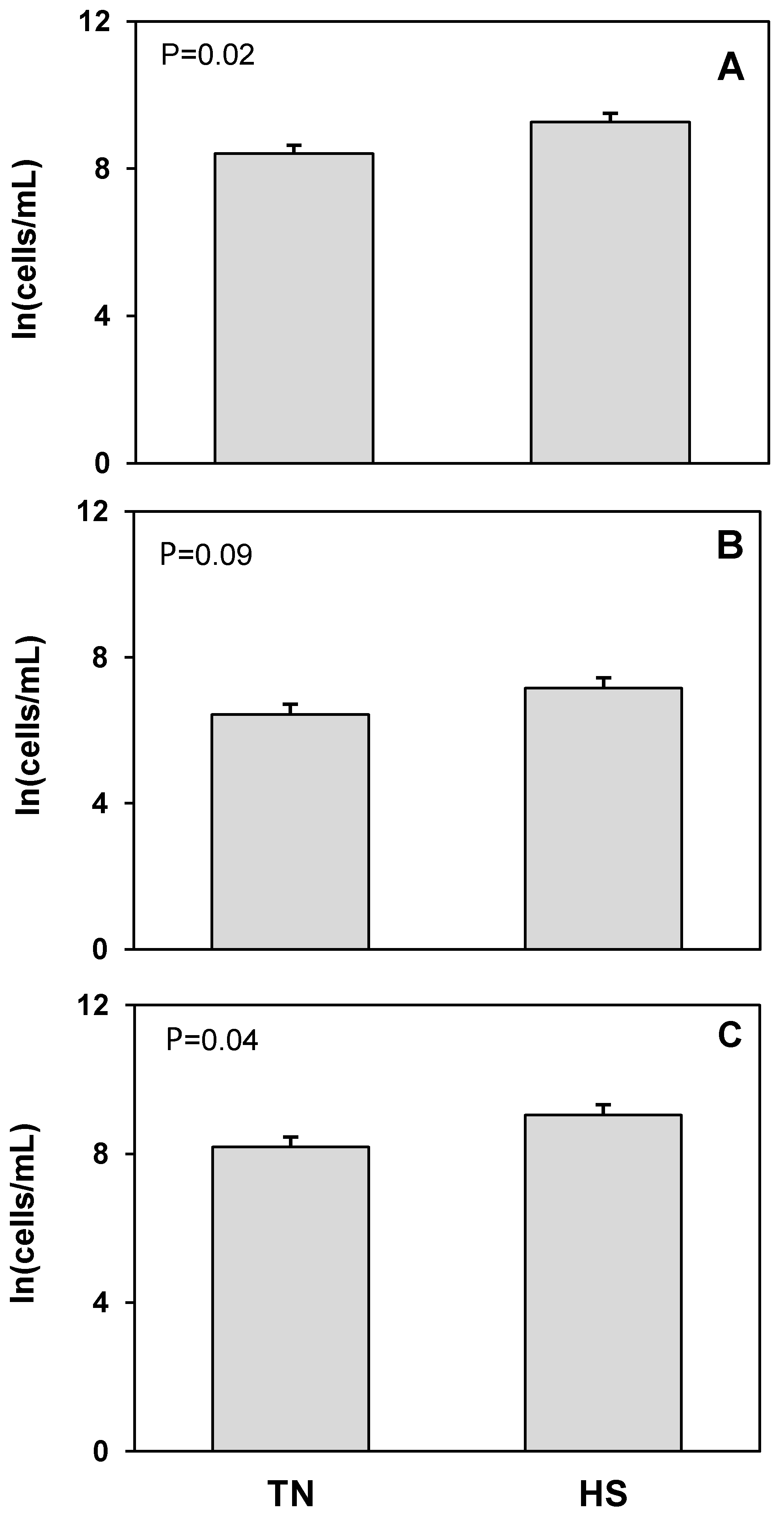
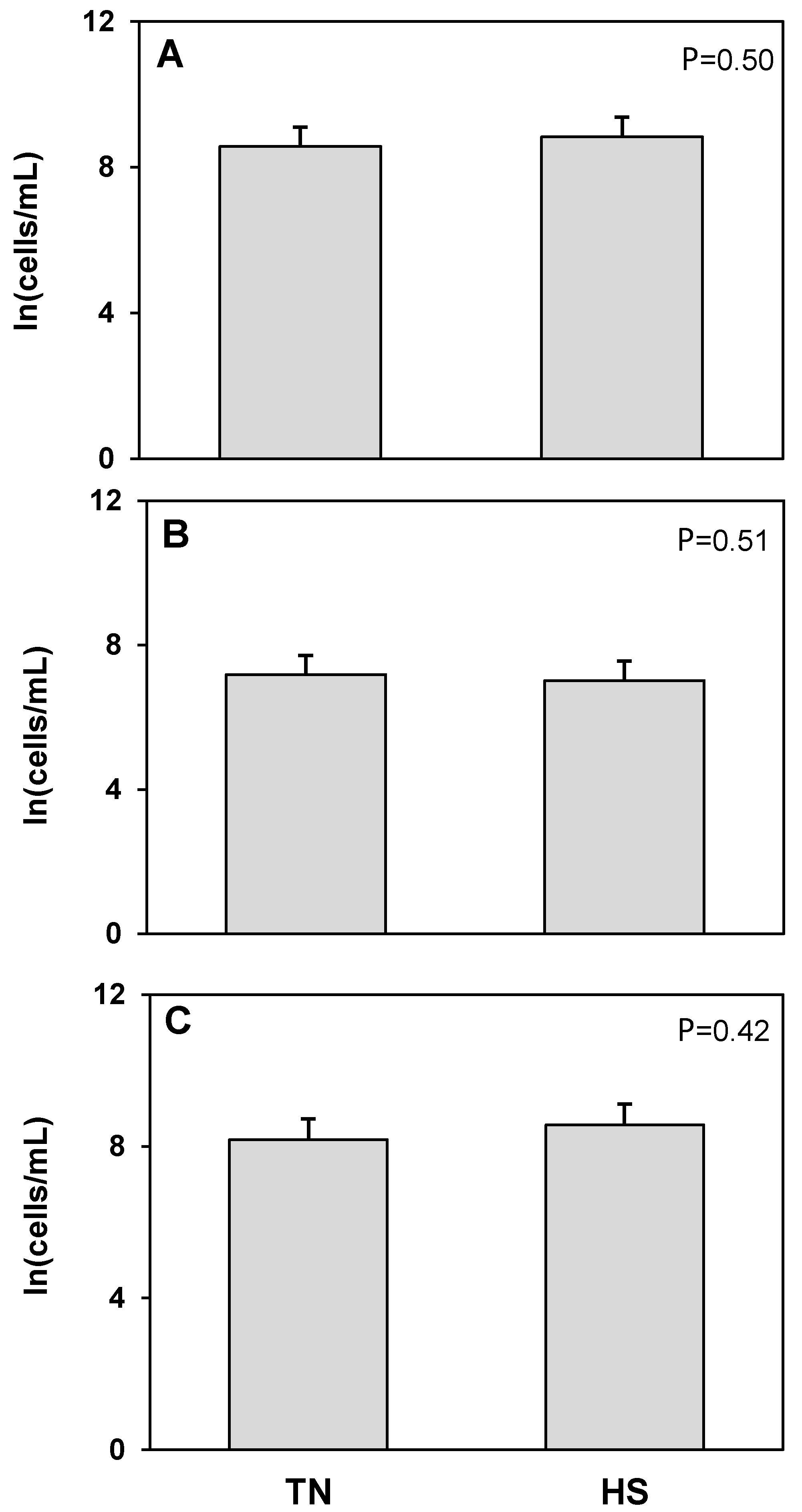
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Vet. World 2018, 11, 562–577. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, J.L.; Cue, R.I.; Botaro, B.G.; Horst, J.A.; Valloto, A.A.; Santos, M.V. Milk losses associated with somatic cell counts by parity and stage of lactation. J. Dairy Sci. 2018, 101, 4357–4366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trend, S.; de Jong, E.; Lloyd, M.L.; Kok, C.H.; Richmond, P.; Doherty, D.A.; Simmer, K.; Kakulas, F.; Strunk, T.; Currie, A. Leukocyte populations in human preterm and term breast milk identified by multicolour flow cytometry. PLoS ONE 2015, 10, e0135580. [Google Scholar] [CrossRef] [Green Version]
- Leitner, G.; Shoshani, E.; Krifucks, O.; Chaffer, M.; Saran, A. Milk leucocyte population patterns in bovine udder infection of different aetiology. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Herve, L.; Quesnel, H.; Lollivier, V.; Boutinaud, M. Regulation of cell number in the mammary gland by controlling the exfoliation process in milk in ruminants. J. Dairy Sci. 2016, 99, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Herve, L.; Quesnel, H.; Lollivier, V.; Portanguen, J.; Bruckmaier, R.M.; Boutinaud, M. Mammary epithelium disruption and mammary epithelial cell exfoliation during milking in dairy cows. J. Dairy Sci. 2017, 100, 9824–9834. [Google Scholar] [CrossRef] [Green Version]
- Boutinaud, M.; Ben Chedly, M.H.; Delamaire, E.; Guinard-Flament, J. Milking and feed restriction regulate transcripts of mammary epithelial cells purified from milk. J. Dairy Sci. 2008, 91, 988–998. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Impact of different seasons on the milk somatic and differential cell counts, milk cortisol and neutrophils functionality of three Indian native breeds of cattle. J. Therm. Biol. 2018, 78, 27–35. [Google Scholar] [CrossRef]
- Olde Riekerink, R.G.; Barkema, H.W.; Stryhn, H. The effect of season on somatic cell count and the incidence of clinical mastitis. J. Dairy Sci. 2007, 90, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Basirico, L.; Morera, P.; Nardone, A. Heat stress elicits different responses in peripheral blood mononuclear cells from Brown Swiss and Holstein cows. J. Dairy Sci. 2006, 89, 4606–4612. [Google Scholar] [CrossRef] [Green Version]
- Elvinger, F.; Hansen, P.J.; Natzke, R.P. Modulation of function of bovine polymorphonuclear leukocytes and lymphocytes by high temperature in vitro and in vivo. Am. J. Vet. Res. 1991, 52, 1692–1698. [Google Scholar]
- Lecchi, C.; Rota, N.; Vitali, A.; Ceciliani, F.; Lacetera, N. In vitro assessment of the effects of temperature on phagocytosis, reactive oxygen species production and apoptosis in bovine polymorphonuclear cells. Vet. Immunol. Immunopathol. 2016, 182, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kamwanja, L.A.; Chase, C.C., Jr.; Gutierrez, J.A.; Guerriero, V., Jr.; Olson, T.A.; Hammond, A.C.; Hansen, P.J. Responses of bovine lymphocytes to heat shock as modified by breed and antioxidant status. J. Anim. Sci. 1994, 72, 438–444. [Google Scholar] [CrossRef]
- Capuco, A.V.; Ellis, S.E.; Hale, S.A.; Long, E.; Erdman, R.A.; Zhao, X.; Paape, M.J. Lactation persistency: Insights from mammary cell proliferation studies. J. Anim. Sci. 2003, 81 (Suppl. 3), 18–31. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.W.; Arneson, A.G.; Byrd, M.K.H.; Negron-Perez, V.M.; Newberne, H.M.; White, R.R.; El-Kadi, S.W.; Ealy, A.D.; Rhoads, R.P.; Rhoads, M.L. Comparison of production-related responses to hyperinsulinemia and hypoglycemia induced by clamp procedures or heat stress of lactating dairy cattle. J. Dairy Sci. 2022, 105, 8439–8453. [Google Scholar] [CrossRef] [PubMed]
- Zimbelman, R.B.; Rhoads, R.P.; Rhoads, M.L.; Duff, G.C.; Baumgard, L.H.; Collier, R.J. A re-evaluation of the impact of temperature humidity index (THI) and Black Globe Humidity Index (BGHI) on milk production in high producing dairy cows. In Proceedings of the 24th Annual Southwest Nutrition and Management Conference, Tempe, AZ, USA, 26–27 February 2009; pp. 158–168. [Google Scholar]
- Lengi, A.J.; Makris, M.; Corl, B.A. A flow cytometric method for measuring and isolating mammary epithelial cells from bovine milk. JDS Commun. 2021, in press. [Google Scholar] [CrossRef]
- DasDas, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Paape, M.J.; Schultze, W.D.; Miller, R.H.; Smith, J.W. Thermal stress and circulating erythrocytes, leucocytes, and milk somatic cells. J. Dairy Sci. 1973, 56, 84–91. [Google Scholar] [CrossRef]
- Wegner, T.N.; Schuh, J.D.; Nelson, F.E.; Stott, G.H. Effect of stress on blood leucocyte and milk somatic cell counts in dairy cows. J. Dairy Sci. 1976, 59, 949–956. [Google Scholar] [CrossRef]
- Smith, D.L.; Smith, T.; Rude, B.J.; Ward, S.H. Short communication: Comparison of the effects of heat stress on milk and component yields and somatic cell score in Holstein and Jersey cows. J. Dairy Sci. 2013, 96, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Ravagnolo, O.; Misztal, I.; Hoogenboom, G. Genetic component of heat stress in dairy cattle, development of heat index function. J. Dairy Sci. 2000, 83, 2120–2125. [Google Scholar] [CrossRef]
- Knapp, D.M.; Grummer, R.R. Response of lactating dairy cows to fat supplementation during heat stress. J. Dairy Sci. 1991, 74, 2573–2579. [Google Scholar] [CrossRef]
- Garner, J.B.; Douglas, M.L.; Williams, S.R.; Wales, W.J.; Marett, L.C.; Nguyen, T.T.; Reich, C.M.; Hayes, B.J. Genomic selection improves heat tolerance in dairy cattle. Sci. Rep. 2016, 6, 34114. [Google Scholar] [CrossRef]
- Ogg, S.L.; Weldon, A.K.; Dobbie, L.; Smith, A.J.; Mather, I.H. Expression of butyrophilin (Btn1a1) in lactating mammary gland is essential for the regulated secretion of milk-lipid droplets. Proc. Natl. Acad. Sci. USA 2004, 101, 10084–10089. [Google Scholar] [CrossRef] [Green Version]
- Capuco, A.V.; Wood, D.L.; Baldwin, R.; McLeod, K.; Paape, M.J. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relation to milk production and effect of bST. J. Dairy Sci. 2001, 84, 2177–2187. [Google Scholar] [CrossRef]
- Herve, L.; Quesnel, H.; Veron, M.; Portanguen, J.; Gross, J.J.; Bruckmaier, R.M.; Boutinaud, M. Milk yield loss in response to feed restriction is associated with mammary epithelial cell exfoliation in dairy cows. J. Dairy Sci. 2019, 102, 2670–2685. [Google Scholar] [CrossRef] [Green Version]
- Donovan, J.A.; Koretzky, G.A. CD45 and the immune response. J. Am. Soc. Nephrol. 1993, 4, 976–985. [Google Scholar] [CrossRef]
- Woodford-Thomas, T.; Thomas, M.L. The leukocyte common antigen, CD45 and other protein tyrosine phosphatases in hematopoietic cells. Semin. Cell Biol. 1993, 4, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.K.; Nguyen, V.; Muller, H.K.; Walker, A.M. Maternal milk T cells drive development of transgenerational th1 immunity in offspring thymus. J. Immunol. 2016, 197, 2290–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, M.R.; Yang, T.J. Chemotactic activities in nonmastitic and mastitic mammary secretions: Presence of interleukin-8 in mastitic but not nonmastitic secretions. Clin. Diagn. Lab. Immunol. 1998, 5, 82–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler-Heitbrock, H.W.; Ulevitch, R.J. CD14: Cell surface receptor and differentiation marker. Immunol. Today 1993, 14, 121–125. [Google Scholar] [CrossRef]
- Nicod, L.P. Cytokines. 1. Overview. Thorax 1993, 48, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Wang, J.M.; Colotta, F.; Dejana, E.; Mantovani, A.; Oppenheim, J.J.; Larsen, C.G.; Zachariae, C.O.; Matsushima, K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J. Immunol. 1990, 144, 3034–3038. [Google Scholar]
- Vidal, K.; Labeta, M.O.; Schiffrin, E.J.; Donnet-Hughes, A. Soluble CD14 in human breast milk and its role in innate immune responses. Acta Odontol. Scand 2001, 59, 330–334. [Google Scholar] [CrossRef]
- Vidal, K.; Donnet-Hughes, A. CD14: A soluble pattern recognition receptor in milk. Adv. Exp. Med. Biol. 2008, 606, 195–216. [Google Scholar] [CrossRef]
- Frey, E.A.; Miller, D.S.; Jahr, T.G.; Sundan, A.; Bazil, V.; Espevik, T.; Finlay, B.B.; Wright, S.D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J. Exp. Med. 1992, 176, 1665–1671. [Google Scholar] [CrossRef] [Green Version]
- Dawod, B.; Marshall, J.S. Cytokines and soluble receptors in breast milk as enhancers of oral tolerance development. Front. Immunol. 2019, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Ward, T.L.; Goto, K.; Altosaar, I. Ingested soluble CD14 contributes to the functional pool of circulating sCD14 in mice. Immunobiology 2014, 219, 537–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, T.L.; Spencer, W.J.; Davis, L.D.; Harrold, J.; Mack, D.R.; Altosaar, I. Ingested soluble CD14 from milk is transferred intact into the blood of newborn rats. Pediatr. Res. 2014, 75, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Spencer, W.J.; Binette, A.; Ward, T.L.; Davis, L.D.; Blais, D.R.; Harrold, J.; Mack, D.R.; Altosaar, I. Alpha-lactalbumin in human milk alters the proteolytic degradation of soluble CD14 by forming a complex. Pediatr. Res. 2010, 68, 490–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugin, J.; Schurer-Maly, C.C.; Leturcq, D.; Moriarty, A.; Ulevitch, R.J.; Tobias, P.S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 1993, 90, 2744–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin Carli, J.F.; Trahan, G.D.; Jones, K.L.; Hirsch, N.; Rolloff, K.P.; Dunn, E.Z.; Friedman, J.E.; Barbour, L.A.; Hernandez, T.L.; MacLean, P.S.; et al. Single cell rna sequencing of human milk-derived cells reveals sub-populations of mammary epithelial cells with molecular signatures of progenitor and mature states: A novel, non-invasive framework for investigating human lactation physiology. J. Mammary Gland Biol. Neoplasia 2020, 25, 367–387. [Google Scholar] [CrossRef]
- Garcia Sola, M.E.; Stedile, M.; Beckerman, I.; Kordon, E.C. An integrative single-cell transcriptomic atlas of the post-natal mouse mammary gland allows discovery of new developmental trajectories in the luminal compartment. J. Mammary Gland Biol. Neoplasia 2021, 26, 29–42. [Google Scholar] [CrossRef]
- Hughes, K.; Wickenden, J.A.; Allen, J.E.; Watson, C.J. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J. Pathol. 2012, 227, 106–117. [Google Scholar] [CrossRef]
- Stein, T.; Morris, J.S.; Davies, C.R.; Weber-Hall, S.J.; Duffy, M.A.; Heath, V.J.; Bell, A.K.; Ferrier, R.K.; Sandilands, G.P.; Gusterson, B.A. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004, 6, R75-91. [Google Scholar] [CrossRef] [Green Version]
- Monks, J.; Smith-Steinhart, C.; Kruk, E.R.; Fadok, V.A.; Henson, P.M. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol. Reprod. 2008, 78, 586–594. [Google Scholar] [CrossRef]




| Antibody Target | Catalog Number | Clone | Concentration |
|---|---|---|---|
| Primary Antibodies | |||
| BTN1A1 | MAB8467APC a | 2151C | 7.0 ng/µL |
| CD45 | WS0544B-100 b | CACTB51A | 3.1 ng/µL |
| CD3 | WS0561B-100 b | MM1A | 6.25 ng/µL |
| Granulocyte | WS0609B-100 b | MM20A | 1.25 ng/µL |
| CD14 | WS0564B-100 b | CAM36A | 1.25 ng/µL |
| Secondary Antibodies | |||
| Rat anti-mouse IgG2a-PE | 1155-09 c | SB84a | 1.0 ng/µL |
| Goat anti-mouse IgG1-AF488 | 1070-30 c | Polyclonal | 1.25 ng/µL |
| Stains | |||
| Hoechst33342 | H3570 d | 1:1000 | |
| Propidium Iodide | 556463 e | 1:10 | |
| TN | HS | SEM | p | |
|---|---|---|---|---|
| Milk yield (kg per milking) | 28.2 | 21.4 | 1.29 | 0.001 |
| Milk fat | ||||
| % | 4.23 | 3.88 | 0.33 | 0.48 |
| g per milking | 1163 | 836 | 89 | 0.02 |
| Milk protein | ||||
| % | 2.61 | 2.52 | 0.06 | 0.28 |
| g per milking | 737 | 536 | 32 | 0.003 |
| Milk other solids | ||||
| % | 5.87 | 5.73 | 0.04 | 0.02 |
| g per milking | 1656 | 1227 | 78 | 0.001 |
| Somatic Cells b | ||||
| Ln(cells per mL) | 4.17 | 4.24 | 0.54 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengi, A.J.; Stewart, J.W.; Makris, M.; Rhoads, M.L.; Corl, B.A. Heat Stress Increases Mammary Epithelial Cells and Reduces Viable Immune Cells in Milk of Dairy Cows. Animals 2022, 12, 2810. https://doi.org/10.3390/ani12202810
Lengi AJ, Stewart JW, Makris M, Rhoads ML, Corl BA. Heat Stress Increases Mammary Epithelial Cells and Reduces Viable Immune Cells in Milk of Dairy Cows. Animals. 2022; 12(20):2810. https://doi.org/10.3390/ani12202810
Chicago/Turabian StyleLengi, Andrea J., Jacob W. Stewart, Melissa Makris, Michelle L. Rhoads, and Benjamin A. Corl. 2022. "Heat Stress Increases Mammary Epithelial Cells and Reduces Viable Immune Cells in Milk of Dairy Cows" Animals 12, no. 20: 2810. https://doi.org/10.3390/ani12202810
APA StyleLengi, A. J., Stewart, J. W., Makris, M., Rhoads, M. L., & Corl, B. A. (2022). Heat Stress Increases Mammary Epithelial Cells and Reduces Viable Immune Cells in Milk of Dairy Cows. Animals, 12(20), 2810. https://doi.org/10.3390/ani12202810





