The Effect of Chiropractic Treatment on Limb Lameness and Concurrent Axial Skeleton Pain and Dysfunction in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion-Exclusion Criteria
2.3. Study Design
2.4. Subjective Lameness Evaluation
2.5. Objective Lameness Evaluation
2.6. Spinal Evaluation
2.7. Active Range of Motion
- Lateral bending of the cranial cervical region (Figure 1a): The treat was initially positioned approximately 12–18 inches lateral to the head and held at the height of the withers to maintain cervical extension. The treat was then moved caudally to direct the muzzle of the horse laterally and caudally until the horse’s head was facing caudally (i.e., perpendicular to the long axis of the trunk) or until the horse was no longer able to follow the treat (i.e., end range of motion).
- Lateral bending of the middle cervical region (Figure 1b): The treat was initially positioned approximately 12–18 inches lateral to the head and the horses’ muzzle was directed laterally and caudally toward the point of the elbow at the girth region with the neck maintained in a neutral flexion-extension position. Attention was focused on the ability to laterally bend the middle cervical region.
- Lateral bending of the caudal cervical region (Figure 1c): The treat was initially positioned approximately 12–18 inches lateral to the head and the horses’ muzzle was directed laterally and ventrally toward the lateral surface of the ipsilateral carpus to induce concurrent cervical flexion. Attention was focused on the ability to laterally bend the caudal cervical region around the ipsilateral scapula and shoulder region.
- Combined lateral bending of the cervical and thoracolumbar regions (Figure 2a): The horse’s tail was grasped with the caudal hand and lateral tension was applied until quadriceps muscle activation of the ipsilateral hind limb was observed. Simultaneously, the treat was positioned approximately 24–36 inches lateral to the girth region and the horses’ muzzle was directed laterally and caudally toward the stifle region. Attention was focused on the ability of the horse to touch and maintain the muzzle position at the stifle region.
- Combined flexion and lateral bending of the cervical and thoracolumbar regions: The same procedure was repeated, except that the treat was directed toward the ipsilateral tarsal region to induce concurrent trunk flexion and lateral bending. Attention was focused on the ability to activate the internal abdominal oblique muscle (Figure 2b).
2.8. Spinal Reflexes
2.9. Mechanical Nociceptive Thresholds
2.10. Chiropractic Treatment
2.11. Statistical Analysis
3. Results
3.1. Subjects
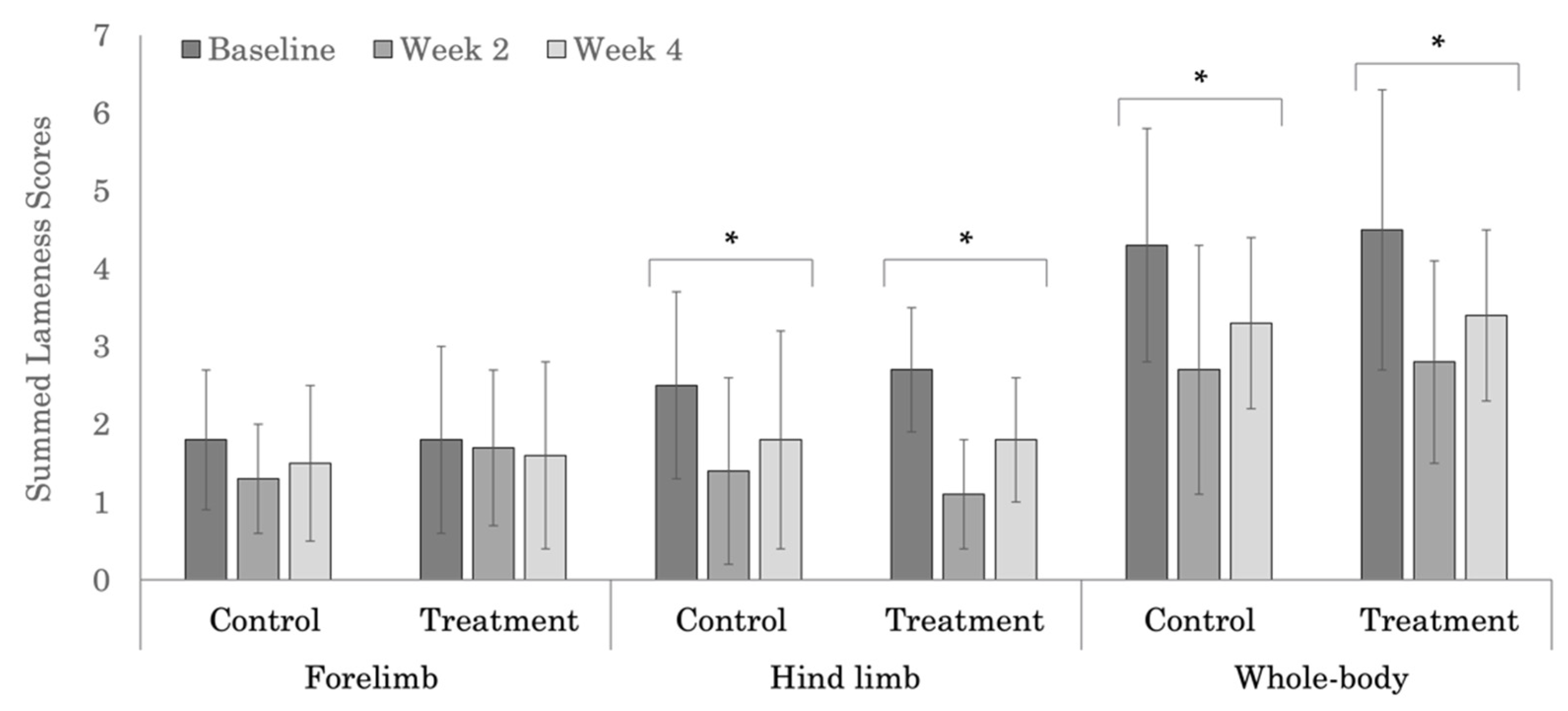
3.2. Subjective Lameness Evaluation
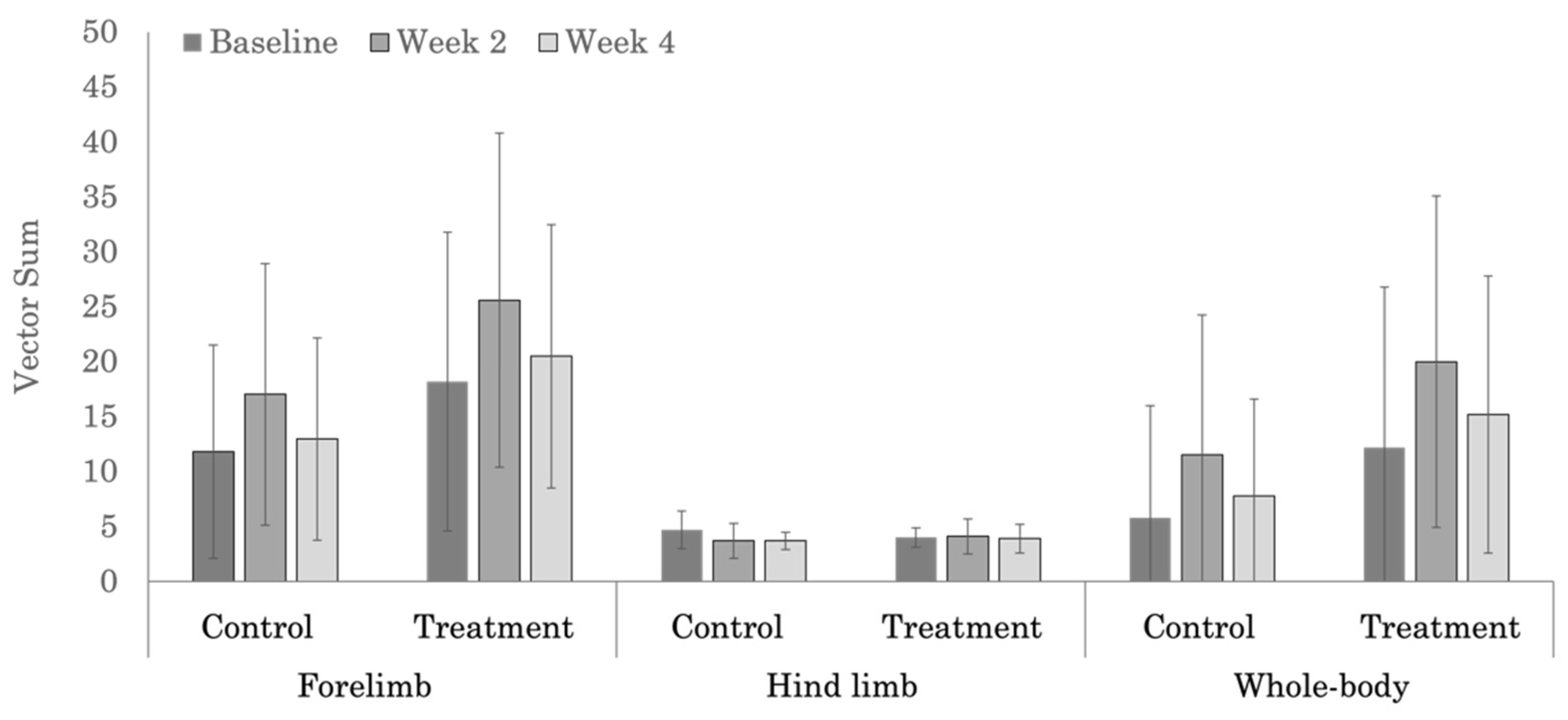
3.3. Objective Lameness Evaluation
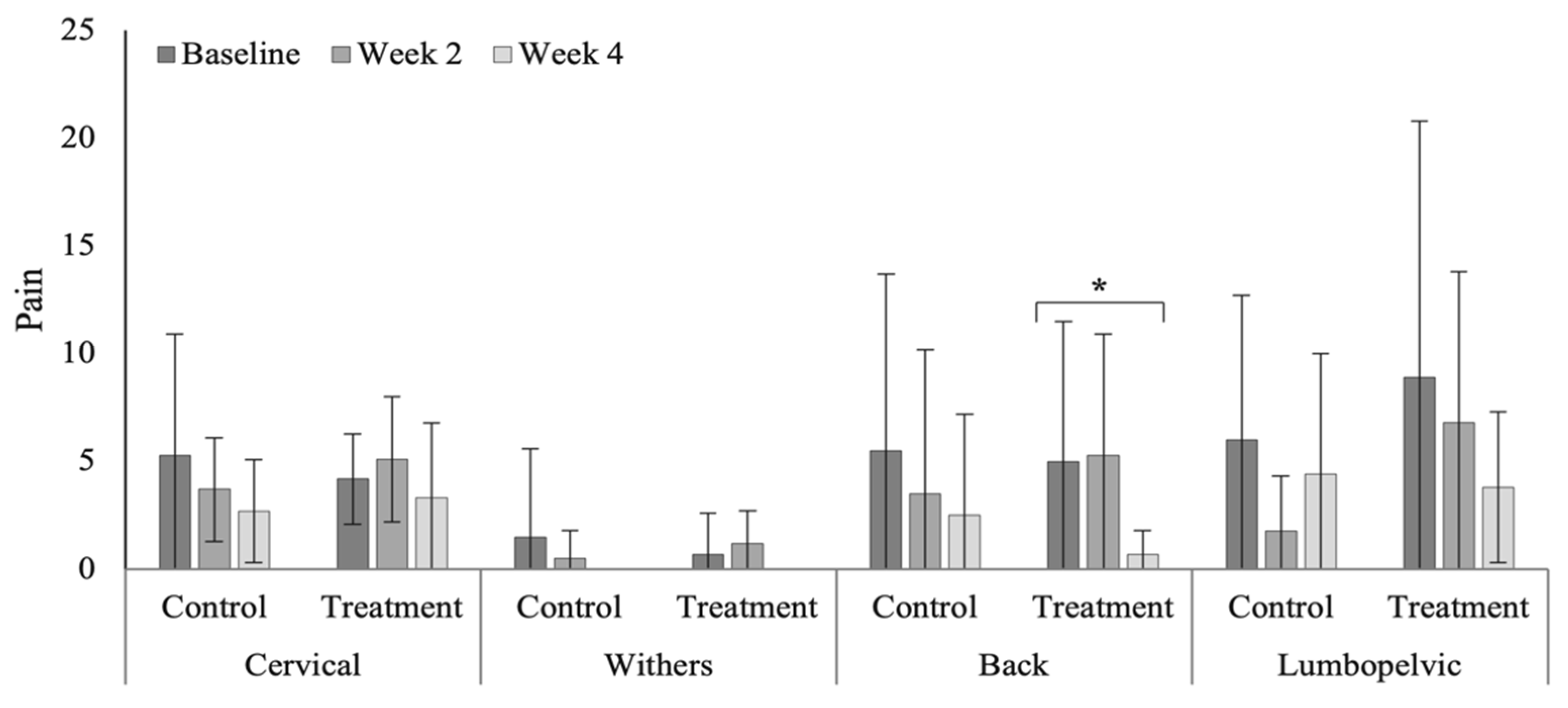
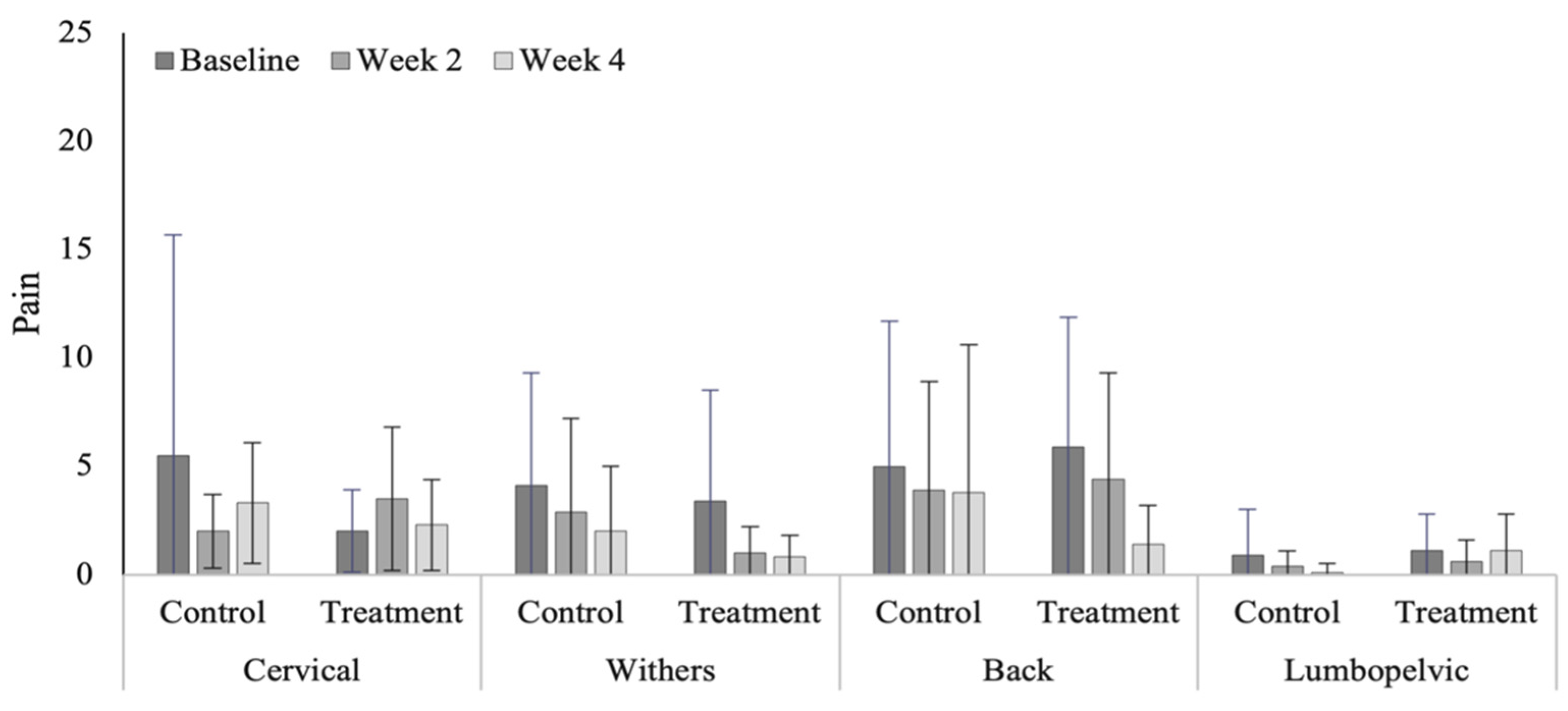
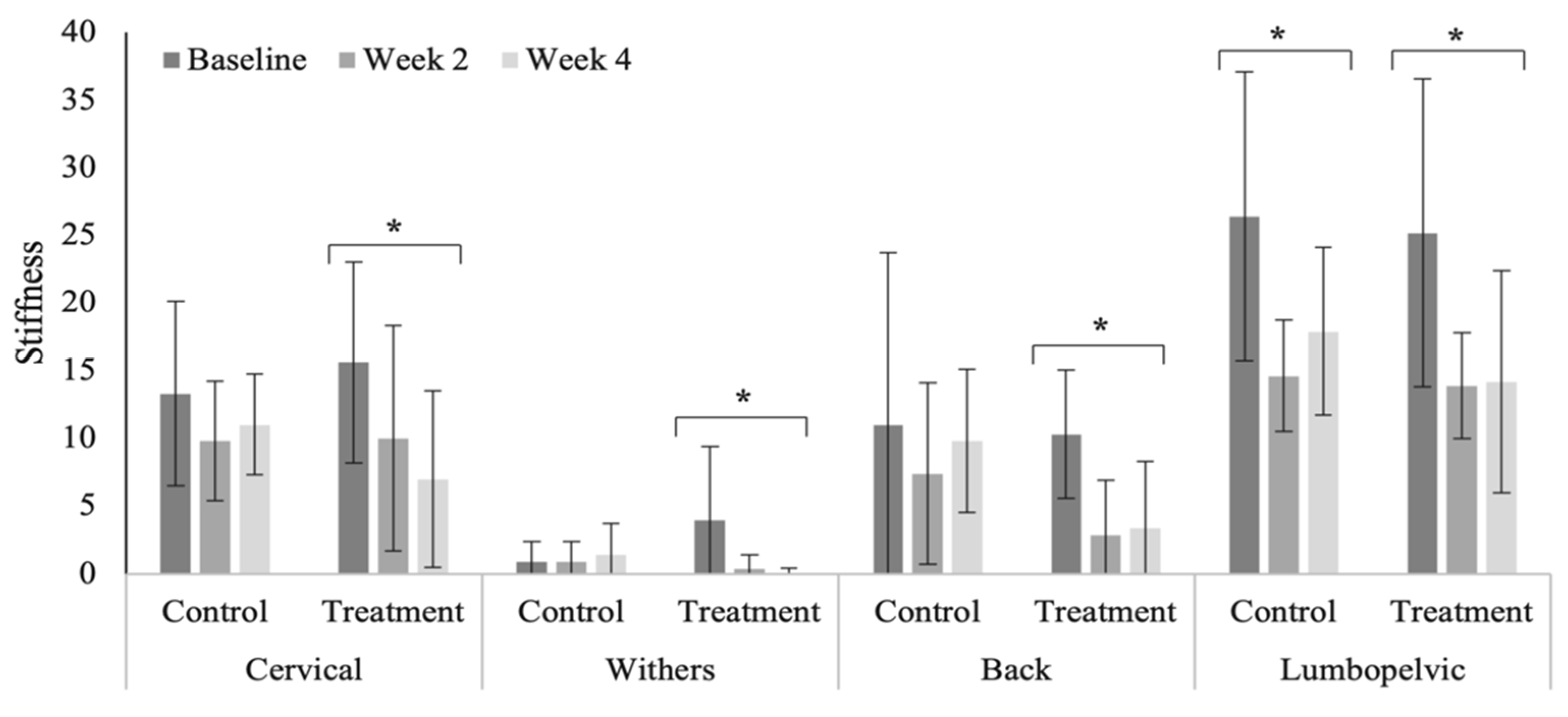
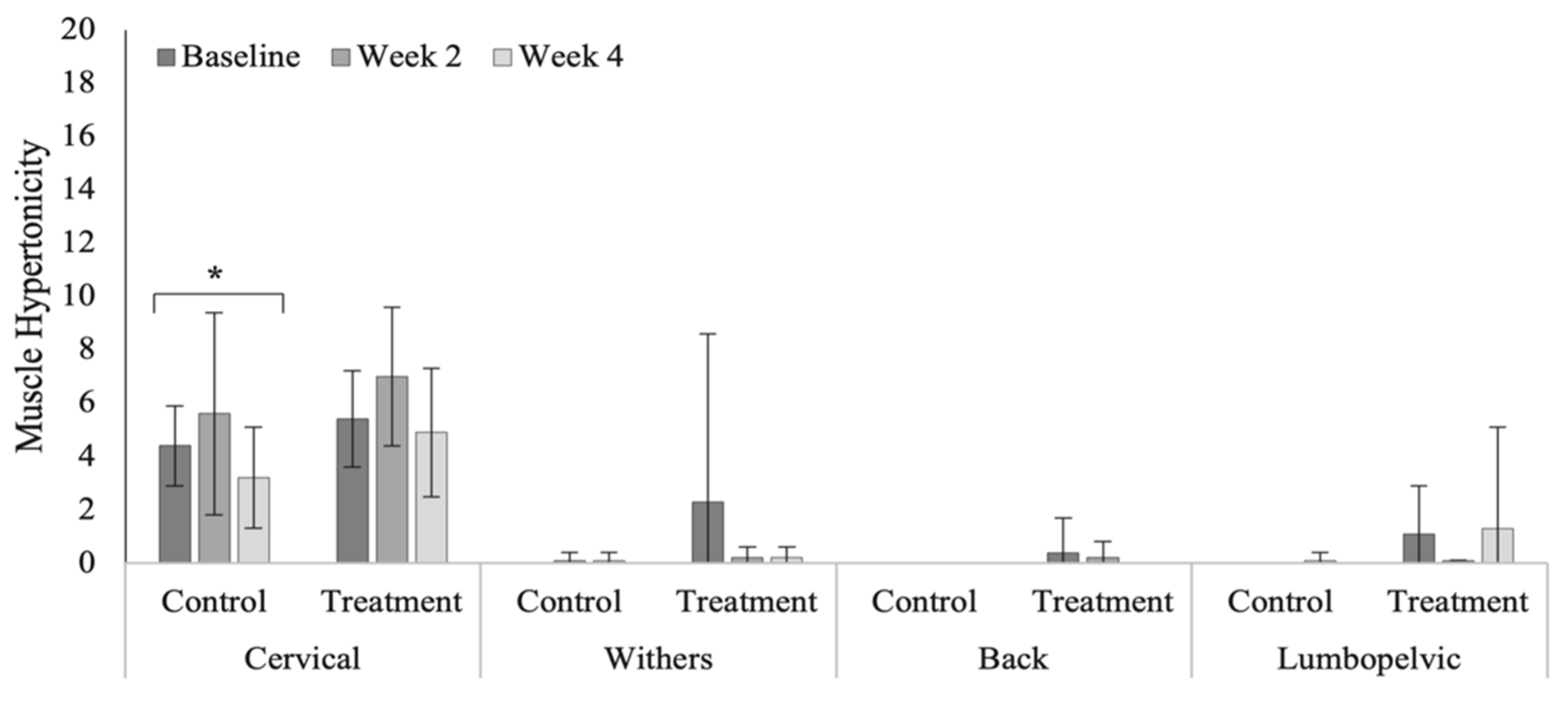
3.4. Spinal Evaluation
3.5. Active Range of Motion
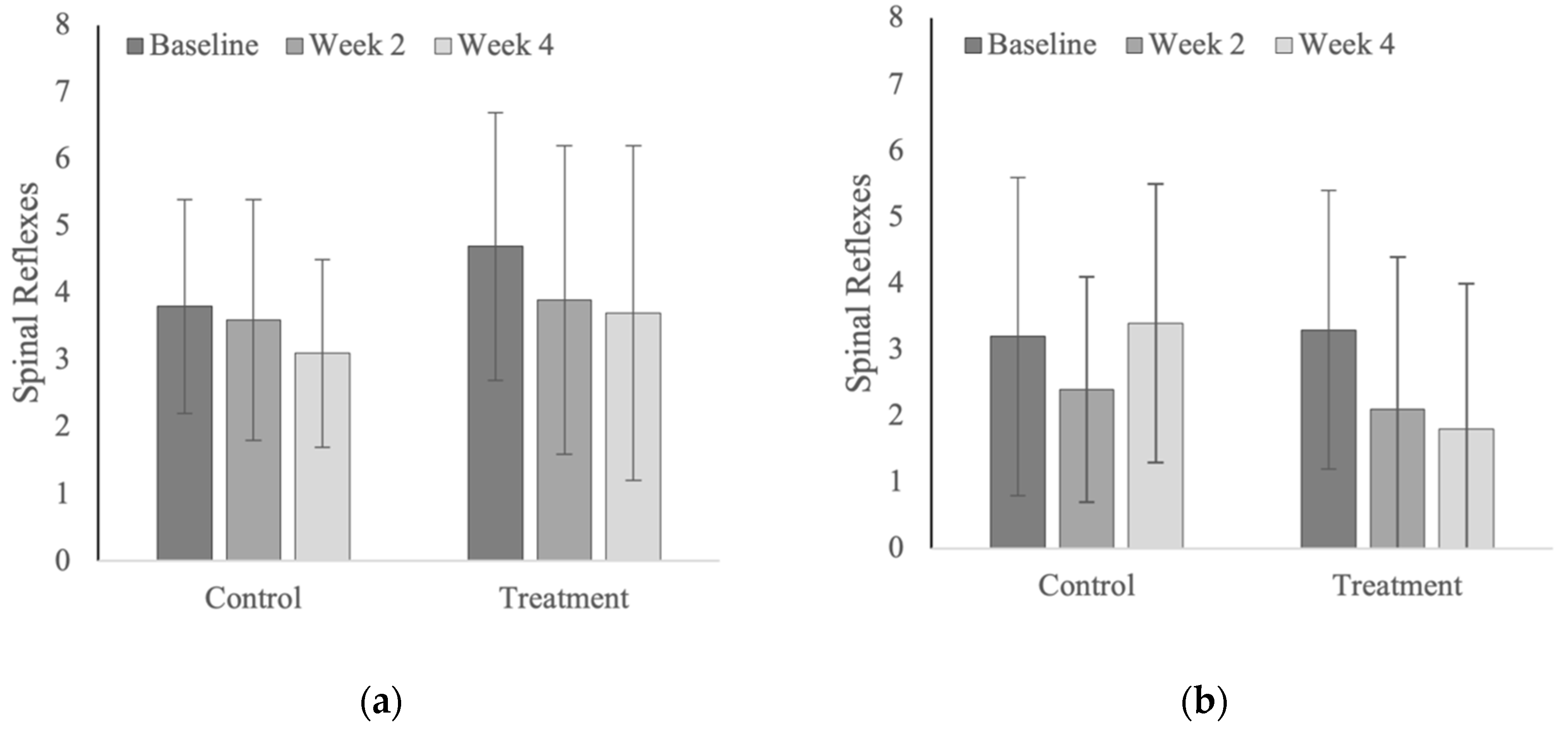
3.6. Spinal Reflexes
3.7. Mechanical Nociceptive Thresholds
4. Discussion
4.1. Subjects
4.2. Subjective Lameness Evaluation
4.3. Objective Lameness Evaluation
4.4. Spinal Evaluation
4.5. Active Range of Motion
4.6. Spinal Reflexes
4.7. Mechanical Nociceptive Thresholds
4.8. Chiropractic Treatment
4.9. Limitations
4.10. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year 1 | ||||
|---|---|---|---|---|
| Group | Baseline | Week 2 | Week 4 | p-values |
| Treatment | 1.8 ± 1.2 | 1.7 ± 1.0 | 1.6 ± 1.2 | 0.840 |
| Control | 1.8 ± 0.9 | 1.3 ± 0.7 | 1.5 ± 1.0 | 0.341 |
| p-values | 1.000 | 0.378 | 0.825 | |
| Year 2 | ||||
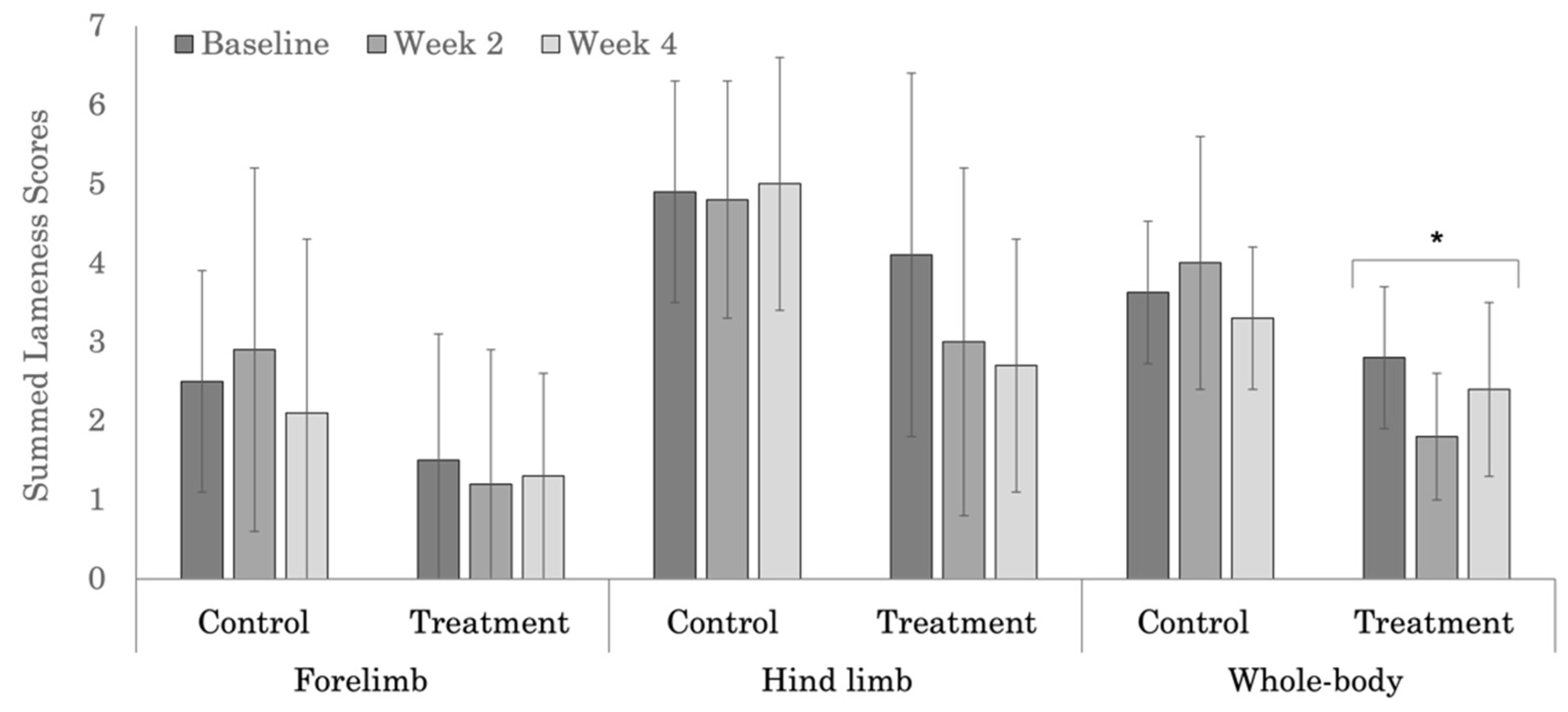
| Treatment | 1.5 ± 1.6 | 1.2 ± 1.7 | 1.3 ± 1.3 | 0.845 |
| Control | 2.5 ± 1.4 | 2.9 ± 2.3 | 2.1 ± 2.2 | 0.450 |
| p-values | 0.239 | 0.053 | 0.330 | |
| Year 1 | ||||
|---|---|---|---|---|
| Group | Baseline | Week 2 | Week 4 | p-values |
| Treatment | 2.7 ± 0.8 | 1.1 ± 0.7 | 1.8 ± 0.8 | <0.0001 |
| Control | 2.5 ± 1.2 | 1.4 ± 1.2 | 1.8 ± 1.4 | 0.006 |
| p-values | 0.672 | 0.526 | 1.000 | |
| Year 2 | ||||
| Treatment | 4.1 ± 2.3 | 3.0 ± 2.2 | 2.7 ± 1.6 | 0.078 |
| Control | 4.9 ± 1.4 | 4.8 ± 1.5 | 5.0 ± 1.6 | 0.939 |
| p-values | 0.376 | 0.050 | 0.012 | |
| Year 1 | ||||
|---|---|---|---|---|
| Group | Baseline | Week 2 | Week 4 | p-values |
| Treatment | 4.5 ± 1.8 | 2.8 ± 1.3 | 3.4 ± 1.1 | 0.002 |
| Control | 4.3 ± 1.5 | 2.7 ± 1.6 | 3.3 ± 1.1 | 0.005 |
| p-values | 0.776 | 0.887 | 0.887 | |
| Year 2 | ||||
| Treatment | 2.8 ± 0.9 | 1.8 ± 0.8 | 2.4 ± 1.1 | 0.027 |
| Control | 3.6 ± 0.9 | 4.0 ± 1.6 | 3.3 ± 0.9 | 0.182 |
| p-values | 0.107 | <0.0001 | 0.097 | |
| Year 1 | ||||
|---|---|---|---|---|
| Group | Baseline | Week 2 | Week 4 | p-values |
| Treatment | 18.2 ± 13.6 | 25.6 ± 15.2 | 20.5 ± 12.0 | 0.812 |
| Control | 11.8 ± 9.7 | 17.0 ± 11.9 | 13.0 ± 9.2 | 0.292 |
| p-values | 0.122 | 0.488 | 0.117 | |
| Year 2 | ||||
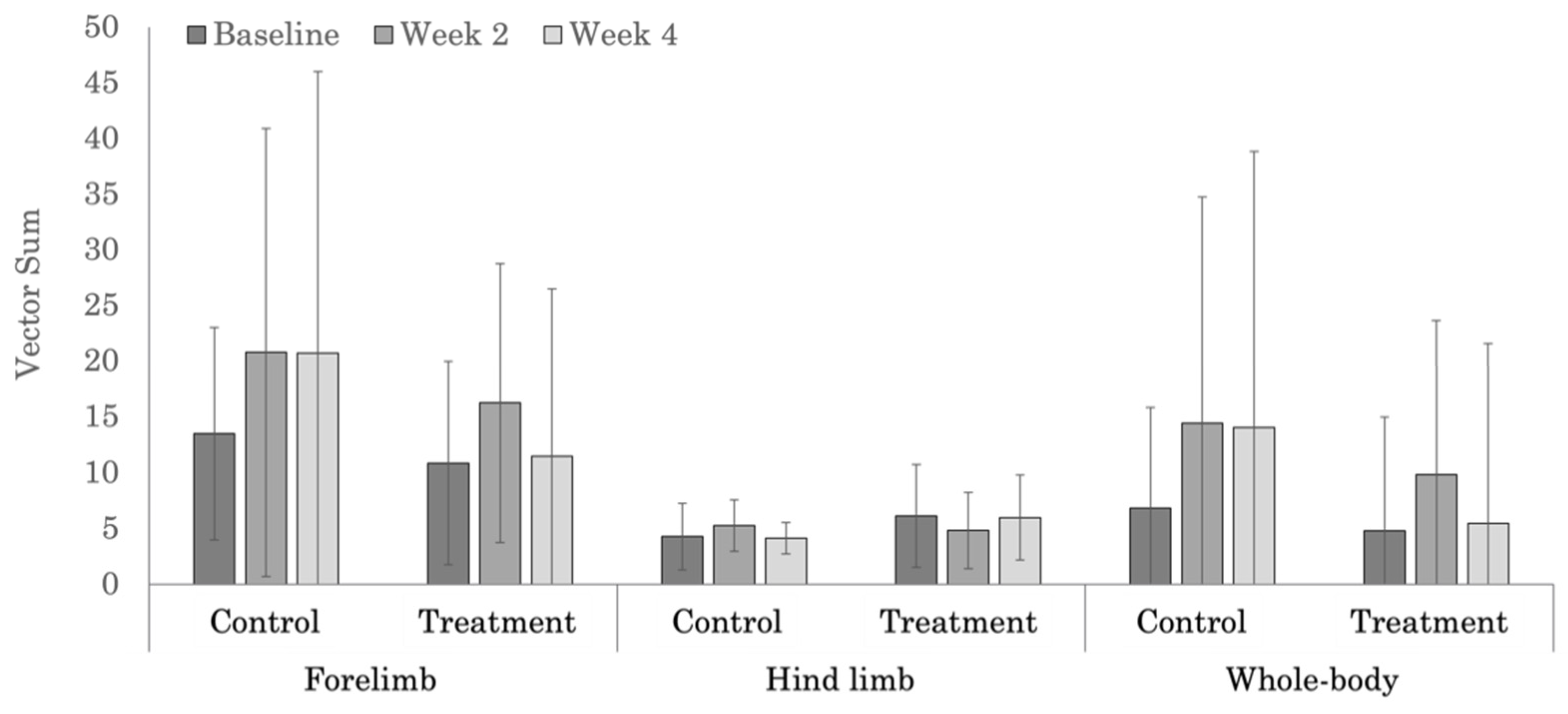
| Treatment | 10.8 ± 9.1 | 16.2 ± 12.5 | 11.5 ± 15.0 | 0.286 |
| Control | 13.5 ± 9.5 | 20.8 ± 20.1 | 20.7 ± 25.3 | 0.139 |
| p-values | 0.729 | 0.552 | 0.232 | |
| Year 1 | ||||
|---|---|---|---|---|
| Group | Baseline | Week 2 | Week 4 | p-values |
| Treatment | 4.0 ± 0.9 | 4.1 ± 1.6 | 3.9 ± 1.3 | 0.521 |
| Control | 4.7 ± 1.7 | 3.7 ± 1.6 | 3.7 ± 0.8 | 0.613 |
| p-values | 0.824 | 0.887 | 0.883 | |
| Year 2 | ||||
| Treatment | 6.1 ± 4.6 | 4.8 ± 3.4 | 6.0 ± 3.8 | 0.346 |
| Control | 4.3 ± 3.0 | 5.3 ± 2.3 | 4.1 ± 1.4 | 0.525 |
| p-values | 0.248 | 0.777 | 0.255 | |
| Year 1 | ||||
|---|---|---|---|---|
| Group | Baseline | Week 2 | Week 4 | p-values |
| Treatment | 12.2 ± 14.6 | 20.0 ± 15.1 | 15.2 ± 12.6 | 0.228 |
| Control | 5.8 ± 10.2 | 11.5 ± 12.7 | 7.8 ± 8.8 | 0.440 |
| p-values | 0.261 | 0.138 | 0.193 | |
| Year 2 | ||||
| Treatment | 4.8 ± 10.2 | 9.8 ± 13.8 | 5.4 ± 16.1 | 0.365 |
| Control | 6.8 ± 9.0 | 14.4 ± 20.3 | 14.0 ± 24.8 | 0.148 |
| p-values | 0.794 | 0.557 | 0.279 | |
| Year 1 | |||||
|---|---|---|---|---|---|
| Region | Group | Baseline | Week 2 | Week 4 | p-values |
| Cervical | Treatment | 4.2 ± 2.1 | 5.1 ± 2.9 | 3.3 ± 3.5 | 0.446 |
| Control | 5.3 ± 5.6 | 3.7 ± 2.4 | 2.7 ± 2.4 | 0.188 | |
| p-values | 0.467 | 0.356 | 0.691 | ||
| Cranial thoracic | Treatment | 0.7 ± 1.9 | 1.2 ± 1.5 | 0.0 ± 0.0 | 0.365 |
| Control | 1.5 ± 4.1 | 0.5 ± 1.3 | 0.0 ± 0.0 | 0.256 | |
| p-values | 0.665 | 0.387 | 1.000 | ||
| Caudal thoracic | Treatment | 5.0 ± 6.5 | 5.3 ± 5.6 | 0.7 ± 1.1 | 0.029 |
| Control | 5.5 ± 8.2 | 3.5 ± 6.7 | 2.5 ± 4.7 | 0.266 | |
| p-values | 0.851 | 0.501 | 0.501 | ||
| Lumbopelvic | Treatment | 8.9 ± 11.9 | 6.8 ± 7.0 | 3.8 ± 3.5 | 0.164 |
| Control | 6.0 ± 6.7 | 1.8 ± 2.5 | 4.4 ± 5.6 | 0.285 | |
| p-values | 0.352 | 0.112 | 0.847 | ||
| Year 2 | |||||
| Cervical | Treatment | 2.0 ± 1.9 | 3.5 ± 3.3 | 2.3 ± 2.1 | 0.636 |
| Control | 5.5 ± 10.2 | 2.0 ± 1.7 | 3.3 ± 2.8 | 0.176 | |
| p-values | 0.110 | 0.488 | 0.660 | ||
| Cranial thoracic | Treatment | 3.4 ± 5.1 | 1.0 ± 1.2 | 0.8 ± 1.0 | 0.075 |
| Control | 4.1 ± 5.2 | 2.9 ± 4.3 | 2.0 ± 3.0 | 0.310 | |
| p-values | 0.679 | 0.287 | 0.494 | ||
| Caudal thoracic | Treatment | 5.9 ± 6.0 | 4.4 ± 4.9 | 1.4 ± 1.8 | 0.052 |
| Control | 5.0 ± 6.7 | 3.9 ± 5.0 | 3.8 ± 6.8 | 0.793 | |
| p-values | 0.726 | 0.838 | 0.362 | ||
| Lumbopelvic | Treatment | 1.1 ± 1.7 | 0.6 ± 1.0 | 1.1 ± 1.7 | 0.579 |
| Control | 0.9 ± 2.1 | 0.4 ± 0.7 | 0.1 ± 0.4 | 0.468 | |
| p-values | 0.736 | 0.736 | 0.149 | ||
| Year 1 | |||||
|---|---|---|---|---|---|
| Region | Group | Baseline | Week 2 | Week 4 | p-values |
| Cervical | Treatment | 4.7 ± 3.2 | 7.0 ± 10.2 | 1.9 ± 1.9 | 0.055 |
| Control | 5.4 ± 2.4 | 3.6 ± 2.5 | 3.1 ± 2.3 | 0.501 | |
| p-values | 0.744 | 0.116 | 0.575 | ||
| Cranial thoracic | Treatment | 1.7 ± 3.1 | 0.9 ± 1.4 | 1.7 ± 2.4 | 0.620 |
| Control | 1.2 ± 1.9 | 1.0 ± 1.9 | 0.5 ± 0.8 | 0.746 | |
| p-values | 0.585 | 0.913 | 0.193 | ||
| Caudal thoracic | Treatment | 7.1 ± 12.2 | 3.4 ± 7.5 | 3.2 ± 10.1 | 0.558 |
| Control | 4.5 ± 7.5 | 3.0 ± 6.3 | 5.4 ± 8.8 | 0.835 | |
| p-values | 0.519 | 0.921 | 0.585 | ||
| Lumbopelvic | Treatment | 11.6 ± 15.5 | 5.9 ± 7.5 | 2.8 ± 6.8 | 0.091 |
| Control | 10.4 ± 9.5 | 0.9 ± 1.2 | 4.4 ± 7.2 | 0.064 | |
| p-values | 0.767 | 0.219 | 0.692 | ||
| Year 2 | |||||
| Cervical | Treatment | 15.6 ± 7.4 | 10.0 ± 8.3 | 7.0 ± 6.5 | 0.024 |
| Control | 13.3 ± 6.8 | 9.8 ± 4.4 | 11.0 ± 3.7 | 0.580 | |
| p-values | 0.453 | 0.936 | 0.204 | ||
| Cranial thoracic | Treatment | 4.0 ± 5.4 | 0.4 ± 1.0 | 0.1 ± 0.3 | 0.001 |
| Control | 0.9 ± 1.5 | 0.9 ± 1.5 | 1.4 ± 2.3 | 0.882 | |
| p-values | 0.017 | 0.707 | 0.315 | ||
| Caudal thoracic | Treatment | 10.3 ± 4.7 | 2.9 ± 4.0 | 3.4 ± 4.9 | 0.004 |
| Control | 11.0 ± 12.7 | 7.4 ± 6.7 | 9.8 ± 5.3 | 0.370 | |
| p-values | 0.828 | 0.172 | 0.055 | ||
| Lumbopelvic | Treatment | 25.2 ± 11.4 | 13.9 ± 3.9 | 14.2 ± 8.2 | 0.002 |
| Control | 26.4 ± 10.7 | 14.6 ± 4.1 | 17.9 ± 6.2 | 0.009 | |
| p-values | 0.760 | 0.850 | 0.340 | ||
| Year 1 | |||||
|---|---|---|---|---|---|
| Region | Group | Baseline | Week 2 | Week 4 | p-values |
| Cervical | Treatment | 5.4 ± 1.8 | 7.0 ± 2.6 | 4.9 ± 2.4 | 0.073 |
| Control | 4.4 ± 1.5 | 5.6 ± 3.8 | 3.2 ± 1.9 | 0.046 | |
| p-values | 0.366 | 0.207 | 0.127 | ||
| Cranial thoracic | Treatment | 2.3 ± 6.3 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.119 |
| Control | 0.0 ± 0.0 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.995 | |
| p-values | 0.052 | 0.931 | 0.931 | ||
| Caudal thoracic | Treatment | 0.4 ± 1.3 | 0.2 ± 0.6 | 0.0 ± 0.0 | 0.328 |
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.000 | |
| p-value | 0.127 | 0.442 | 1.000 | ||
| Lumbopelvic | Treatment | 1.1 ± 1.8 | 0.1 ± 0.0 | 1.3 ± 3.8 | 0.293 |
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.3 | 0.990 | |
| p-values | 0.157 | 0.897 | 0.124 | ||
| Year 2 | |||||
| Cervical | Treatment | 7.3 ± 7.0 | 4.4 ± 3.5 | 4.1 ± 2.7 | 0.149 |
| Control | 3.4 ± 3.4 | 5.0 ± 2.7 | 6.0 ± 3.0 | 0.413 | |
| p-values | 0.051 | 0.761 | 0.337 | ||
| Cranial thoracic | Treatment | 6.5 ± 4.9 | 5.3 ± 4.3 | 8.3 ± 5.1 | 0.437 |
| Control | 5.3 ± 3.3 | 6.0 ± 4.4 | 9.4 ± 7.6 | 0.252 | |
| p-values | 0.605 | 0.772 | 0.656 | ||
| Caudal thoracic | Treatment | 3.8 ± 3.2 | 5.7 ± 2.8 | 7.0 ± 3.4 | 0.256 |
| Control | 4.1 ± 5.2 | 5.5 ± 4.6 | 9.8 ± 5.6 | 0.034 | |
| p-values | 0.869 | 0.919 | 0.166 | ||
| Lumbopelvic | Treatment | 7.7 ± 7.5 | 6.7 ± 4.0 | 4.4 ± 2.9 | 0.195 |
| Control | 3.6 ± 5.6 | 3.4 ± 2.9 | 9.1 ± 6.1 | 0.012 | |
| p-values | 0.102 | 0.179 | 0.059 | ||
| Year 1 | ||||||
|---|---|---|---|---|---|---|
| Parameter | Attribute | Group | Baseline | Week 2 | Week 4 | p-values |
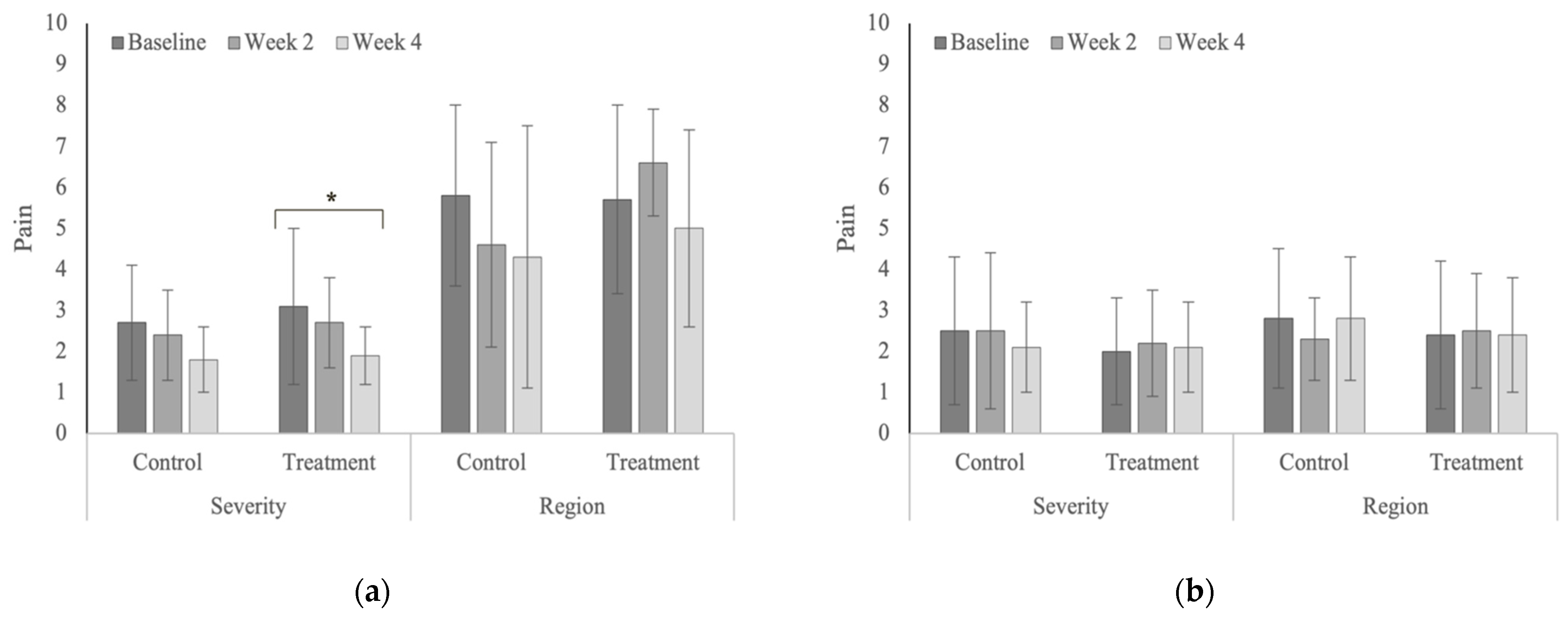
| Pain | Severity | Treatment | 3.1 ± 1.85 | 2.7 ± 1.1 | 1.9 ± 0.7 | 0.041 |
| Control | 2.7 ± 1.4 | 2.4 ± 1.1 | 1.8 ± 0.8 | 0.154 | ||
| p-values | 0.466 | 0.584 | 0.855 | |||
| Region | Treatment | 5.7 ± 2.3 | 6.6 ± 1.3 | 5.0 ± 2.4 | 0.198 | |
| Control | 5.8 ± 2.2 | 4.6 ± 2.5 | 4.3 ± 3.2 | 0.204 | ||
| p-values | 0.924 | 0.063 | 0.508 | |||
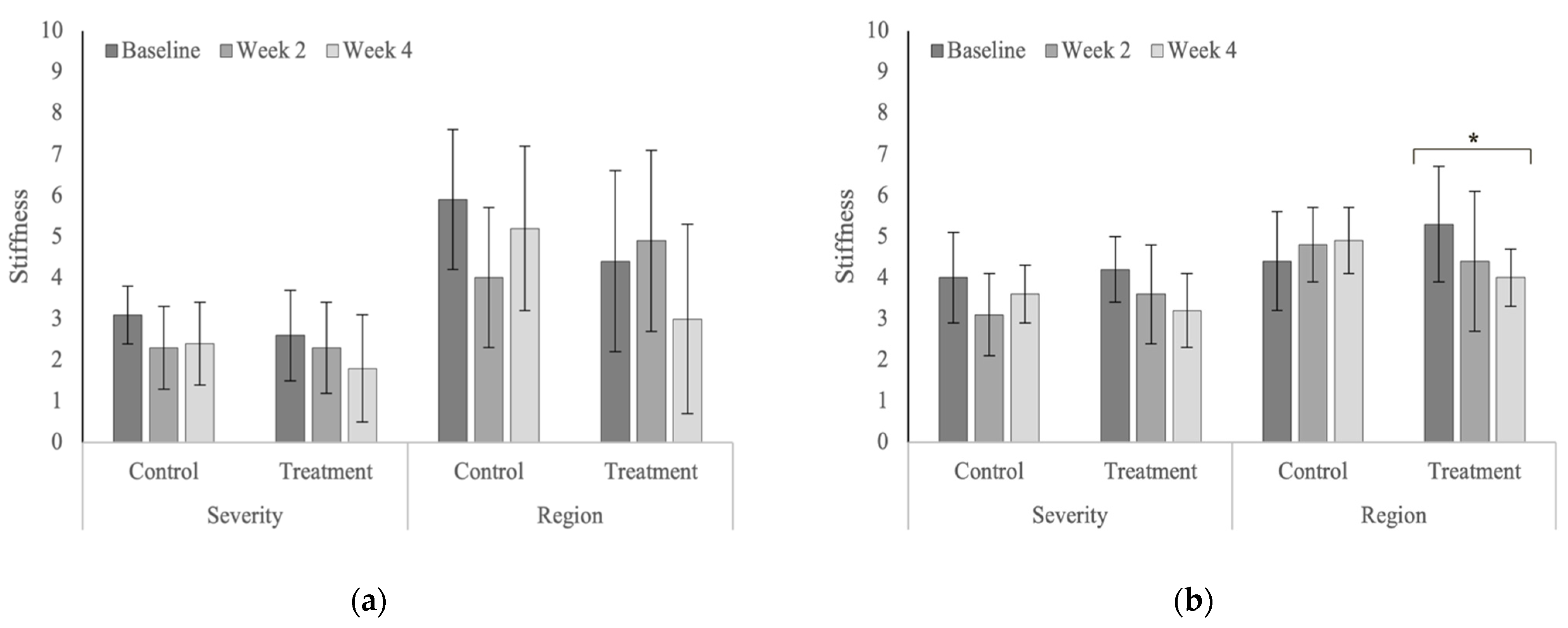
| Stiffness | Severity | Treatment | 2.6 ± 1.1 | 2.3 ± 1.1 | 1.8 ± 1.3 | 0.200 |
| Control | 3.1 ± 0.7 | 2.3 ± 1.0 | 2.4 ± 1.0 | 0.156 | ||
| p-value | 0.284 | 1.000 | 0.199 | |||
| Region | Treatment | 4.4 ± 2.2 | 4.9 ± 2.2 | 3.0 ± 2.3 | 0.085 | |
| Control | 5.9 ± 1.7 | 4.0 ± 1.7 | 5.2 ± 2.0 | 0.095 | ||
| p-values | 0.105 | 0.327 | 0.019 | |||
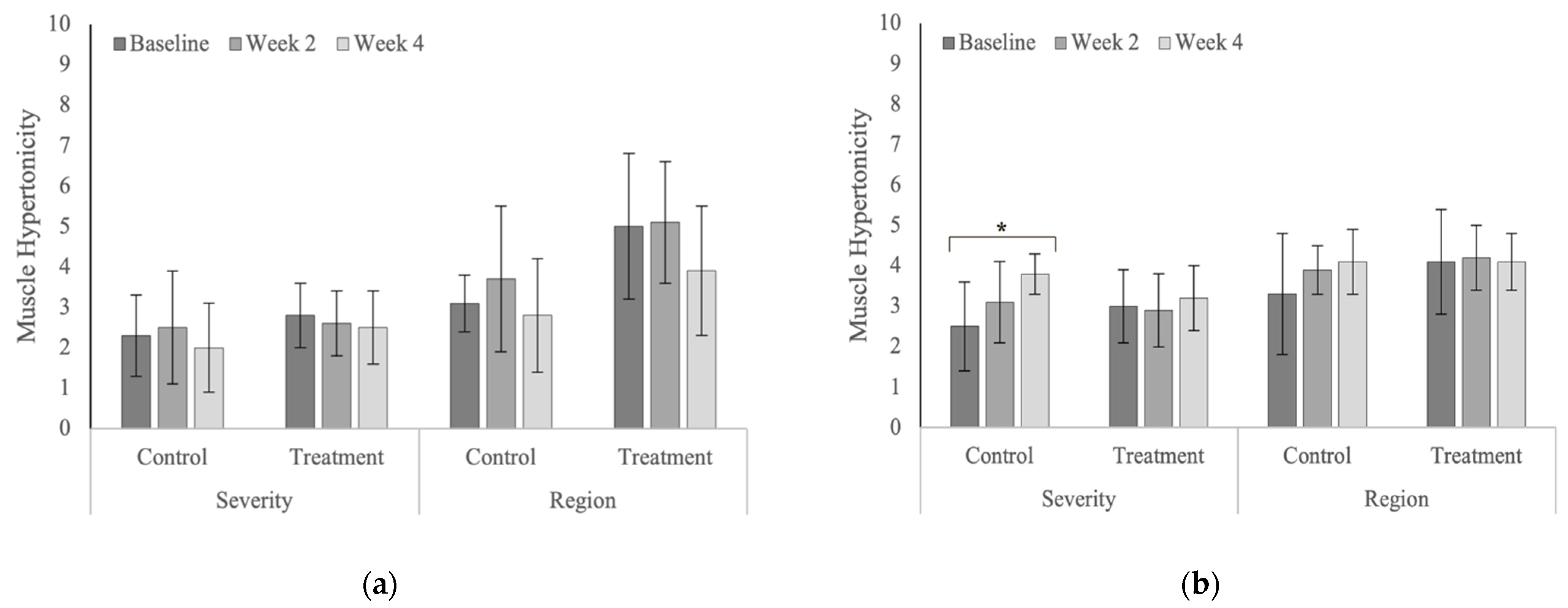
| Hypertonicity | Severity | Treatment | 2.8 ± 0.8 | 2.6 ± 0.8 | 2.5 ± 0.9 | 0.688 |
| Control | 2.3 ± 1.0 | 2.5 ± 1.4 | 2.0 ± 1.1 | 0.370 | ||
| p-values | 0.266 | 0.823 | 0.266 | |||
| Region | Treatment | 5.0 ± 1.8 | 5.1 ± 1.5 | 3.9 ± 1.6 | 0.074 | |
| Control | 3.1 ± 0.7 | 3.7 ± 1.8 | 2.8 ± 1.4 | 0.278 | ||
| p-values | 0.008 | 0.045 | 0.112 | |||
| Year 2 | ||||||
| Pain | Severity | Treatment | 2.0 ± 1.3 | 2.2 ± 1.3 | 2.1 ± 1.1 | 0.925 |
| Control | 2.5 ± 1.8 | 2.5 ± 1.9 | 2.1 ± 1.1 | 0.749 | ||
| p-values | 0.468 | 0.663 | 0.971 | |||
| Region | Treatment | 2.4 ± 1.8 | 2.5 ± 1.4 | 2.4 ± 1.4 | 0.978 | |
| Control | 2.8 ± 1.7 | 2.3 ± 1.0 | 2.8 ± 1.5 | 0.645 | ||
| p-values | 0.625 | 0.727 | 0.625 | |||
| Stiffness | Severity | Treatment | 4.2 ± 0.8 | 3.6 ± 1.2 | 3.2 ± 0.9 | 0.086 |
| Control | 4.0 ± 1.1 | 3.1 ± 1.0 | 3.6 ± 0.7 | 0.214 | ||
| p-value | 0.663 | 0.303 | 0.356 | |||
| Region | Treatment | 5.3 ± 1.4 | 4.4 ± 1.7 | 4.0 ± 0.7 | 0.030 | |
| Control | 4.4 ± 1.2 | 4.8 ± 0.9 | 4.9 ± 0.8 | 0.623 | ||
| p-values | 0.105 | 0.535 | 0.125 | |||
| Hypertonicity | Severity | Treatment | 3.0 ± 0.9 | 2.9 ± 0.9 | 3.2 ± 0.8 | 0.721 |
| Control | 2.5 ± 1.1 | 3.1 ± 1.0 | 3.8 ± 0.5 | 0.020 | ||
| p-values | 0.235 | 0.591 | 0.192 | |||
| Region | Treatment | 4.1 ± 1.3 | 4.2 ± 0.8 | 4.1 ± 0.7 | 0.967 | |
| Control | 3.3 ± 1.5 | 3.9 ± 0.6 | 4.1 ± 0.8 | 0.206 | ||
| p-values | 0.081 | 0.499 | 0.959 | |||
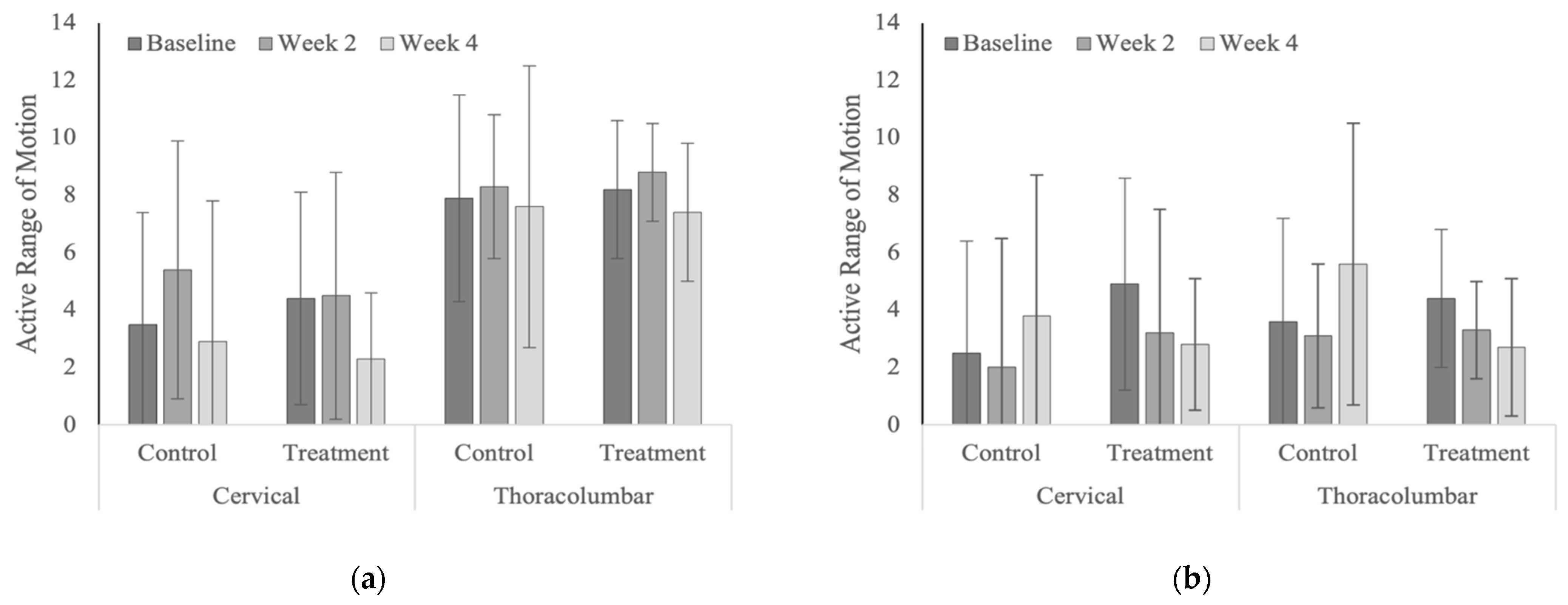
| Year 1 | |||||
|---|---|---|---|---|---|
| Region | Group | Baseline | Week 2 | Week 4 | p-values |
| Cervical | Treatment | 4.4 ± 3.7 | 4.5 ± 4.3 | 2.3 ± 2.3 | 0.248 |
| Control | 3.5 ± 3.9 | 5.4 ± 4.5 | 2.9 ± 4.9 | 0.210 | |
| p-values | 0.601 | 0.601 | 0.741 | ||
| Thoracolumbar | Treatment | 8.2 ± 2.4 | 8.8 ± 1.7 | 7.4 ± 2.4 | 0.429 |
| Control | 7.9 ± 3.6 | 8.3 ± 2.5 | 7.6 ± 4.9 | 0.607 | |
| p-values | 0.834 | 0.727 | 0.917 | ||
| Year 2 | |||||
| Cervical | Treatment | 4.9 ± 5.6 | 3.2 ± 4.5 | 2.8 ± 3.8 | 0.059 |
| Control | 2.5 ± 1.9 | 2.0 ± 2.3 | 3.8 ± 3.3 | 0.170 | |
| p-values | 0.209 | 0.524 | 0.568 | ||
| Thoracolumbar | Treatment | 4.4 ± 3.1 | 3.3 ± 4.8 | 2.7 ± 4.2 | 0.301 |
| Control | 3.6 ± 2.5 | 3.1 ± 2.5 | 5.6 ± 4.1 | 0.098 | |
| p-values | 0.683 | 0.921 | 0.121 | ||
| Year 1 | ||||
|---|---|---|---|---|
| Group | Baseline | Week 2 | Week 4 | p-values |
| Treatment | 4.7 ± 2.0 | 3.9 ± 2.3 | 3.7 ± 2.5 | 0.435 |
| Control | 3.8 ± 1.6 | 3.6 ± 1.8 | 3.1 ± 1.4 | 0.121 |
| p-values | 0.569 | 0.751 | 0.257 | |
| Year 2 | ||||
| Treatment | 3.3 ± 2.1 | 2.1 ± 2.3 | 1.8 ± 2.2 | 0.090 |
| Control | 3.2 ± 2.4 | 2.4 ± 1.7 | 3.4 ± 2.1 | 0.390 |
| p-values | 0.913 | 0.790 | 0.109 | |
| Year 1 | |||||
|---|---|---|---|---|---|
| Region | Group | Baseline | Week 2 | Week 4 | p-values |
| Cervical | Treatment | 14.9 ± 1.6 | 14.0 ± 1.8 | 13.4 ± 2.1 | 0.010 |
| Control | 16.3 ± 2.4 | 15.6 ± 2.0 | 14.6 ± 2.0 | 0.002 | |
| p-values | 0.112 | 0.087 | 0.185 | ||
| Thoracic | Treatment | 19.7 ± 3.4 | 19.7 ± 3.4 | 18.0 ± 2.7 | 0.073 |
| Control | 20.1 ± 3.0 | 20.3 ± 3.4 | 19.0 ± 2.5 | 0.264 | |
| p-values | 0.808 | 0.649 | 0.470 | ||
| Lumbosacral | Treatment | 24.3 ± 3.9 | 26.2 ± 3.3 | 24.6 ± 2.8 | 0.405 |
| Control | 25.9 ± 2.8 | 29.2 ± 4.3 | 25.4 ± 6.4 | 0.038 | |
| p-values | 0.398 | 0.127 | 0.682 | ||
| Year 2 | |||||
| Cervical | Treatment | 11.9 ± 2.1 | 11.7 ± 2.3 | 13.2 ± 2.3 | 0.064 |
| Control | 11.0 ± 3.0 | 11.1 ± 3.1 | 12.2 ± 3.0 | 0.224 | |
| p-values | 0.448 | 0.662 | 0.427 | ||
| Thoracic | Treatment | 18.9 ± 4.7 | 18.8 ± 3.9 | 19.6 ± 2.8 | 0.656 |
| Control | 18.3 ± 4.0 | 17.4 ± 3.6 | 18.5 ± 4.2 | 0.541 | |
| p-values | 0.745 | 0.439 | 0.536 | ||
| Lumbosacral | Treatment | 21.9 ± 4.5 | 26.6 ± 5.5 | 26.9 ± 2.9 | <0.0001 |
| Control | 25.1 ± 6.3 | 25.6 ± 6.0 | 26.7 ± 6.6 | 0.349 | |
| p-values | 0.215 | 0.704 | 0.936 | ||
References
- Slater, J. Findings from the National Equine Health Survey, 2013. Vet. Rec. 2014, 175, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Jordana, M.; Martens, A.; Duchateau, L.; Haspeslagh, M.; Vanderperren, K.; Oosterlinck, M.; Pille, F. Diffusion of mepivacaine to adjacent synovial structures after intrasynovial analgesia of the digital flexor tendon sheath. Equine Vet. J. 2016, 48, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Miagkoff, L.; Bonilla, A.G. Desensitisation of the distal forelimb following intrathecal anaesthesia of the carpal sheath in horses. Equine Vet. J. 2021, 53, 167–176. [Google Scholar] [CrossRef]
- Pilsworth, R.; Dyson, S. Where does it hurt? Problems with interpretation of regional and intra-synovial diagnostic analgesia. Equine Vet. Educ. 2015, 27, 595–603. [Google Scholar] [CrossRef]
- Byam-Cook, K.L.; Singer, E.R. Is there a relationship between clinical presentation, diagnostic and radiographic findings and outcome in horses with osteoarthritis of the small tarsal joints? Equine Vet. J. 2009, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.; Dyson, S.; Murray, R. Close, impinging and overriding spinous processes in the thoracolumbar spine: The relationship between radiological and scintigraphic findings and clinical signs. Equine Vet. J. 2012, 44, 178–184. [Google Scholar] [CrossRef]
- Broster, C.E.; Burn, C.C.; Barr, A.R.S.; Whay, H.R. The range and prevalence of pathological abnormalities associated with lameness in working horses from developing countries. Equine Vet. J. 2009, 41, 474–481. [Google Scholar] [CrossRef]
- Swor, T.M.; Dabareiner, R.M.; Honnas, C.M.; Cohen, N.D.; Black, J.B. Musculoskeletal problems associated with lameness and poor performance in cutting horses: 200 cases (2007–2015). J. Am. Vet. Med. Assoc. 2019, 254, 619–625. [Google Scholar] [CrossRef]
- Gorgas, D.; Luder, P.; Lang, J.; Doherr, M.G.; Ueltschi, G.; Kircher, P. Scintigraphic and radiographic appearance of the sacroiliac region in horses with gait abnormalities or poor performance. Vet. Radiol. Ultrasound 2009, 50, 208–214. [Google Scholar] [CrossRef]
- Bentz, B. How to evaluate clinically indistinct gait deficits to differentiate musculoskeletal and neurological causes. Proc. Am. Assoc. Equine Pract. 2009, 56, 50–56. [Google Scholar]
- Dyson, S. Evaluation of poor performance in competition horses: A musculoskeletal perspective. Part 2: Further investigation. Equine Vet. Educ. 2016, 28, 379–387. [Google Scholar] [CrossRef]
- Dyson, S. The interrelationships between back pain and lameness: A diagnostic challenge. Proc. Congr. Br. Equine Vet. Assoc. 2005, 44, 137–138. [Google Scholar]
- Landman, M.A.; de Blaauw, J.A.; van Weeren, P.R.; Hofland, L.J. Field study of the prevalence of lameness in horses with back problems. Vet. Rec. 2004, 155, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Steckel, R.R.; Kraus-Hansen, A.E.; Fackelman, G.E.; Mitchell, S.M. Scintigraphic diagnosis of thoracolumbar spinal disease in horses: A review of 50 cases. Proc. Am. Assoc. Equine Pract. 1991, 37, 583–591. [Google Scholar]
- Girodroux, M.; Dyson, S.; Murray, R. Osteoarthritis of the thoracolumbar synovial intervertebral articulations: Clinical and radiographic features in 77 horses with poor performance and back pain. Equine Vet. J. 2009, 41, 130–138. [Google Scholar] [CrossRef]
- Sullivan, H.M.; Acutt, E.V.; Barrett, M.F.; Salman, M.D.; Ellis, K.L.; King, M.R. Influence of chronic lameness on thoracolumbar musculus multifidus structure in the horse. J. Equine Vet. Sci. 2022, 117, 104053. [Google Scholar] [CrossRef]
- Keegan, K.G.; Messer, N.T.; Reed, S.K.; Wilson, D.A.; Kramer, J. Effectiveness of administration of phenylbutazone alone or concurrent administration of phenylbutazone and flunixin meglumine to alleviate lameness in horses. Am. J. Vet. Res. 2008, 69, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Smit, Y.; Marais, H.J.; Thompson, P.N.; Mahne, A.T.; Goddard, A. Clinical findings, synovial fluid cytology and growth factor concentrations after intra-articular use of a platelet-rich product in horses with osteoarthritis. J. S. Afr. Vet. Assoc. 2019, 90, e1–e9. [Google Scholar] [CrossRef]
- Magri, C.; Schramme, M.; Febre, M.; Cauvin, E.; Labadie, F.; Saulnier, N.; François, I.; Lechartier, A.; Aebischer, D.; Moncelet, A.-S.; et al. Comparison of efficacy and safety of single versus repeated intra-articular injection of allogeneic neonatal mesenchymal stem cells for treatment of osteoarthritis of the metacarpophalangeal/metatarsophalangeal joint in horses: A clinical pilot study. PLoS ONE 2019, 14, e0221317. [Google Scholar] [CrossRef]
- McIlwraith, C.W. The use of intra-articular corticosteroids in the horse: What is known on a scientific basis? Equine Vet. J. 2010, 42, 563–571. [Google Scholar] [CrossRef]
- Davis, J.L. Nonsteroidal anti-inflammatory drug associated right dorsal colitis in the horse. Equine Vet. Educ. 2017, 29, 104–113. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Hill, A.E.; Haussler, K.K. The effects of chiropractic, massage and phenylbutazone on spinal mechanical nociceptive thresholds in horses without clinical signs. Equine Vet. J. 2008, 40, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Colahan, P.; Ott, E.A. Evaluation of electroacupuncture treatment of horses with signs of chronic thoracolumbar pain. J. Am. Vet. Med. Assoc. 2005, 227, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Driessen, B.; Zarucco, L. Treatment of acute and dhronic pain in horses. In Pain Management in Veterinary Practice; Egger, C.M., Love, L.T.D., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 323–348. [Google Scholar] [CrossRef]
- Lange, C.D.; Flammer, S.A.; Gerber, V.; Kindt, D.; Koch, C. Complementary and alternative medicine for the management of orthopaedic problems in Swiss Warmblood horses. Vet. Med. Sci. 2017, 3, 125–133. [Google Scholar] [CrossRef]
- Davies, L. Equine rehabilitation. In Pain Management in Veterinary Practice; Egger, C.M., Love, L.T.D., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 147–154. [Google Scholar] [CrossRef]
- Haussler, K.K. Back problems. Chiropractic evaluation and management. Vet. Clin. N. Am. Equine Pract. 1999, 15, 195–209. [Google Scholar] [CrossRef]
- Haussler, K.K. Equine manual therapies in sport horse practice. Vet. Clin. N. Am. Equine Pract. 2018, 34, 375–389. [Google Scholar] [CrossRef]
- Haussler, K.K.; Hesbach, A.L.; Romano, L.; Goff, L.; Bergh, A. A systematic review of musculoskeletal mobilization and manipulation techniques used in veterinary medicine. Animals 2021, 11, 2787. [Google Scholar] [CrossRef]
- Acutt, E.V.; le Jeune, S.S.; Pypendop, B.H. Evaluation of the effects of chiropractic on static and dynamic muscle variables in sport horses. J. Equine Vet. Sci. 2019, 73, 84–90. [Google Scholar] [CrossRef]
- Gomez Alvarez, C.B.; L’Ami, J.J.; Moffat, D.; Back, W.; van Weeren, P.R. Effect of chiropractic manipulations on the kinematics of back and limbs in horses with clinically diagnosed back problems. Equine Vet. J. 2008, 40, 153–159. [Google Scholar] [CrossRef]
- Keegan, K.G.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Dent, E.V.; Kellerman, T.E.; Wilson, D.A.; Reed, S.K. Assessment of repeatability of a wireless, inertial sensor-based lameness evaluation system for horses. Am. J. Vet. Res. 2011, 72, 1156–1163. [Google Scholar] [CrossRef]
- Hoerdemann, M.; Smith, R.L.; Hosgood, G. Duration of action of mepivacaine and lidocaine in equine palmar digital perineural blocks in an experimental lameness model. Vet. Surg. 2017, 46, 986–993. [Google Scholar] [CrossRef]
- Rettig, M.J.; Leelamankong, P.; Rungsri, P.; Lischer, C.J. Effect of sedation on fore- and hindlimb lameness evaluation using body-mounted inertial sensors. Equine Vet. J. 2016, 48, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G.; MacAllister, C.G.; Wilson, D.A.; Gedon, C.A.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F. Comparison of an inertial sensor system with a stationary force plate for evaluation of horses with bilateral forelimb lameness. Am. J. Vet. Res. 2012, 73, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Mayaki, A.M.; Razak, I.S.A.; Adzahan, N.M.; Mazlan, M.; Rasedee, A. Clinical assessment and grading of back pain in horses. J. Vet. Sci. 2020, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Munroe, G.A. Clinical examination. In Equine Neck and Back Pathology; Henson, F.M.D., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 81–94. [Google Scholar] [CrossRef]
- Haussler, K.K.; Manchon, P.T.; Donnell, J.R.; Frisbie, D.D. Effects of low-level laser therapy and chiropractic care on back pain in Quarter Horses. J. Equine Vet. Sci. 2020, 86, 102891. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, N.C.; Kaiser, L.J.; Hauptman, J.; Clayton, H.M. Dynamic mobilisation exercises increase cross sectional area of musculus multifidus. Equine Vet. J. 2011, 43, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H.M.; Kaiser, L.J.; Lavagnino, M.; Stubbs, N.C. Dynamic mobilisations in cervical flexion: Effects on intervertebral angulations. Equine Vet. J. Suppl. 2010, 42, 688–694. [Google Scholar] [CrossRef]
- Haussler, K.K.; Erb, H.N. Mechanical nociceptive thresholds in the axial skeleton of horses. Equine Vet. J. 2006, 38, 70–75. [Google Scholar] [CrossRef]
- Haussler, K.K.; Hill, A.E.; Puttlitz, C.M.; McIlwraith, C.W. Effects of vertebral mobilization and manipulation on kinematics of the thoracolumbar region. Am. J. Vet. Res. 2007, 68, 508–516. [Google Scholar] [CrossRef]
- Haussler, K.K.; Martin, C.E.; Hill, A.E. Efficacy of spinal manipulation and mobilisation on trunk flexibility and stiffness in horses: A randomised clinical trial. Equine Vet. J. Suppl. 2010, 38, 695–702. [Google Scholar] [CrossRef]
- Van de Water, E.; Oosterlinck, M.; Korthagen, N.M.; Duchateau, L.; Dumoulin, M.; van Weeren, P.R.; Olijve, J.; van Doorn, D.A.; Pille, F. The lipopolysaccharide model for the experimental induction of transient lameness and synovitis in Standardbred horses. Vet. J. 2021, 270, 105626. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, M.; Persson-Sjödin, E.; Egenvall, A.; Serra Braganca, F.M.; Pfau, T.; Roepstorff, L.; Weishaupt, M.A.; Thomsen, M.H.; van Weeren, P.R.; Hernlund, E. Vertical movement symmetry of the withers in horses with induced forelimb and hindlimb lameness at trot. Equine Vet. J. 2018, 50, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Wennerstrand, J.; Gomez Alvarez, C.B.; Meulenbelt, R.; Johnston, C.; van Weeren, P.R.; Roethlisberger-Holm, K.; Drevemo, S. Spinal kinematics in horses with induced back pain. Vet. Comp. Orthop. Traumatol. 2009, 22, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Haussler, K.K.; Erb, H.N. Pressure algometry for the detection of induced back pain in horses: A preliminary study. Equine Vet. J. 2006, 38, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.C.; Walters, J.M.; Snart, H.; Dyson, S.J.; Parkin, T.D. Identification of risk factors for lameness in dressage horses. Vet. J. 2010, 184, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Parkes, R.S.; Burden, E.R.; Bell, N.; Fairhurst, H.; Witte, T.H. Movement asymmetry in working polo horses. Equine Vet. J. 2016, 48, 517–522. [Google Scholar] [CrossRef]
- Elmeua González, M.; Šarabon, N. Shock attenuation and electromyographic activity of advanced and novice equestrian riders’ trunk. Appl. Sci. 2021, 11, 2304. [Google Scholar] [CrossRef]
- Lagarde, J.; Peham, C.; Licka, T.; Kelso, J.A.S. Coordination dynamics of the horse-rider system. J. Mot. Behav. 2005, 37, 418–424. [Google Scholar] [CrossRef][Green Version]
- Meneses, C.S.; Müller, H.Y.; Herzberg, D.E.; Uberti, B.; Werner, M.P.; Bustamante, H.A. Microglia and astrocyte activation in the spinal cord of lame horses. Vet. Anaesth. Analg. 2018, 45, 92–102. [Google Scholar] [CrossRef]
- Story, M.R.; Nout-Lomas, Y.S.; Aboellail, T.A.; Selberg, K.T.; Barrett, M.F.; McLlwraith, C.W.; Haussler, K.K. Dangerous behavior and intractable axial skeletal pain in performance horses: A possible role for ganglioneuritis (14 cases; 2014–2019). Front. Vet. Sci. 2021, 8, 734218. [Google Scholar] [CrossRef]
- Keegan, K.G. The Biomechanics of Multiple Limb Lameness: Separating Secondary from Compensatory. Available online: https://equinosis.com/multiple-limb-lameness-separating-secondary-from-compensatory/ (accessed on 10 August 2022).
- Serra Bragança, F.M.; Broomé, S.; Rhodin, M.; Björnsdóttir, S.; Gunnarsson, V.; Voskamp, J.P.; Persson-Sjodin, E.; Back, W.; Lindgren, G.; Novoa-Bravo, M.; et al. Improving gait classification in horses by using inertial measurement unit (IMU) generated data and machine learning. Sci. Rep. 2020, 10, 17785. [Google Scholar] [CrossRef]
- Greve, L.; Dyson, S.; Pfau, T. Alterations in thoracolumbosacral movement when pain causing lameness has been improved by diagnostic analgesia. Vet. J. 2017, 224, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Scott, W.M.; Sternberg Allen, T. Uppe rbody movement symmetry in reining quarter horses during trot in-hand, on the lunge and during ridden exercise. Animals 2022, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- MacKechnie-Guire, R.; Pfau, T. Differential rotational movement and symmetry values of the thoracolumbosacral region in high-level dressage horses when trotting. PLoS ONE 2021, 16, e0251144. [Google Scholar] [CrossRef] [PubMed]
- Wennerstrand, J.; Johnston, C.; Roethlisberger-Holm, K.; Erichsen, C.; Eksell, P.; Drevemo, S. Kinematic evaluation of the back in the sport horse with back pain. Equine Vet. J. 2004, 36, 707–711. [Google Scholar] [CrossRef]
- Pfau, T.; Noordwijk, K.; Sepulveda Caviedes, M.F.; Persson-Sjodin, E.; Barstow, A.; Forbes, B.; Rhodin, M. Head, withers and pelvic movement asymmetry and their relative timing in trot in racing Thoroughbreds in training. Equine Vet. J. 2018, 50, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, A.; Egenvall, A.; Roepstorff, L.; Rhodin, M.; Braganca, F.S.; Hernlund, E.; van Weeren, R.; Weishaupt, M.A.; Clayton, H.M. Biomechanical findings in horses showing asymmetrical vertical excursions of the withers at walk. PLoS ONE 2018, 13, e0204548. [Google Scholar] [CrossRef]
- Goff, L.M.; Jasiewicz, J.; Jeffcott, L.B.; Condie, P.; McGowan, T.W.; McGowan, C.M. Movement between the equine ilium and sacrum: In vivo and in vitro studies. Equine Vet. J. Suppl. 2006, 36, 457–461. [Google Scholar] [CrossRef]
- Ricardi, G.; Dyson, S.J. Forelimb lameness associated with radiographic abnormalities of the cervical vertebrae. Equine Vet. J. 1993, 25, 422–426. [Google Scholar] [CrossRef]
- Dyson, S.J. Lesions of the equine neck resulting in lameness or poor performance. Vet. Clin. N. Am. Equine Pract. 2011, 27, 417–437. [Google Scholar] [CrossRef]
- Merrifield-Jones, M.; Tabor, G.; Williams, J. Inter- and intra-rater reliability of soft tissue palpation scoring in the equine thoracic epaxial region. J. Equine Vet. Sci. 2019, 83, 102812. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.B., Jr.; Klide, A.M. Physical examination of horses with back pain. Vet. Clin. N. Am. Equine Pract. 1999, 15, 61–70. [Google Scholar] [CrossRef]
- Haussler, K.K.; Erb, H.N. Pressure algometry: Objective assessment of back pain and effects of chiropractic treatment. Proc. Am. Assoc. Equine Pract. 2003, 49, 66–70. [Google Scholar]
- Wakeling, J.M.; Barnett, K.; Price, S.; Nankervis, K. Effects of manipulative therapy on the longissimus dorsi in the equine back. Equine Comp. Exerc. Physiol. 2006, 3, 153–160. [Google Scholar] [CrossRef]
- Long, K.; McGowan, C.M.; Hyytiäinen, H.K. Effect of caudal traction on mechanical nociceptive thresholds of epaxial and pelvic musculature on a group of horses with signs of back pain. J. Equine Vet. Sci. 2020, 93, 103197. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, S.D.; Zanotto, G.M.; Maldonado, M.D.; King, M.R.; Haussler, K.K. The effect of capacitive-resistive electrical therapy on neck pain and dysfunction in horses. J. Equine Vet. Sci. 2022, 117, 104091. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.; Soutello, R.V.G.; da Fonseca, R.; Costa, C.; de L. Meirelles, P.R.; Fachiolli, D.F.; Clayton, H.M. Gymnastic training and dynamic mobilization exercises improve stride quality and increase epaxial muscle size in therapy horses. J. Equine Vet. Sci. 2015, 35, 888–893. [Google Scholar] [CrossRef]
- Onifer, S.M.; Sozio, R.S.; DiCarlo, D.M.; Li, Q.; Donahue, R.R.; Taylor, B.K.; Long, C.R. Spinal manipulative therapy reduces peripheral neuropathic pain in the rat. Neuroreport 2018, 29, 191–196. [Google Scholar] [CrossRef]
- Sullivan, K.; Hill, A.; Haussler, K.K. Effect of different treatment modalities on spinal nociceptive thresholds in horses. Proc. Am. Assoc. Equine Pract. 2007, 53, 58–59. [Google Scholar]
- Bathe, A.P. Lameness in the three day event horse. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier Inc.: St. Louis, MO, USA, 2011; pp. 1123–1137. [Google Scholar] [CrossRef]
- Jorgensen, H.S.; Proschowsky, H.; Falk-Ronne, J.; Willeberg, R.; Hesselholt, M. The significance of routine radiographic findings with respect to subsequent racing performance and longevity in Standardbred trotters. Equine Vet. J. 1997, 29, 55–59. [Google Scholar] [CrossRef]
- Cohen, N.D.; Carter, G.K.; Watkins, J.P.; O’Conor, M.S. Association of racing performance with specific abnormal radiographic findings in Thoroughbred yearlings sold in Texas. J. Equine Vet. Sci. 2006, 26, 462–474. [Google Scholar] [CrossRef]
- van Loon, J.P.A.M.; Back, W.; Hellebrekers, L.J.; van Weeren, P.R. Application of a composite pain scale to objectively monitor horses with somatic and visceral pain under hospital conditions. J. Equine Vet. Sci. 2010, 30, 641–649. [Google Scholar] [CrossRef]
- van Loon, J.P.A.M.; Van Dierendonck, M.C. Objective pain assessment in horses (2014–2018). Vet. J. 2018, 242, 1–7. [Google Scholar] [CrossRef] [PubMed]
- van Loon, J.P.A.M.; Macri, L. Objective assessment of chronic pain in horses using the Horse Chronic Pain Scale (HCPS): A scale-construction study. Animals 2021, 11, 1826. [Google Scholar] [CrossRef]
- Lopes, M.A.F.; Eleuterio, A.; Mira, M.C. Objective detection and quantification of irregular gait with a portable inertial sensor-based system in horses during an endurance race—A preliminary assessment. J. Equine Vet. Sci. 2018, 70, 123–129. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, M.D.; Parkinson, S.D.; Story, M.R.; Haussler, K.K. The Effect of Chiropractic Treatment on Limb Lameness and Concurrent Axial Skeleton Pain and Dysfunction in Horses. Animals 2022, 12, 2845. https://doi.org/10.3390/ani12202845
Maldonado MD, Parkinson SD, Story MR, Haussler KK. The Effect of Chiropractic Treatment on Limb Lameness and Concurrent Axial Skeleton Pain and Dysfunction in Horses. Animals. 2022; 12(20):2845. https://doi.org/10.3390/ani12202845
Chicago/Turabian StyleMaldonado, Mikaela D., Samantha D. Parkinson, Melinda R. Story, and Kevin K. Haussler. 2022. "The Effect of Chiropractic Treatment on Limb Lameness and Concurrent Axial Skeleton Pain and Dysfunction in Horses" Animals 12, no. 20: 2845. https://doi.org/10.3390/ani12202845
APA StyleMaldonado, M. D., Parkinson, S. D., Story, M. R., & Haussler, K. K. (2022). The Effect of Chiropractic Treatment on Limb Lameness and Concurrent Axial Skeleton Pain and Dysfunction in Horses. Animals, 12(20), 2845. https://doi.org/10.3390/ani12202845





