Generalizability, Robustness and Replicability When Evaluating Wellbeing of Laboratory Mice with Various Methods
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.1.1. Study Concept and Animal Husbandry
2.1.2. Surgical Interventions and Induction of Diseases
2.2. Assessment of Animal Wellbeing
2.3. Data Presentation and Statistical Analysis
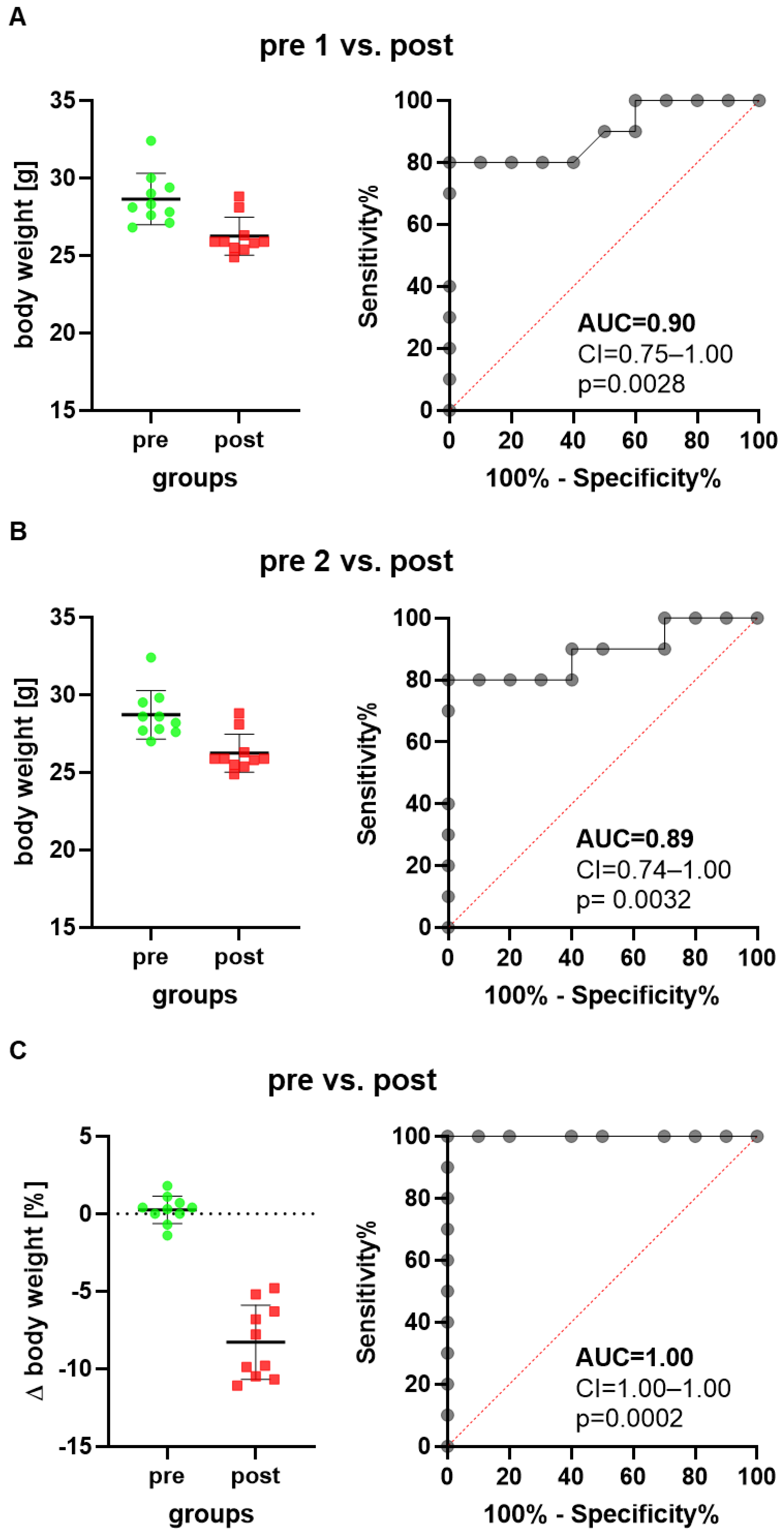
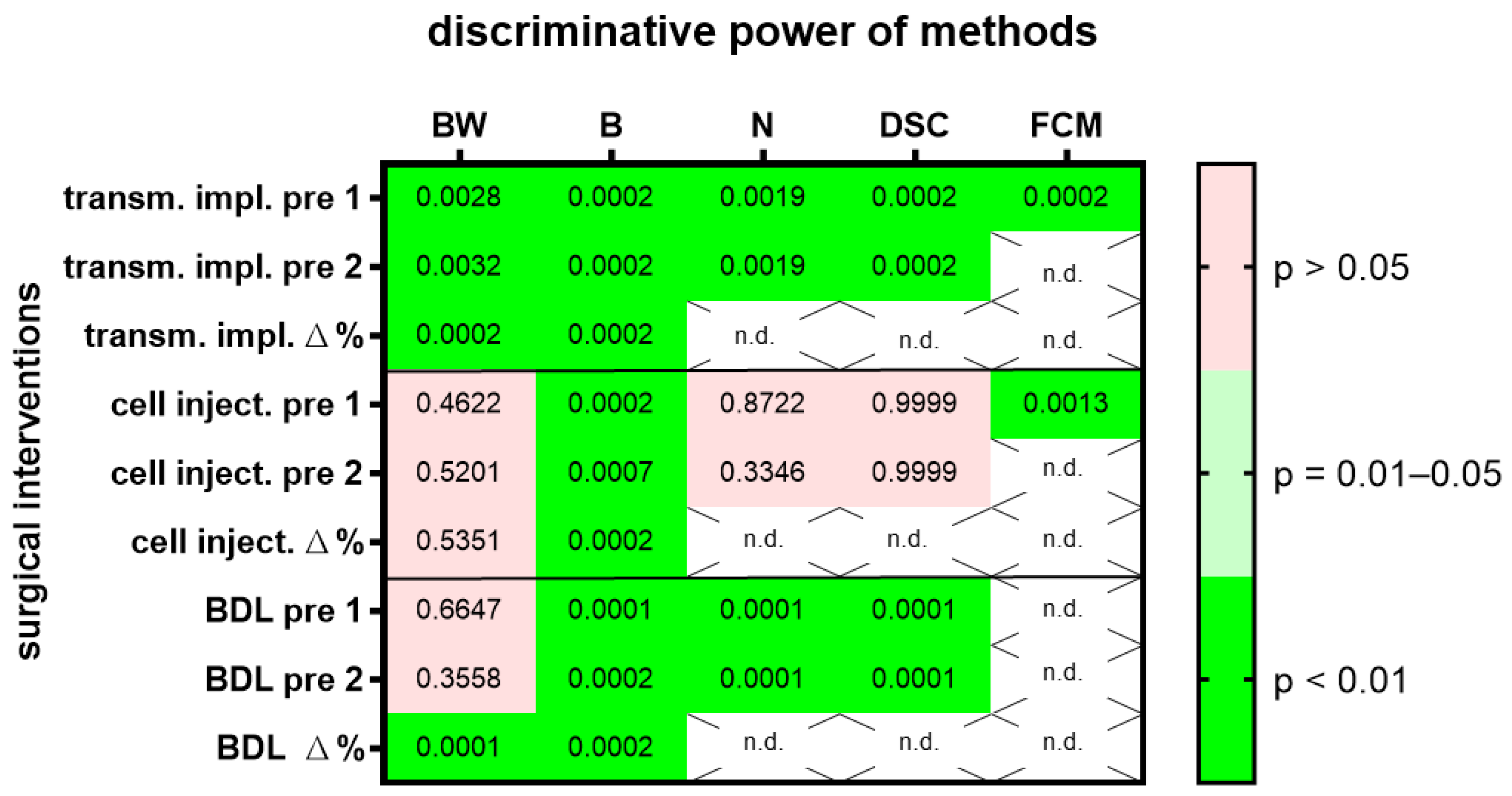
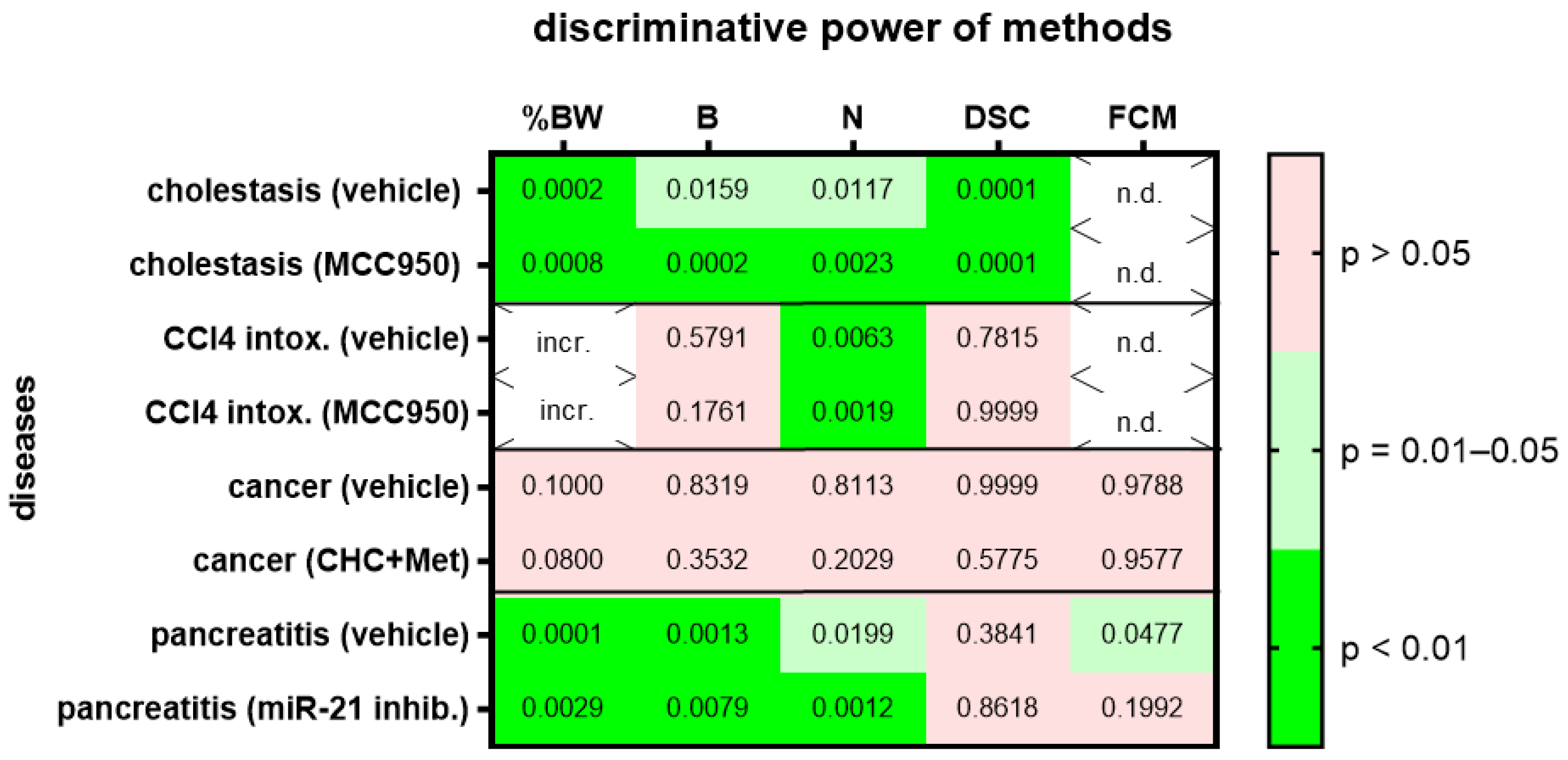
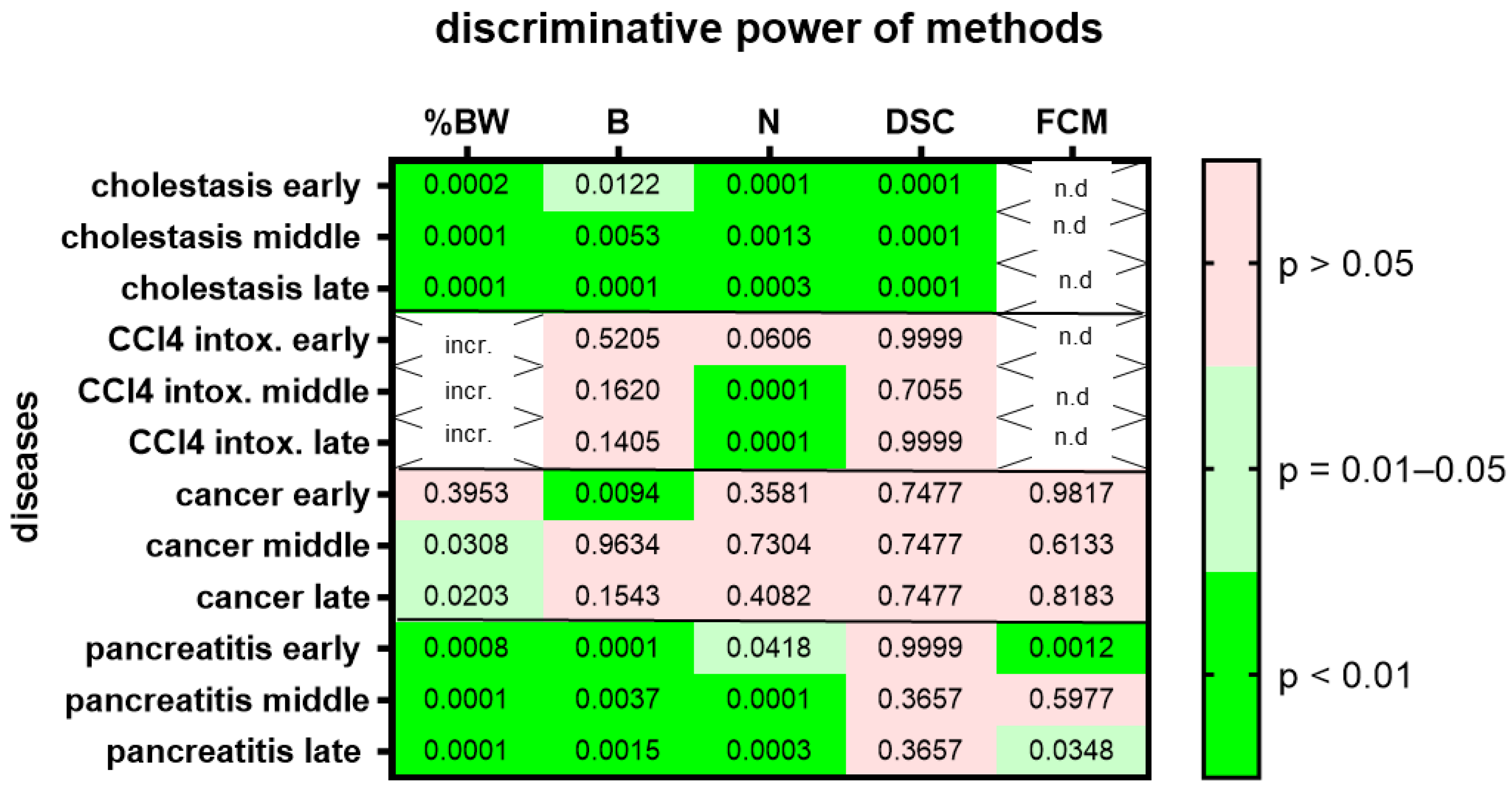
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [CrossRef]
- Office, P. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Text with EEA relevance. Off. J. Eur. Union 2010, L 276, 33–79. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 9780309154000. [Google Scholar]
- Talbot, S.R.; Biernot, S.; Bleich, A.; van Dijk, R.M.; Ernst, L.; Häger, C.; Helgers, S.O.A.; Koegel, B.; Koska, I.; Kuhla, A.; et al. Defining body-weight reduction as a humane endpoint: A critical appraisal. Lab. Anim. 2020, 54, 99–110. [Google Scholar] [CrossRef]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Morton, D.B. A systematic approach for establishing humane endpoints. ILAR J. 2000, 41, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Touma, C.; Palme, R.; Sachser, N. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones. Horm. Behav. 2004, 45, 10–22. [Google Scholar] [CrossRef]
- Auer, K.E.; Kußmaul, M.; Möstl, E.; Hohlbaum, K.; Rülicke, T.; Palme, R. Measurement of Fecal Testosterone Metabolites in Mice: Replacement of Invasive Techniques. Animals 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Kolbe, T.; Palme, R.; Tichy, A.; Rülicke, T. Lifetime Dependent Variation of Stress Hormone Metabolites in Feces of Two Laboratory Mouse Strains. PLoS ONE 2015, 10, e0136112. [Google Scholar] [CrossRef] [Green Version]
- Mallien, A.S.; Becker, L.; Pfeiffer, N.; Terraneo, F.; Hahn, M.; Middelman, A.; Palme, R.; Creutzberg, K.C.; Begni, V.; Riva, M.A.; et al. Dopamine Transporter Knockout Rats Show Impaired Wellbeing in a Multimodal Severity Assessment Approach. Front. Behav. Neurosci. 2022, 16, 924603. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- van Fentener Vlissingen, J.M.; Borrens, M.; Girod, A.; Lelovas, P.; Morrison, F.; Torres, Y.S. The reporting of clinical signs in laboratory animals: FELASA Working Group Report. Lab. Anim. 2015, 49, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Palme, R.; Schafmayer, C.; Zechner, D.; Vollmar, B.; Grambow, E. Distress Analysis of Mice with Cervical Arteriovenous Fistulas. Animals 2021, 11, 3051. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, K.; Boldt, L.; Bleich, A.; van Dijk, R.M.; Helgers, S.O.A.; Häger, C.; Nowakowska, M.; Riedesel, A.-K.; Schönhoff, K.; Struve, B.; et al. Nest-building performance in rats: Impact of vendor, experience, and sex. Lab. Anim. 2020, 54, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J. Assessing nest building in mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef]
- Gaskill, B.N.; Karas, A.Z.; Garner, J.P.; Pritchett-Corning, K.R. Nest building as an indicator of health and welfare in laboratory mice. J. Vis. Exp. 2013, 82, e51012. [Google Scholar] [CrossRef] [Green Version]
- Jirkof, P.; Fleischmann, T.; Cesarovic, N.; Rettich, A.; Vogel, J.; Arras, M. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab. Anim. 2013, 47, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Deacon, R. Assessing burrowing, nest construction, and hoarding in mice. J. Vis. Exp. 2012, 59, e2607. [Google Scholar] [CrossRef] [Green Version]
- Deacon, R.M.J. Burrowing in rodents: A sensitive method for detecting behavioral dysfunction. Nat. Protoc. 2006, 1, 118–121. [Google Scholar] [CrossRef]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Gjendal, K.; Ottesen, J.L.; Olsson, I.A.S.; Sørensen, D.B. Burrowing and nest building activity in mice after exposure to grid floor, isoflurane or ip injections. Physiol. Behav. 2019, 206, 59–66. [Google Scholar] [CrossRef]
- Weegh, N.; Füner, J.; Janke, O.; Winter, Y.; Jung, C.; Struve, B.; Wassermann, L.; Lewejohann, L.; Bleich, A.; Häger, C. Wheel running behaviour in group-housed female mice indicates disturbed wellbeing due to DSS colitis. Lab. Anim. 2020, 54, 63–72. [Google Scholar] [CrossRef]
- Weegh, N.; Zentrich, E.; Zechner, D.; Struve, B.; Wassermann, L.; Talbot, S.R.; Kumstel, S.; Heider, M.; Vollmar, B.; Bleich, A.; et al. Voluntary wheel running behaviour as a tool to assess the severity in a mouse pancreatic cancer model. PLoS ONE 2021, 16, e0261662. [Google Scholar] [CrossRef] [PubMed]
- Keubler, L.M.; Hoppe, N.; Potschka, H.; Talbot, S.R.; Vollmar, B.; Zechner, D.; Häger, C.; Bleich, A. Where are we heading? Challenges in evidence-based severity assessment. Lab. Anim. 2020, 54, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.R.; Struve, B.; Wassermann, L.; Heider, M.; Weegh, N.; Knape, T.; Hofmann, M.C.J.; von Knethen, A.; Jirkof, P.; Keubler, L.; et al. One Score to Rule Them All: Severity Assessment in Laboratory Mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tang, G.; Seume, N.; Häger, C.; Kumstel, S.; Abshagen, K.; Bleich, A.; Vollmar, B.; Talbot, S.R.; Zhang, X.; Zechner, D. Comparing distress of mouse models for liver damage. Sci. Rep. 2020, 10, 19814. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Ovadia, C.; Seed, P.T.; Sklavounos, A.; Geenes, V.; Di Ilio, C.; Chambers, J.; Kohari, K.; Bacq, Y.; Bozkurt, N.; Brun-Furrer, R.; et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: Results of aggregate and individual patient data meta-analyses. Lancet 2019, 393, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Janssens, A.C.J.W.; Martens, F.K. Reflection on modern methods: Revisiting the area under the ROC Curve. Int. J. Epidemiol. 2020, 49, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.N.; Fanelli, D.; Ioannidis, J.P.A. What does research reproducibility mean? Sci. Transl. Med. 2016, 8, 341ps12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollen, K.; Cacioppo, J.T.; Kaplan, R.M.; Krosnick, J.A.; Olds, J.L.; Dean, H. Social, behavioral, and economic sciences perspectives on robust and reliable science. Report of the Subcommittee on Replicability in Science Advisory Committee to the National Science Foundation Directorate for Social, Behavioral, and Economic Sciences. 2015. Available online: https://nsf.gov/sbe/AC_Materials/SBE_Robust_and_Reliable_Research_Report.pdf (accessed on 12 October 2022).
- Kumstel, S.; Vasudevan, P.; Palme, R.; Zhang, X.; Wendt, E.H.U.; David, R.; Vollmar, B.; Zechner, D. Benefits of non-invasive methods compared to telemetry for distress analysis in a murine model of pancreatic cancer. J. Adv. Res. 2020, 21, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kumstel, S.; Wendt, E.H.U.; Eichberg, J.; Talbot, S.R.; Häger, C.; Zhang, X.; Abdelrahman, A.; Schönrogge, M.; Palme, R.; Bleich, A.; et al. Grading animal distress and side effects of therapies. Ann. N. Y. Acad. Sci. 2020, 1473, 20–34. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, A.; Kumstel, S.; Zhang, X.; Liebig, M.; Wendt, E.H.U.; Eichberg, J.; Palme, R.; Thum, T.; Vollmar, B.; Zechner, D. A novel multi-parametric analysis of non-invasive methods to assess animal distress during chronic pancreatitis. Sci. Rep. 2019, 9, 14084. [Google Scholar] [CrossRef] [Green Version]
- Paster, E.V.; Villines, K.A.; Hickman, D.L. Endpoints for mouse abdominal tumor models: Refinement of current criteria. Comp. Med. 2009, 59, 234–241. [Google Scholar]
- Kumstel, S.; Tang, G.; Zhang, X.; Kerndl, H.; Vollmar, B.; Zechner, D. Grading Distress of Different Animal Models for Gastrointestinal Diseases Based on Plasma Corticosterone Kinetics. Animals 2019, 9, 145. [Google Scholar] [CrossRef] [Green Version]
- Kroll, T.; Kornadt-Beck, N.; Oskamp, A.; Elmenhorst, D.; Touma, C.; Palme, R.; Bauer, A. Additional Assessment of Fecal Corticosterone Metabolites Improves Visual Rating in the Evaluation of Stress Responses of Laboratory Rats. Animals 2021, 11, 710. [Google Scholar] [CrossRef]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Hadjiiski, L.; Chan, H.P.; Sahiner, B.; Helvie, M.A.; Roubidoux, M.A. Quasi-continuous and discrete confidence rating scales for observer performance studies: Effects on ROC analysis. Acad. Radiol. 2007, 14, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Hanczar, B.; Hua, J.; Sima, C.; Weinstein, J.; Bittner, M.; Dougherty, E.R. Small-sample precision of ROC-related estimates. Bioinformatics 2010, 26, 822–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goksuluk, D.; Korkmaz, S.; Zararsiz, G.; Karaagaoglu, A.E. easyROC: An Interactive Web-tool for ROC Curve Analysis Using R Language Environment. R J. 2016, 8, 213–230. [Google Scholar] [CrossRef]
- easyROC: A Web-Tool for ROC Curve Analysis (Ver. 1.3.1). Available online: http://www.biosoft.hacettepe.edu.tr/easyROC/ (accessed on 12 October 2022).
- Jiménez-Torres, C.; El-Kehdy, H.; Hernández-Kelly, L.C.; Sokal, E.; Ortega, A.; Najimi, M. Acute Liver Toxicity Modifies Protein Expression of Glutamate Transporters in Liver and Cerebellar Tissue. Front. Neurosci. 2020, 14, 613225. [Google Scholar] [CrossRef]
- Altinoz, E.; Erdemli, M.E.; Gul, M.; Aksungur, Z.; Gul, S.; Bag, H.G.; Kaya, G.B.; Turkoz, Y. Neuroprotection against CCl4 induced brain damage with crocin in Wistar rats. Biotech. Histochem. 2018, 93, 623–631. [Google Scholar] [CrossRef]
- National Academies Press (US). Reproducibility and Replicability in Science; National Academies Press: Washington, DC, USA, 2019; ISBN 9780309486163. [Google Scholar]
- Dirnagl, U. The p value wars (again). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2421–2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Clymer, J.W.; Po-Han Chen, B.; Sadeghirad, B.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration is associated with complications: A systematic review and meta-analysis. J. Surg. Res. 2018, 229, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Zhang, C.; Dong, Y.; Zhang, Y.; Nakazawa, H.; Kaneki, M.; Zheng, H.; Shen, Y.; Marcantonio, E.R.; Xie, Z. Battery of behavioral tests in mice to study postoperative delirium. Sci. Rep. 2016, 6, 29874. [Google Scholar] [CrossRef] [Green Version]
- Häger, C.; Keubler, L.M.; Talbot, S.R.; Biernot, S.; Weegh, N.; Buchheister, S.; Buettner, M.; Glage, S.; Bleich, A. Running in the wheel: Defining individual severity levels in mice. PLoS Biol. 2018, 16, e2006159. [Google Scholar] [CrossRef]
- Buchecker, V.; Koska, I.; Pace, C.; Talbot, S.R.; Palme, R.; Bleich, A.; Potschka, H. Toward evidence-based severity assessment in mouse models with repeated seizures: (II.) Impact of surgery and intrahippocampal kainate. Eur. Surg. Res. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Möller, C.; Wolf, F.; van Dijk, R.M.; Di Liberto, V.; Russmann, V.; Keck, M.; Palme, R.; Hellweg, R.; Gass, P.; Otzdorff, C.; et al. Toward evidence-based severity assessment in rat models with repeated seizures: I. Electrical kindling. Epilepsia 2018, 59, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Mallien, A.S.; Pfeiffer, N.; Brandwein, C.; Inta, D.; Sprengel, R.; Palme, R.; Talbot, S.R.; Gass, P. Comparative Severity Assessment of Genetic, Stress-Based, and Pharmacological Mouse Models of Depression. Front. Behav. Neurosci. 2022, 16. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zechner, D.; Schulz, B.; Tang, G.; Abdelrahman, A.; Kumstel, S.; Seume, N.; Palme, R.; Vollmar, B. Generalizability, Robustness and Replicability When Evaluating Wellbeing of Laboratory Mice with Various Methods. Animals 2022, 12, 2927. https://doi.org/10.3390/ani12212927
Zechner D, Schulz B, Tang G, Abdelrahman A, Kumstel S, Seume N, Palme R, Vollmar B. Generalizability, Robustness and Replicability When Evaluating Wellbeing of Laboratory Mice with Various Methods. Animals. 2022; 12(21):2927. https://doi.org/10.3390/ani12212927
Chicago/Turabian StyleZechner, Dietmar, Benjamin Schulz, Guanglin Tang, Ahmed Abdelrahman, Simone Kumstel, Nico Seume, Rupert Palme, and Brigitte Vollmar. 2022. "Generalizability, Robustness and Replicability When Evaluating Wellbeing of Laboratory Mice with Various Methods" Animals 12, no. 21: 2927. https://doi.org/10.3390/ani12212927




