Equine Stomach Development in the Fetal Period: An Anatomical, Topographical, and Morphometric Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preservation and Age Estimation
2.2. Morphological Analysis
- fetus dissection and anatomical preparation of the stomach (laparotomy in the median and transverse line),
- fetus gender identification based on external and internal genital organs,
- topographic analysis of the cranial abdominal region and the stomach location (holotopy, skeletotopy, and syntopy) according to the methods introduced by Chrószcz [25].
2.3. Morphometric Analysis
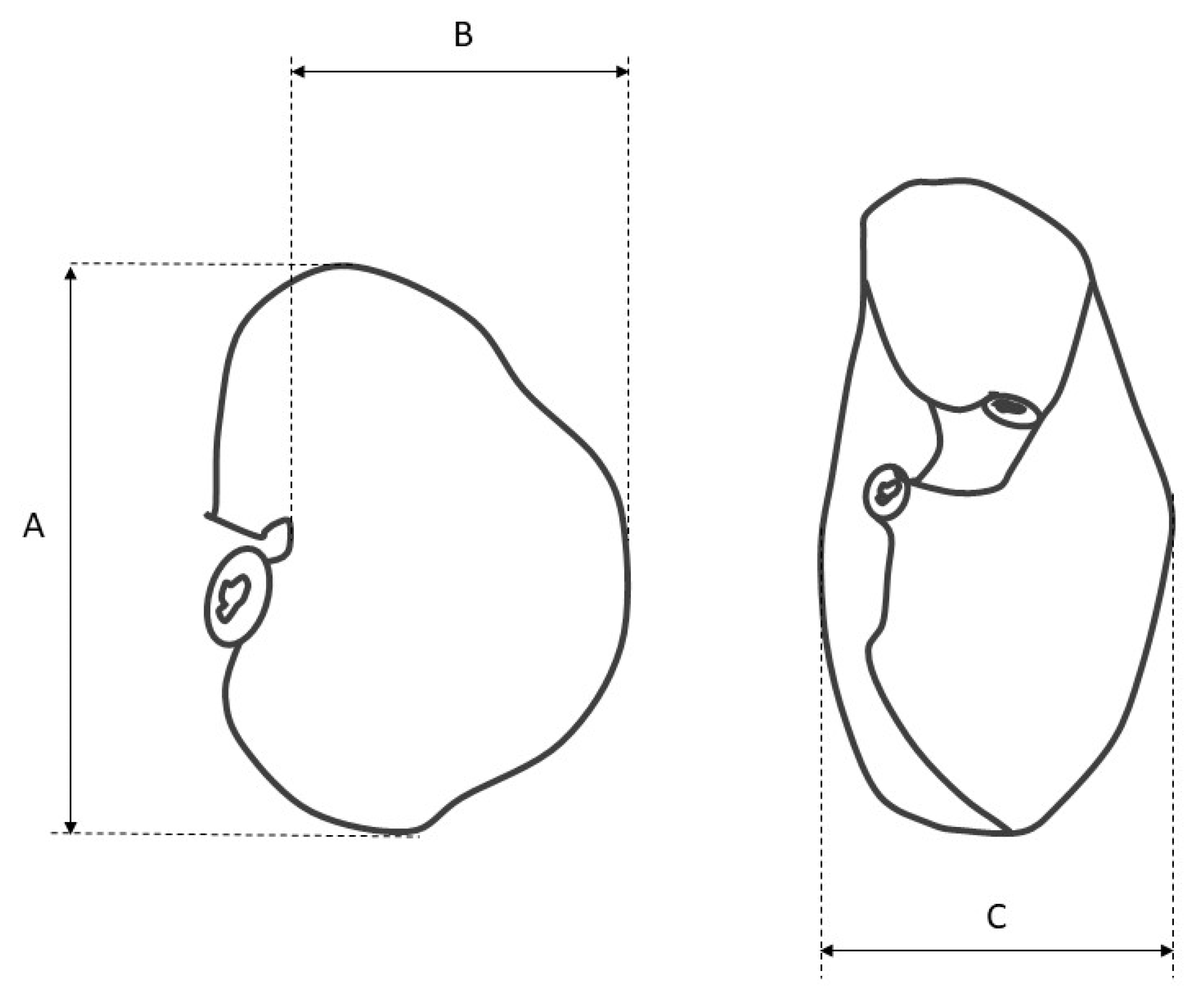
- measurement of the stomach filled with gelatin to estimate its length, width, thickness, and volume (Figure 1),
- measurement of the length of the greater and lesser curvature,
- measurement of the diameter of the cardiac and pyloric orifice,
- gastrotomy along the greater curvature, and measurement of the distance from the cardiac orifice to the fundus and from the cardiac orifice to the greater curvature (along the parietal and visceral surface),
2.4. Statistical Analysis
3. Results
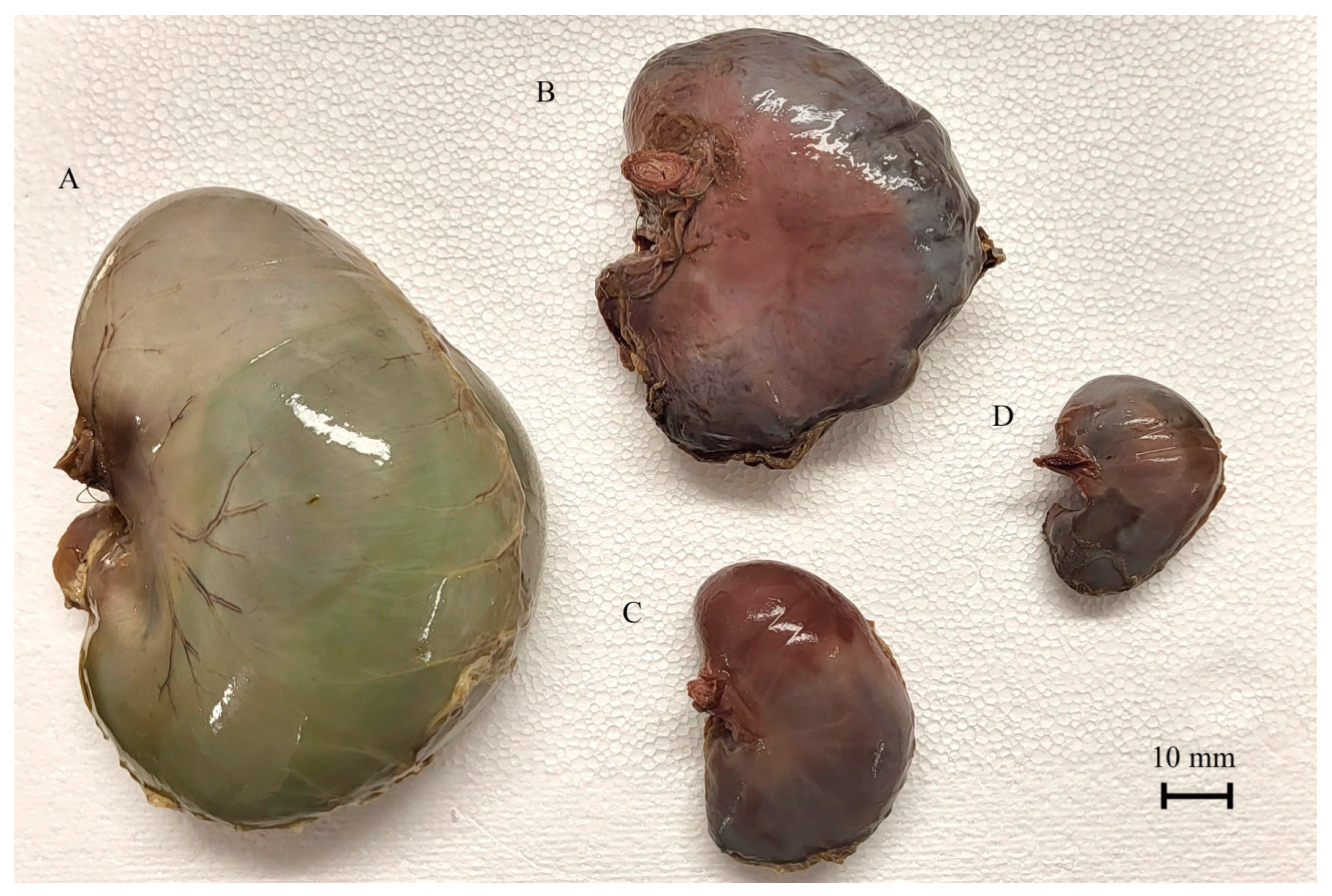
3.1. CRL Analysis
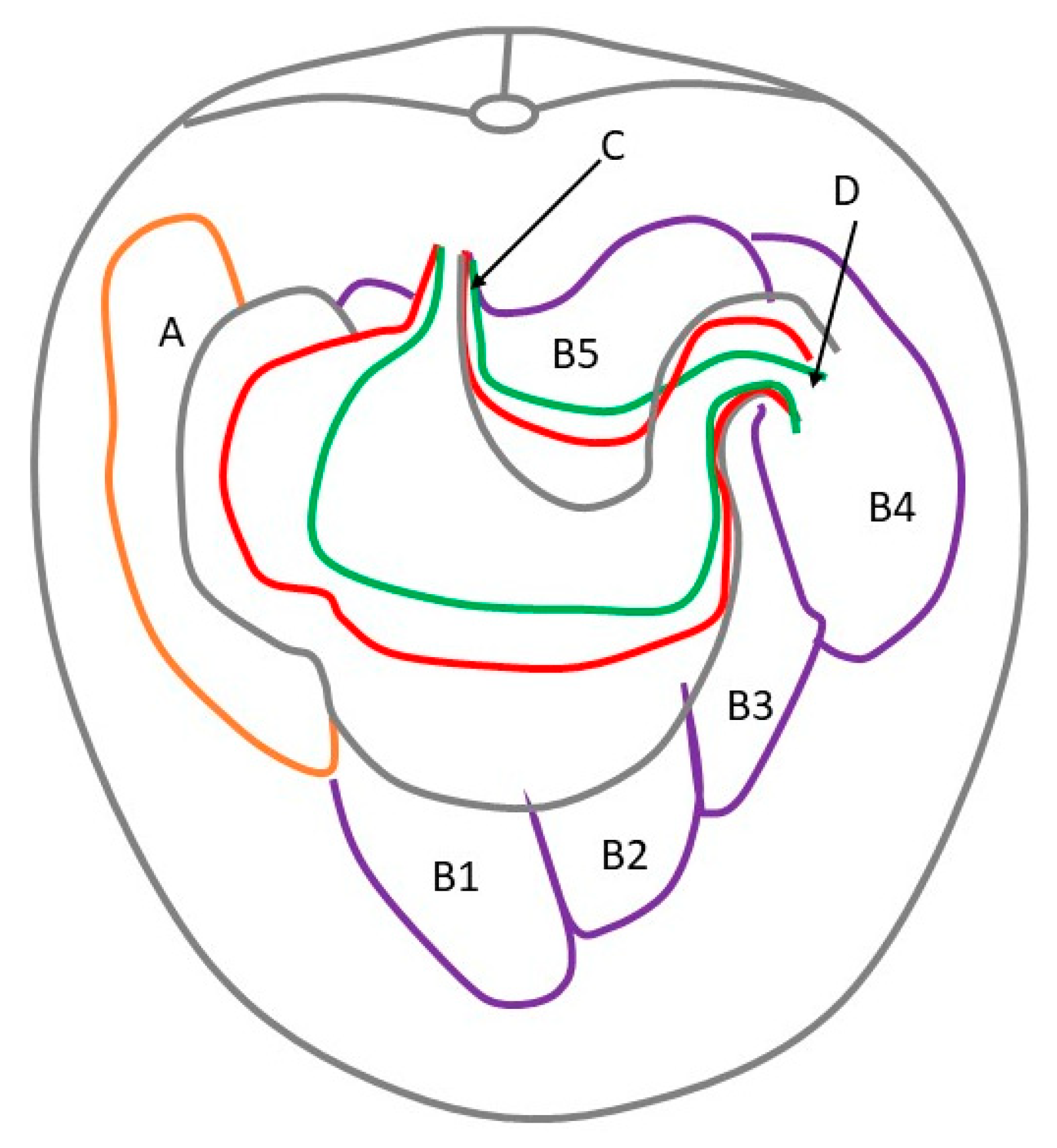
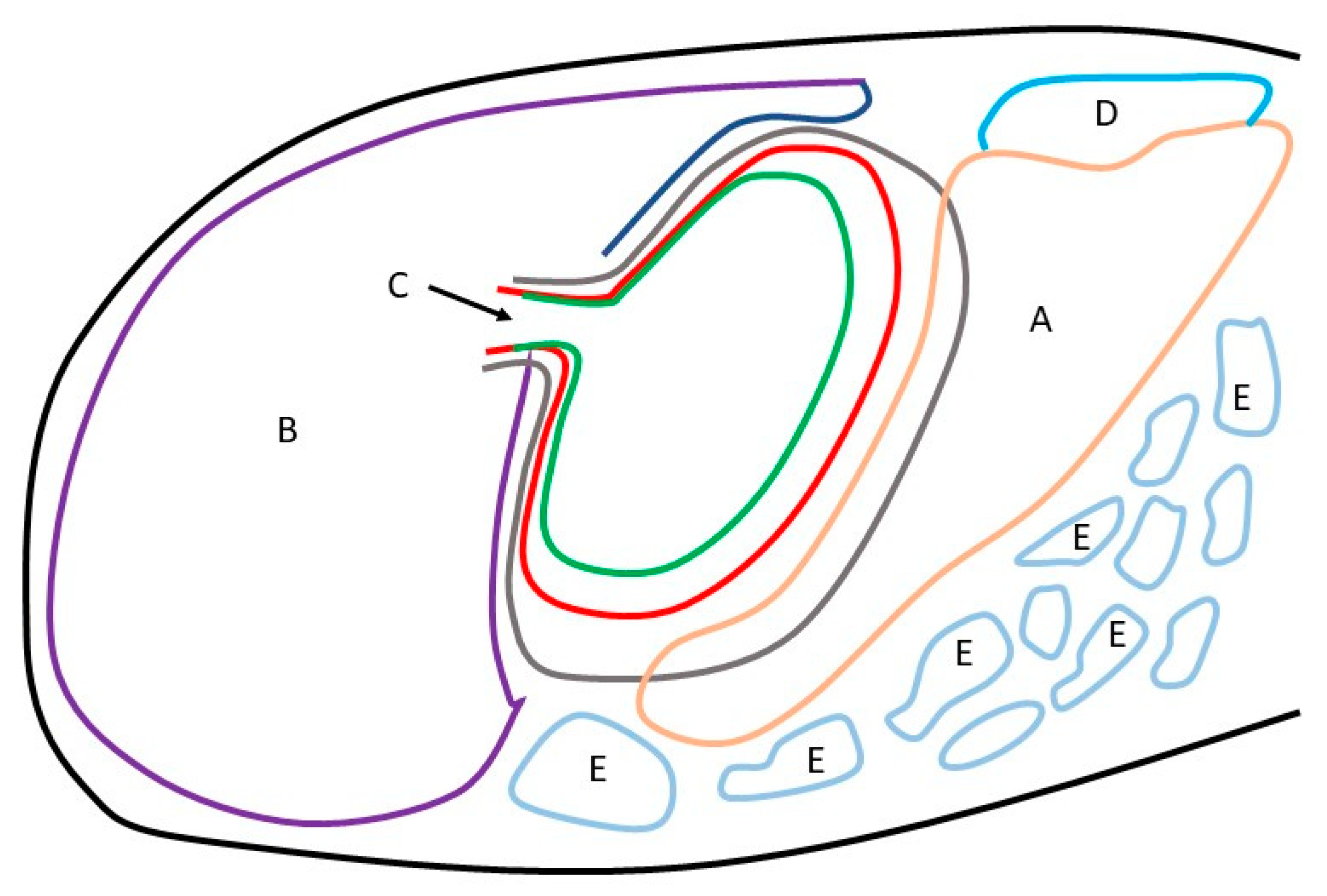
3.2. Anatomy and Topography of the Stomach (Holotopy, Skeletotopy, Syntopy)
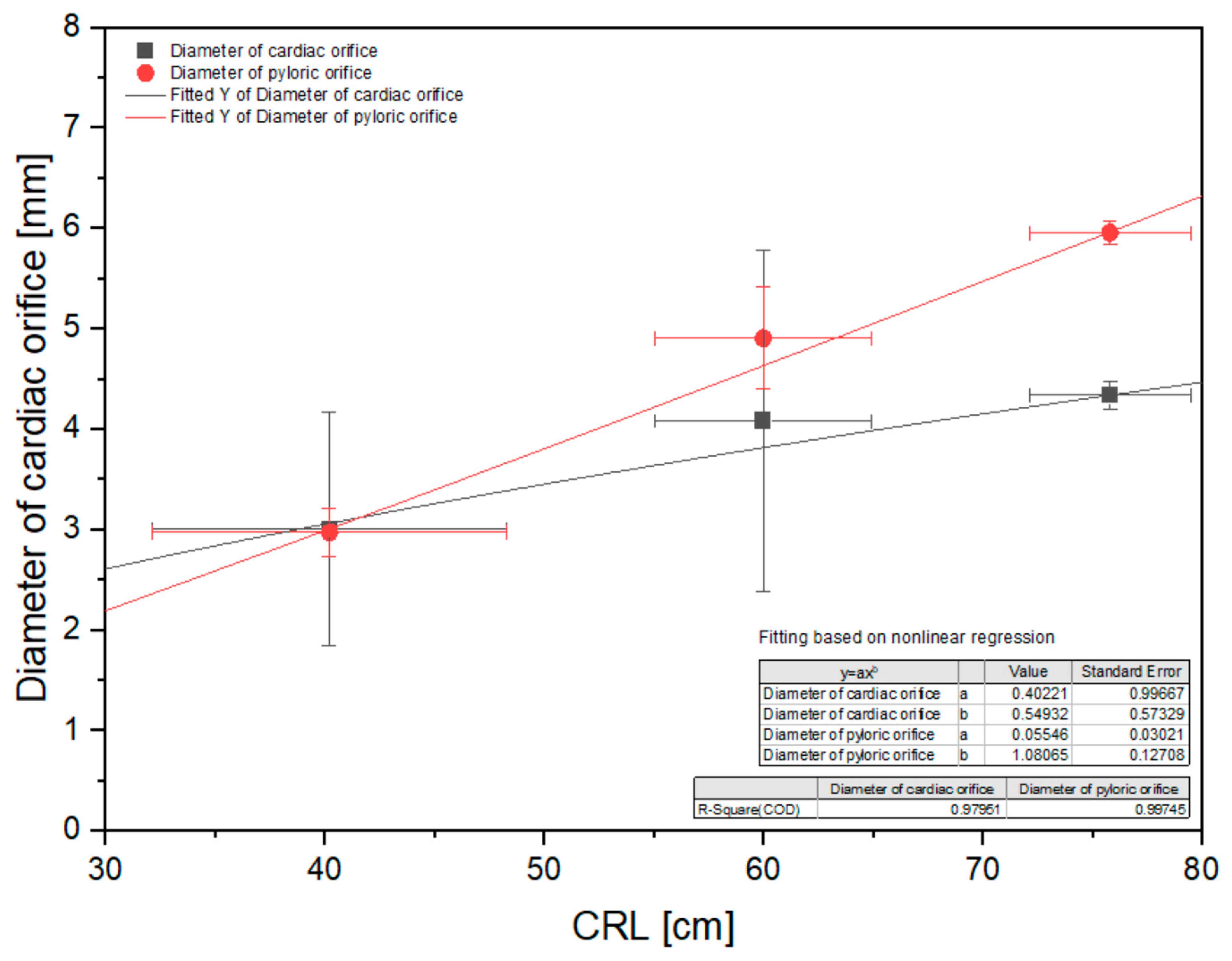
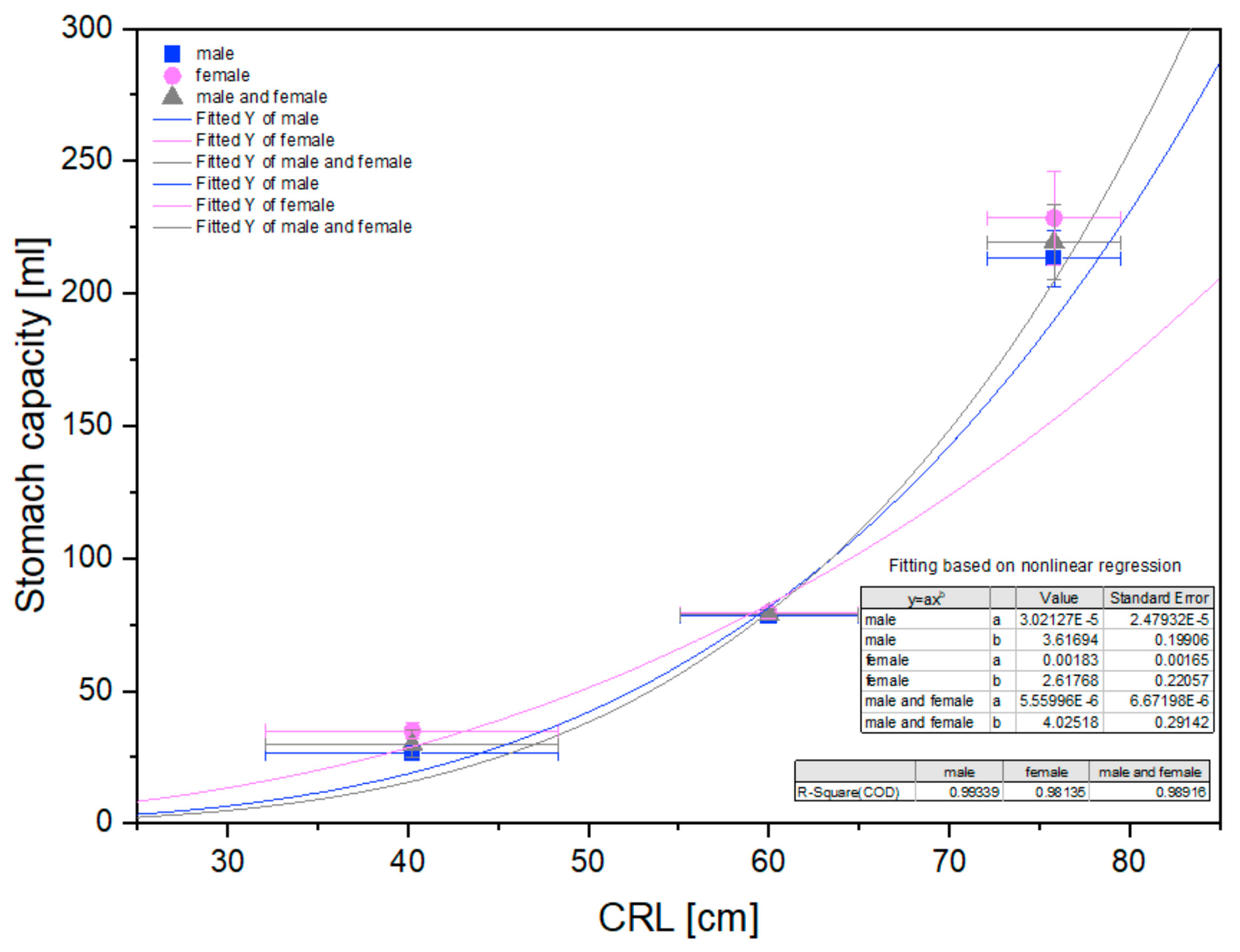
3.3. Morphometry of the Stomach
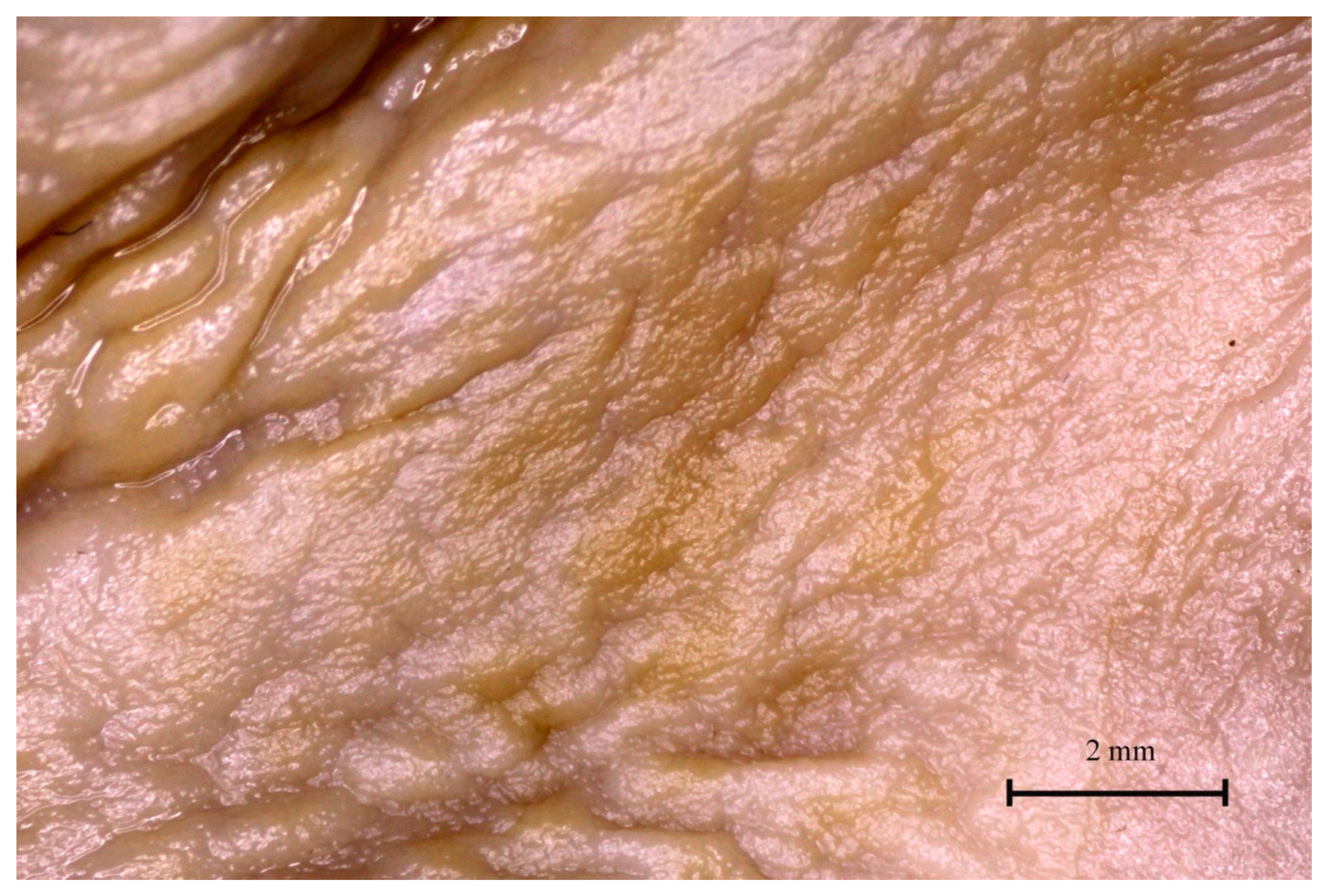
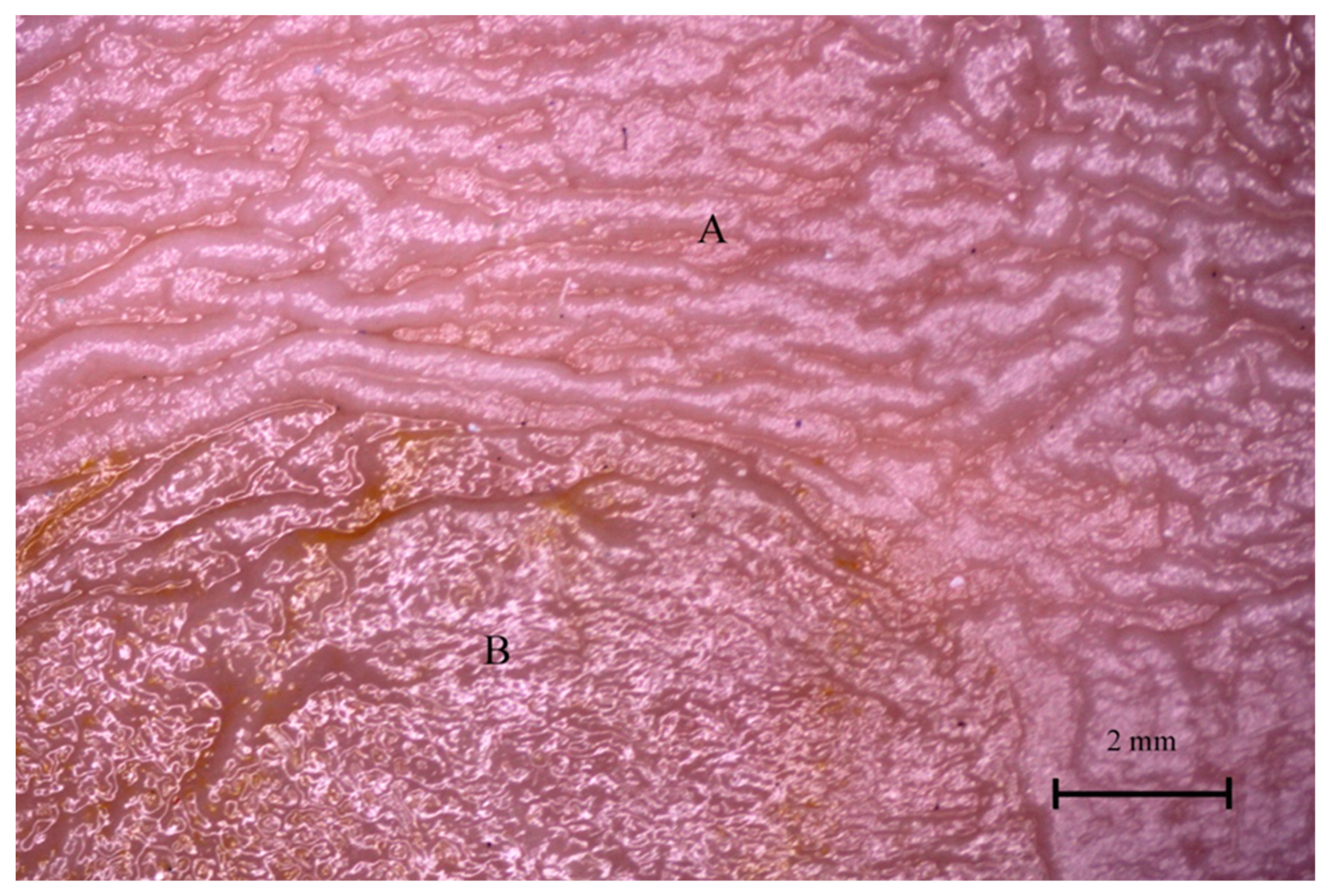
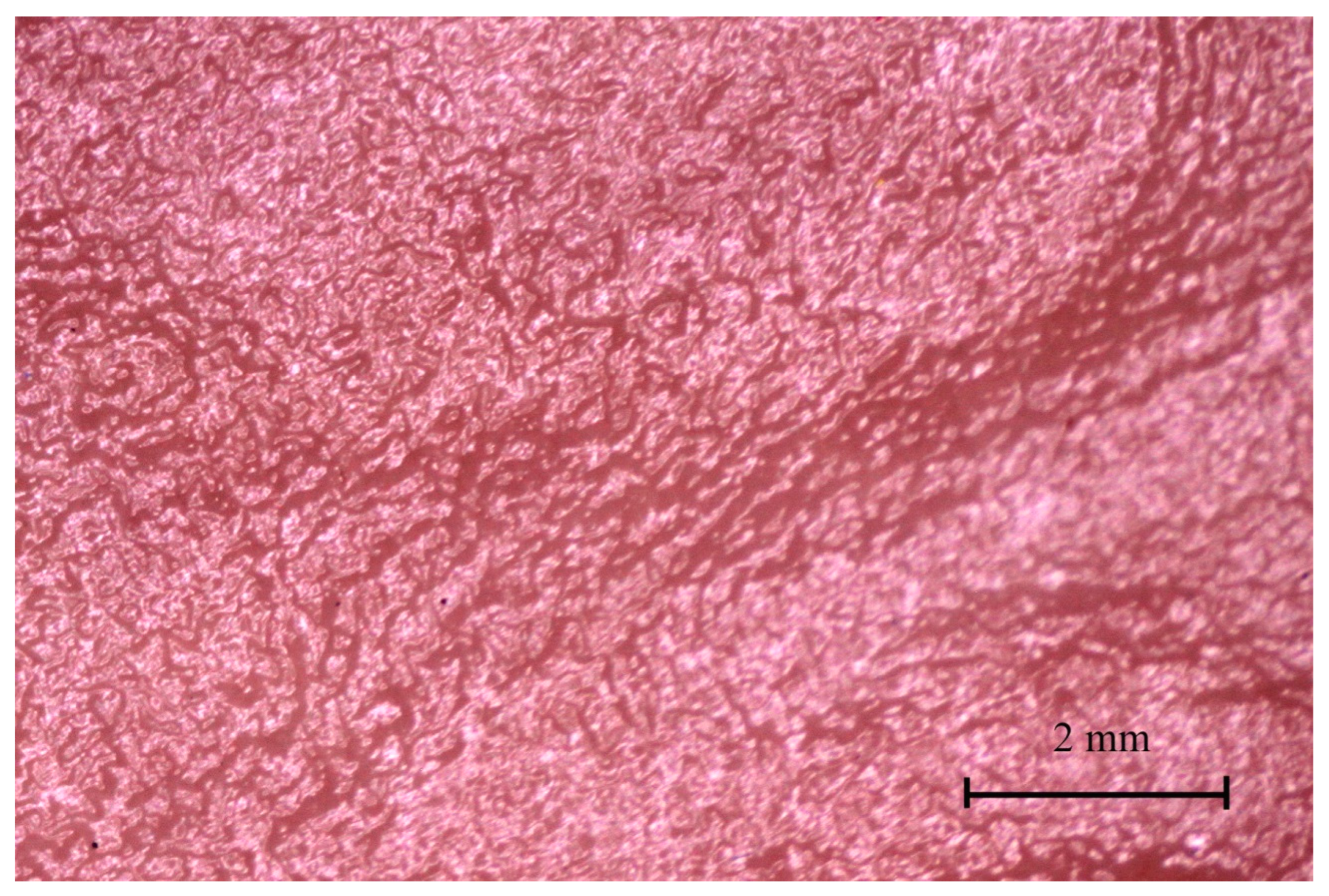
3.4. Stomach Mucosa Macroanatomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucca, S.; Fogarty, U.; Collins, A.; Small, V. Assessment of feto-placental well-being in the mare from mid-gestation to term: Transrectal and transabdominal ultrasonographic features. Theriogenology 2005, 64, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Murase, H.; Endo, Y.; Tsuchiya, T.; Kotoyori, Y.; Shikichi, M.; Ito, K.; Sato, F.; Nambo, Y. Ultrasonographic evaluation of equine fetal growth throughout gestation in normal mares using a convex transducer. J. Vet. Med. Sci. 2014, 76, 947–953. [Google Scholar] [CrossRef]
- Agnew, M.E.; Slack, J.; Stefanovski, D.; Linton, J.K.; Sertich, P.L. Sonographic appearance of the late gestation equine fetal intestine. Theriogenology 2019, 138, 121–126. [Google Scholar] [CrossRef]
- Lanci, A.; Castagnetti, C.; Ranciati, S.; Sergio, C.; Mariella, J. A regression model including fetal orbit measurements to predict parturition in Standardbred mares with normal pregnancy. Theriogenology 2019, 126, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Bucca, S.; De Oliveira, I.R.S.; Cunanan, J.C.; Vinardell, T.; Troedsson, M.H. Doppler indices of the equine fetal carotid artery throughout gestation. Theriogenology 2020, 156, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, B.; Kressin, M. Embriologie der Haustiere, 6th ed.; Enke Verlag: Stuttgart, Germany, 2011. [Google Scholar]
- Rüsse, I.; Sinowatz, F. Lehrbuch der Embriologie der Haustiere; Verlag Paul Parey: Berlin, Germany; Hamburg, Germany, 1991. [Google Scholar]
- Bielańska-Osuchowska, Z. Zarys Organogenezy, 1st ed.; PWN: Warszawa, Poland, 2004. [Google Scholar]
- Franciolli, A.L.R.; Cordeiro, B.M.; Fonseca, E.T.; Rodrigues, M.N.; Sarmento, C.A.P.; Ambrosio, C.E.; Carvalho, A.F.; Miglino, M.A.; Silva, L.A. Characteristics of equine embryo and fetus from days 15 to 107 of pregnancy. Theriogenology 2011, 76, 819–832. [Google Scholar] [CrossRef]
- Cai, W.-Q.; Gabella, G. Structure and innervation of the musculature at the gastroduodenal junction of the guinea pig. J. Anat. 1984, 139, 93–104. [Google Scholar]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse. I–Identification of proliferative cell types and pin pointing of the stem cells. Anat. Rec. 1993, 236, 259–279. [Google Scholar] [CrossRef]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse. II–Outward migration of pit cells. Anat. Rec. 1993, 236, 280–296. [Google Scholar] [CrossRef]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse. III–Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat. Rec. 1993, 236, 297–313. [Google Scholar] [CrossRef]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse. IV–Bidirectional migration of parietal cells ending in their degeneration and loss. Anat. Rec. 1993, 236, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse. V–Behaviour of entero-endocrine and caveolated cells: General conclusions on cell kinetics in the oxyntic epithelium. Anat. Rec. 1993, 236, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.M.; Leblond, C.P. Origin and migratory pathways of the eleven epithelial cell types present in the body of mouse stomach. Microsc Res Tech 1995, 31, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Kablar, B. Structural study on the appearance of innervation in the stomach of mouse and rat embryos. Tissue Cell. 1995, 27, 309–315. [Google Scholar] [CrossRef]
- Asar, M.; Bayram, Z.; Korgun, E.T.; Tertemiz, F.; Akkoyunlu, G.; Demir, R. Immunocytochemical Detection of Synaptophysin in Enteric Neurones during Prenatal Development in the Rat Stomach. Anat. Histol. Embryol. 2004, 33, 135–140. [Google Scholar] [CrossRef]
- Çetin, A.; Eşrefoğlu, M. Prenatal and Postnatal Development of the Stomach in Wistar Albino Rats. J. Turgut Ozal Med. Cent. 2014, 21, 4–11. [Google Scholar]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F.; Guan, L.L. Development and physiology of the rumen and the lower gut: Targets for improving gut health. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef]
- Green, W.W.; Winters, L.M. Prenatal Development of the Sheep; Technical Bulletin 169; University of Minnesota Agricultural Experiment Station: St. Paul, MN, USA, 1945; pp. 3–36. [Google Scholar]
- Michel, G.; Flechsig, G. Zur Histogenese der Vormagenabteilung und des Labmagen beim Rind unter besonderer Beachtung der Entwicklung der Schleimhautbildungen. Anat. Anz. 1969, 124, 403–418. [Google Scholar]
- Georgieva, R.; Gerov, K. The morphological and functional differentiation of the alimentary canal of pig during ontogeny. I–Development and differentiation of the fundic portion of stomach. Anat. Anz. 1975, 137, 12–15. [Google Scholar]
- Liebich, H.G.; Scharrer, E. Entwicklungsbedingte Veränderungen von Struktur und Funktion des Pansenepithels. Anat. Histol. Embryol. 1984, 13, 25–41. [Google Scholar] [CrossRef]
- Chrószcz, A. The innervation and arterial blood supply of pig’s stomach in the fetal period. EJPAU 2008, 11, 3. [Google Scholar]
- Chrószcz, A. The morphometric and topographic study of pig’s stomach development between the 35th and 114th day of gestation. EJPAU 2008, 11, 4. [Google Scholar]
- Chrószcz, A. Morphology, development and histometry of swine gastric wall in the fetal period. EJPAU 2008, 11, 4. [Google Scholar]
- Rodrigues, M.N.; Carvalho, R.C.; Franciolli, A.L.R.; Rodrigues, R.F.; Rigoglio, N.N.; Jacob, J.C.F.; Gastal, E.L.; Miclino, M.A. Prenatal development of the digestive system in the horse. Anat. Rec. 2014, 297, 1218–1227. [Google Scholar] [CrossRef]
- Marrable, A.W. The Embryonic Pig a Chronological Account, 1st ed.; Pitman Medical: London, UK, 1971. [Google Scholar]
- Nickel, R.; Schummer, A.; Seiferle, E. Lehrbuch der Anatomie der Haustiere, 1st ed.; Parey Verlag: Stuttgart, Germany, 2004. [Google Scholar]
- Bergin, W.C. Developmental Horizons and Measurement Useful for Age Determination of Equine Embryos and Fetuses. Master’s Thesis, Kansas State Unversity, Manhattan, KS, USA, 1969. [Google Scholar]
- Platt, H. Growth and maturity in the equine fetus. J. R. Soc. Med. 1978, 71, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Bożiłow, W.; Sawicki, K. Metody Badań Zmienności Cech Anatomicznych Człowieka Podczas Rozwoju Prenatalnego i Okołoporodowego, 1st ed.; Akademia Medyczna: Wrocław, Poland, 1980. [Google Scholar]
- Stelmasiak, M.; Osemlak, J.; Siwek, R. The shape and measurements of the stomach in Cercopithecus aethiops. Folia Morphol. 1980, 39, 233–243. [Google Scholar]
- Biedermann, F. Metrische Untersuchungen an Pferdemagen. Doctoral Thesis, Leipzig, Germany, 1921. [Google Scholar]
- International Committee on Veterinary Histological Nomenclature. Nomina Histologica Veterinaria (NHV); World Association of Veterinary Anatomists: Ghent, Belgium, 2017. [Google Scholar]
- International Committee on Veterinary Histological Nomenclature. Nomina Anatomica Veterinaria (NAV), 6th ed.; World Association of Veterinary Anatomists: Ghent, Belgium, 2017. [Google Scholar]
- International Committee on Veterinary Histological Nomenclature. Nomina Embryologica Veterinaria (NEV), 2nd ed.; World Association of Veterinary Anatomists: Ghent, Belgium, 2006. [Google Scholar]
- Nawrot, J.; Dzięgiel, P. Morphology of lacrimal gland in pig fetuses. Anat. Histol. Embryol. 2007, 37, 74–77. [Google Scholar]
- Pospieszny, N. Morfologia pni błędnych owcy w okresie płodowym. Zesz. Nauk. AR Wroc. S. Wet. 2000, 381, 71–77. [Google Scholar]



| Sex | M | M | M | F | F | M | M | M | F | F | M | M | F | F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age [months] | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 7–8 | 7–8 | 7–8 | 7–8 | 10–11 | 10–11 | 10–11 | 10–11 | 10–11 |
| Intercostal space | 14–17 | 13–16 | 14–16 | 14–17 | 14–16 | 13–16 | 14–16 | 12–16 | 13–17 | 12–16 | 13–16 | 12–16 | 11–16 | 12–16 |
| Sex | M | M | M | F | F | M | M | M | F | F | M | M | F | F | F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age [months] | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 7–8 | 7–8 | 7–8 | 7–8 | 7–8 | 10–11 | 10–11 | 10–11 | 10–11 | 10–11 |
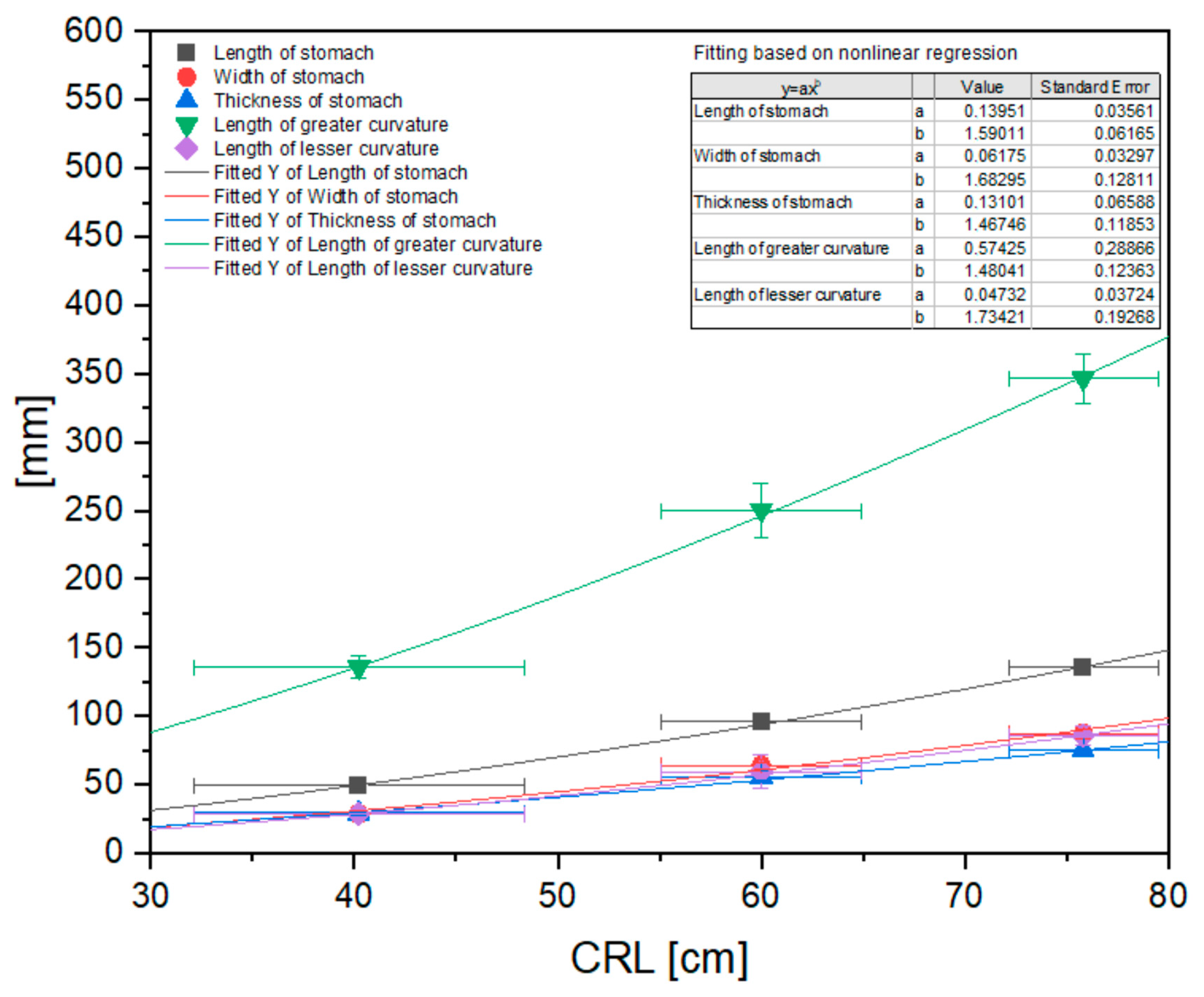
| Stomach length [mm] | 50.46 | 51.65 | 49.11 | 48.25 | 47.68 | 95.65 | 92.41 | 98.42 | 91.49 | 102.3 | 136.11 | 133.34 | 137.11 | 139.25 | 131.98 |
| Stomach width [mm] | 30.04 | 27.71 | 26.74 | 31.89 | 26.25 | 64.36 | 61.89 | 63.45 | 62.53 | 67.91 | 91.02 | 89.39 | 83.49 | 88.11 | 84.23 |
| Stomach thickness [mm] | 29.51 | 26.82 | 30.84 | 31.64 | 27.23 | 54.14 | 53.16 | 58.56 | 51.56 | 62.11 | 72.14 | 74.54 | 77.72 | 74.39 | 75.43 |
| Length of greater curvature [mm] | 141 | 141 | 139 | 136 | 122 | 284 | 245 | 244 | 232 | 247 | 357 | 337 | 368 | 350 | 321 |
| Length of lesser curvature [mm] | 26 | 32 | 27 | 27 | 31 | 47 | 59 | 78 | 50 | 63 | 96 | 88 | 81 | 85 | 79 |
| Diameter of cardiac orifice [mm] | 2.15 | 4.83 | 2.23 | 2.32 | 3.49 | 2.62 | 6.15 | 5.32 | 2.16 | 4.15 | 4.32 | 4.11 | 4.36 | 4.51 | 4.37 |
| Diameter of pyloric orifice [mm] | 3.38 | 2.75 | 2.88 | 2.95 | 2.89 | 5.53 | 4.17 | 5.01 | 5.13 | 4.68 | 6.13 | 5.82 | 5.92 | 6.01 | 5.88 |
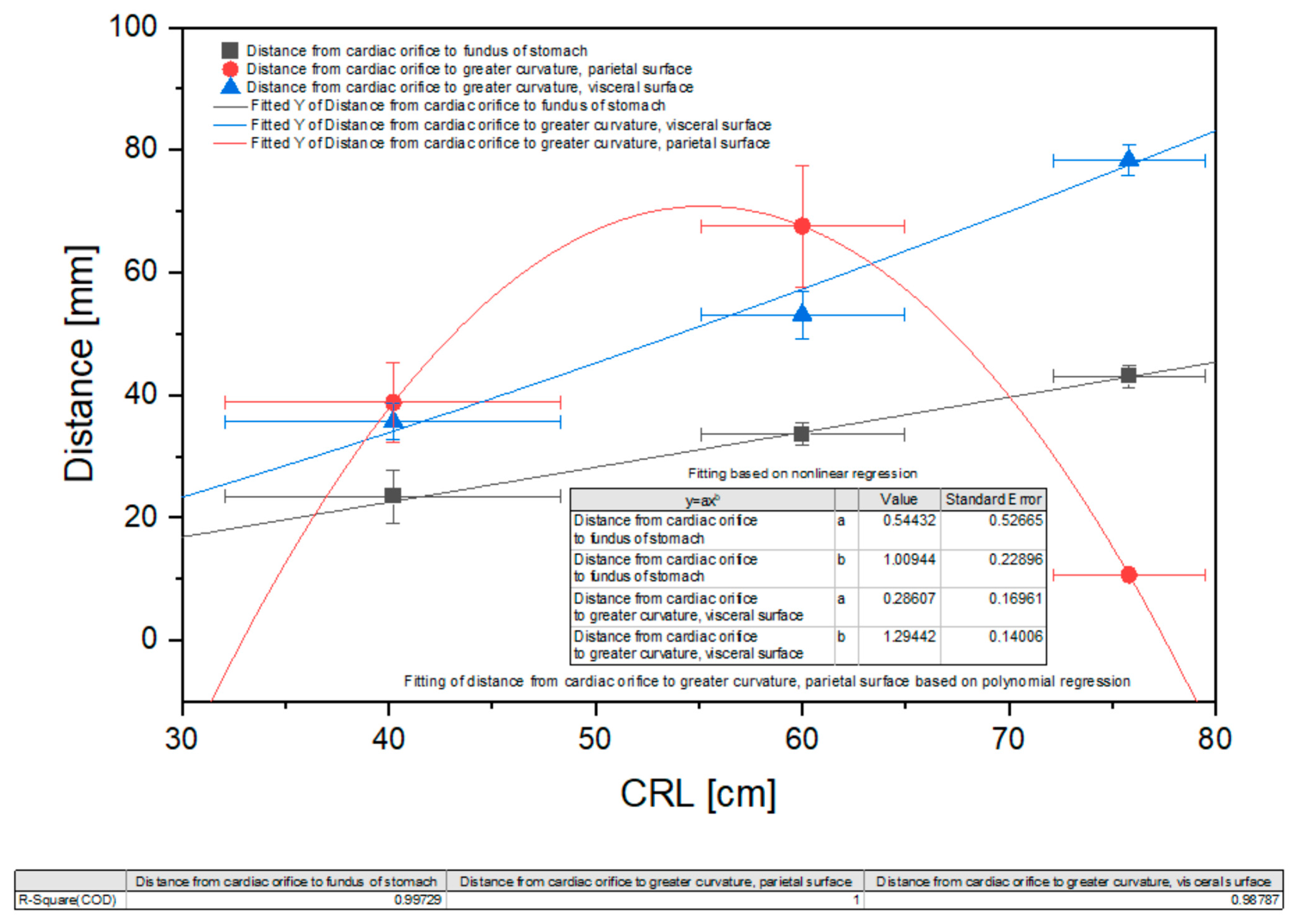
| Distance from cardiac orifice to stomach fundus [mm] | 17.28 | 26.17 | 21.32 | 28.39 | 24.34 | 33.69 | 36.35 | 32.59 | 31.38 | 34.32 | 40.38 | 42.39 | 44.13 | 45.23 | 43.45 |
| Distance from cardiac orifice to greater curvature parietal surface [mm] | 29.61 | 36.17 | 43.11 | 46.39 | 38.72 | 78.57 | 77.49 | 61.21 | 56.46 | 64.32 | 9.84 | 11.39 | 10.01 | 11.93 | 10.32 |
| Distance from cardiac orifice to greater curvature visceral surface [mm] | 34.17 | 31.5 | 37.71 | 39.12 | 35.83 | 58.88 | 49.66 | 53.29 | 49.36 | 54.35 | 79.99 | 76.21 | 78.76 | 75.43 | 81.22 |
| Stomach volume [mL] | 27 | 24 | 29 | 37 | 33 | 80 | 76 | 79 | 81 | 78 | 241 | 216 | 220 | 219 | 201 |
| Stomach Indexes [mm] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | F | F | M | M | M | F | F | M | M | F | F | F |
| Age [months] | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 7–8 | 7–8 | 7–8 | 7–8 | 7–8 | 10–11 | 10–11 | 10–11 | 10–11 | 10–11 |
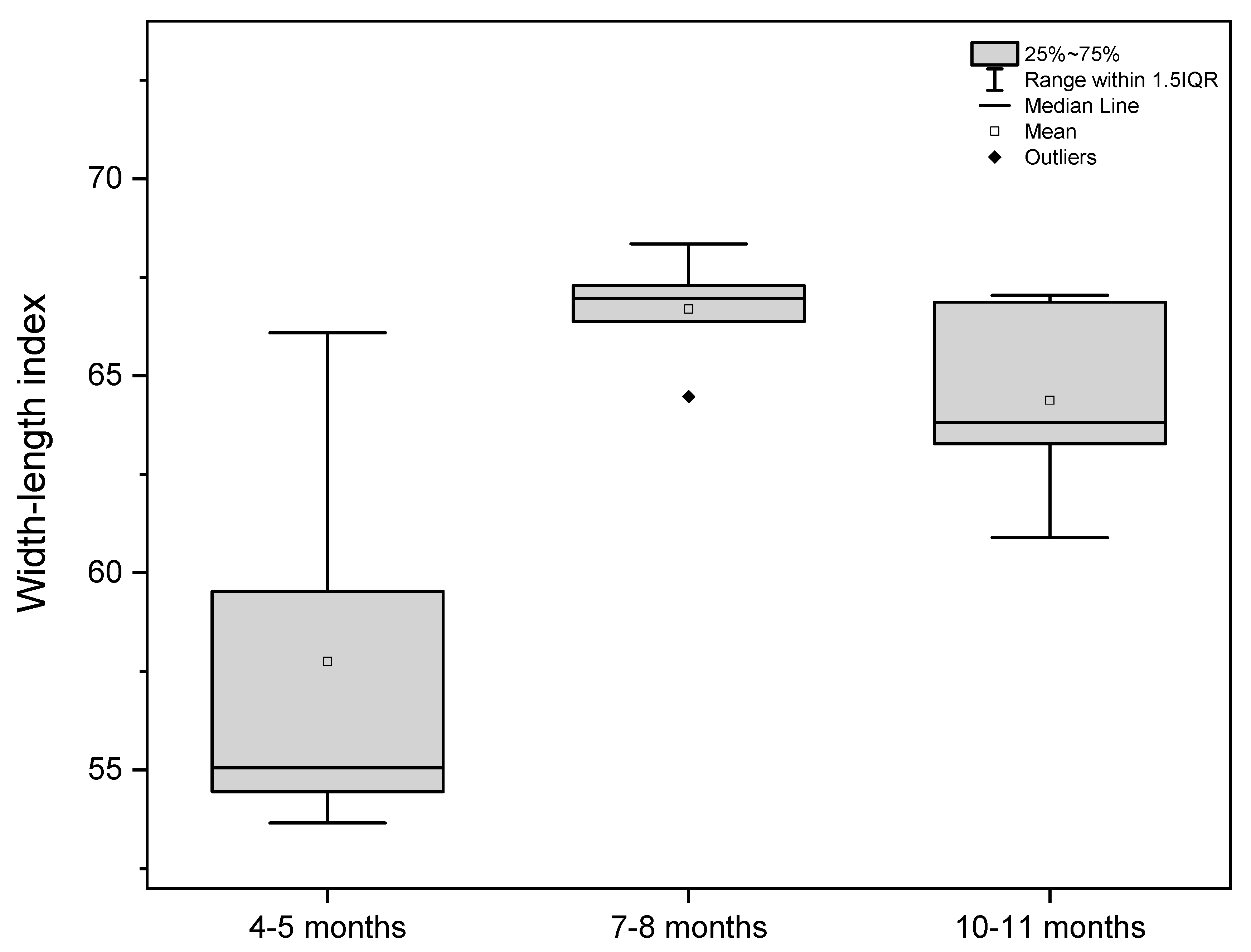
| Width-length | 59.53 | 53.65 | 54.45 | 66.09 | 55.05 | 67.29 | 66.97 | 64.47 | 68.35 | 66.38 | 66.87 | 67.04 | 60.89 | 63.27 | 63.82 |
| Thickness-length | 58.48 | 51.93 | 62.80 | 65.58 | 57.11 | 56.60 | 57.53 | 59.50 | 56.36 | 60.71 | 53.00 | 55.90 | 56.68 | 53.42 | 57.15 |
| Thickness-width | 98.24 | 96.79 | 115.33 | 99.22 | 103.73 | 84.12 | 85.89 | 92.29 | 82.46 | 91.46 | 79.26 | 83.39 | 93.09 | 84.43 | 89.55 |
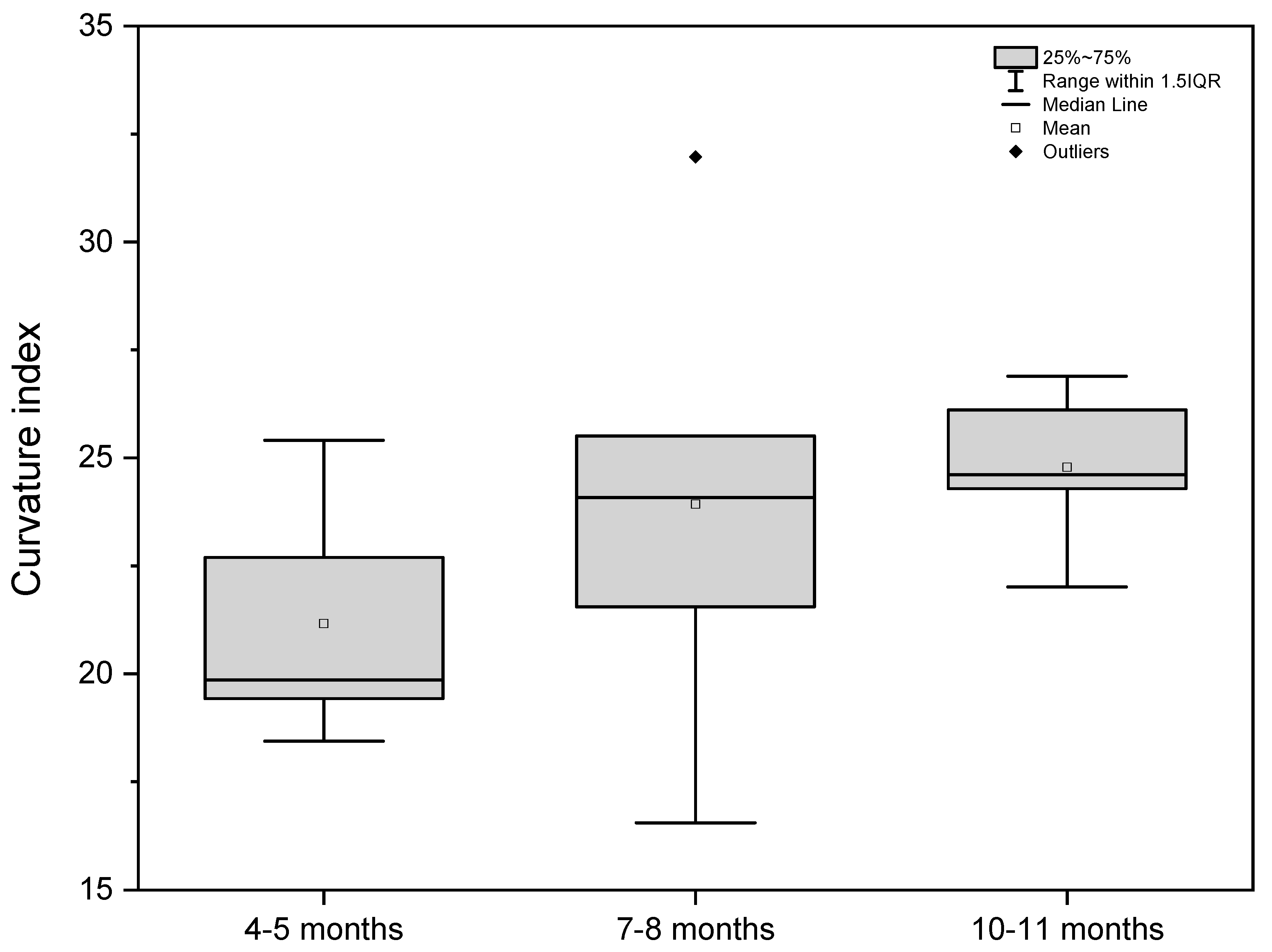
| Curvatures | 18.44 | 22.70 | 19.42 | 19.85 | 25.41 | 16.55 | 24.08 | 31.97 | 21.55 | 25.51 | 26.89 | 26.11 | 22.01 | 24.29 | 24.61 |
| Cardia-pyloric | 63.61 | 175.64 | 77.43 | 78.64 | 120.76 | 47.38 | 147.48 | 106.19 | 42.11 | 88.68 | 70.47 | 70.62 | 73.65 | 75.04 | 74.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poradowski, D.; Chrószcz, A. Equine Stomach Development in the Fetal Period: An Anatomical, Topographical, and Morphometric Study. Animals 2022, 12, 2966. https://doi.org/10.3390/ani12212966
Poradowski D, Chrószcz A. Equine Stomach Development in the Fetal Period: An Anatomical, Topographical, and Morphometric Study. Animals. 2022; 12(21):2966. https://doi.org/10.3390/ani12212966
Chicago/Turabian StylePoradowski, Dominik, and Aleksander Chrószcz. 2022. "Equine Stomach Development in the Fetal Period: An Anatomical, Topographical, and Morphometric Study" Animals 12, no. 21: 2966. https://doi.org/10.3390/ani12212966
APA StylePoradowski, D., & Chrószcz, A. (2022). Equine Stomach Development in the Fetal Period: An Anatomical, Topographical, and Morphometric Study. Animals, 12(21), 2966. https://doi.org/10.3390/ani12212966






