Dietary Oregano Essential Oil Supplementation Influences Production Performance and Gut Microbiota in Late-Phase Laying Hens Fed Wheat-Based Diets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Laying Performance and Egg Quality
2.4. Analysis of Ileal Microbiota
2.5. Statistical Analysis
3. Results
3.1. Laying Performance and Egg Quality
3.2. Yolk Fatty Acids Composition
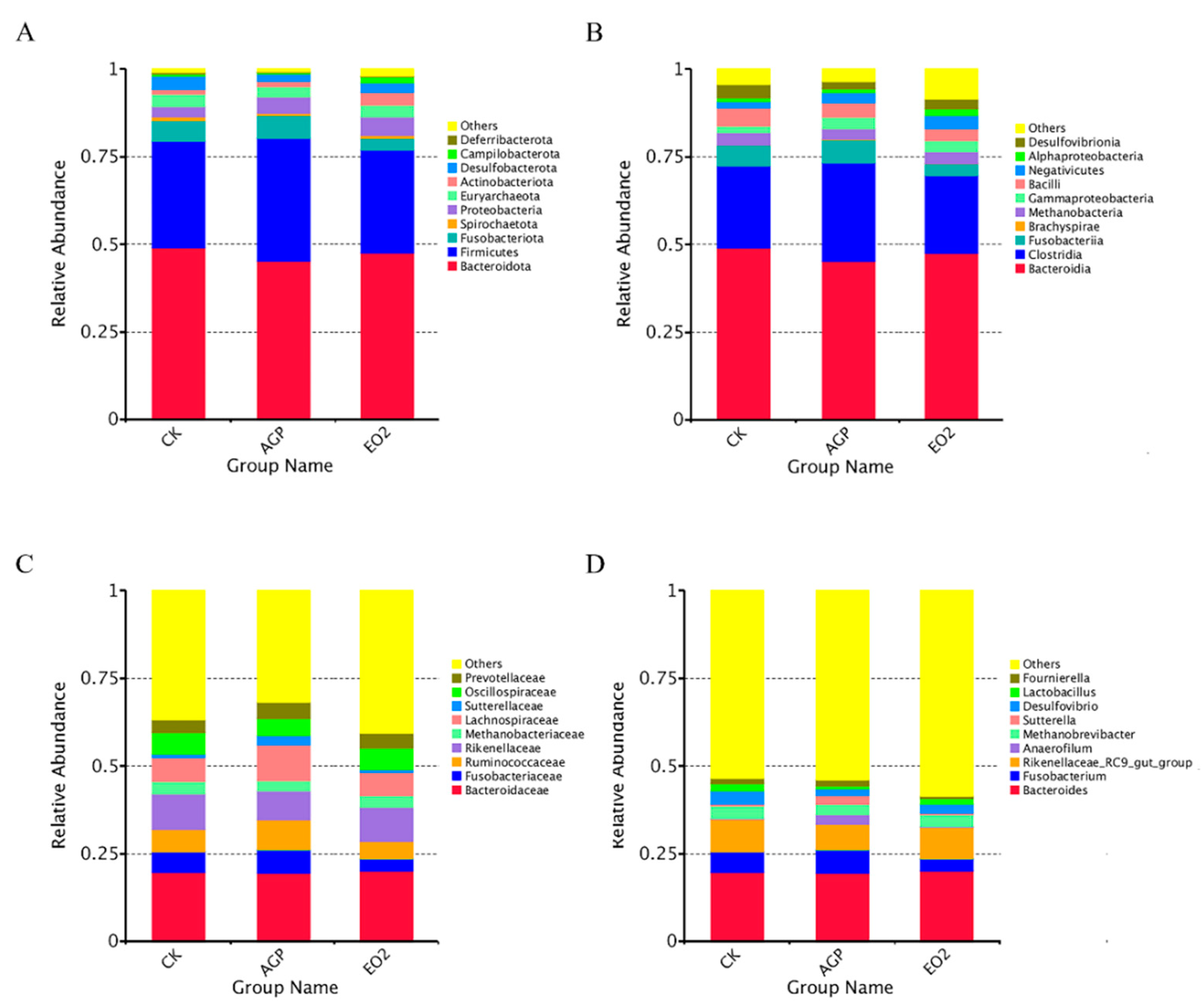
3.3. Cecal Microbial Profile
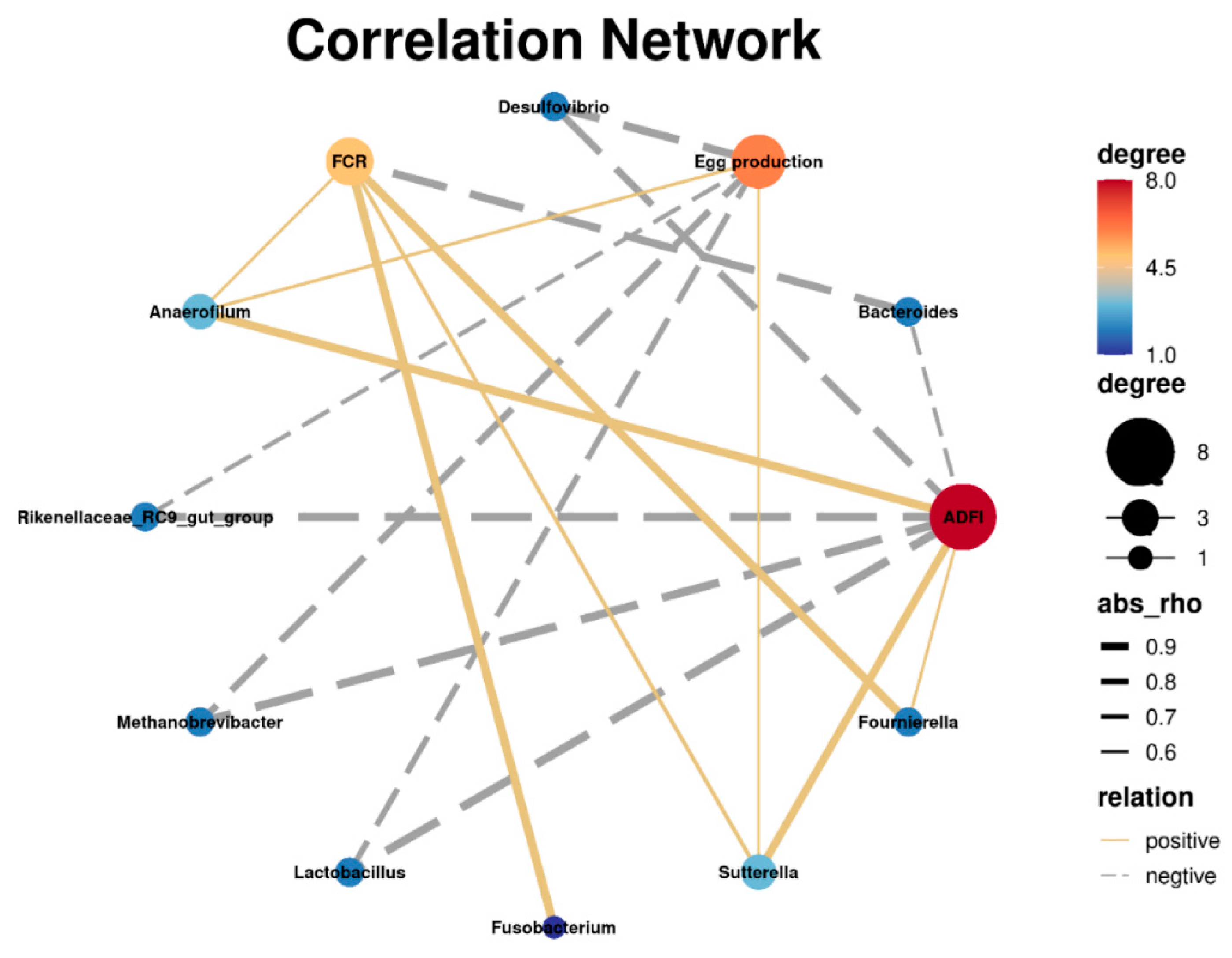
3.4. Correlation between Cecal Microbiota and Laying Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, M.D. Antibiotic use in animal feed and its impact on human healt. Nutr. Res. Rev. 2000, 13, 279–299. [Google Scholar] [CrossRef] [Green Version]
- Phillips, I. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2003, 53, 28–52. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anima. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems–A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Zhang, W.; Sun, M.; Li, H.; Xia, F.; Cui, H.; Bai, H.; Shi, L. Analysis of the chemical profiles and anti-S. aureus activities of essential oils extracted from different parts of three oregano cultivars. Foods 2021, 10, 2328. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Andrey, T.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yang, Y.; Yu, H.D.; Ying, Y.; Zou, G.L. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 2005, 96, 151–158. [Google Scholar]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Coronado, A.C.; Silva-Vázquez, R.; Rangel-Nava, Z.E.; Hernández-Martínez, C.A.; Kawas-Garza, J.R.; Hume, M.E.; Méndez-Zamora, G. Mexican oregano essential oils given in drinking water on performance, carcass traits, and meat quality of broilers. Poult. Sci. 2019, 98, 3050–3058. [Google Scholar] [CrossRef]
- Xue, F.; Shi, L.; Li, Y.; Ni, A.; Ma, H.; Sun, Y.; Chen, J. Effects of replacing dietary Aureomycin with a combination of plant essential oils on production performance and gastrointestinal health of broilers. Poult. Sci. 2020, 99, 4521–4529. [Google Scholar] [CrossRef]
- Peng, Q.Y.; Li, J.D.; Li, Z.; Duan, Z.Y.; Wu, Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed Sci. Technol. 2016, 214, 148–153. [Google Scholar] [CrossRef]
- Abdul, H.; Zia, U.; Rifat, U.K.; Qudrat, U.; Shabana, N. Effect of diet supplemented with coconut essential oil on performance and villus histomorphology in broiler exposed to avian coccidiosis. Trop. Anim. Health Prod. 2020, 52, 2499–2504. [Google Scholar]
- Cheng, H.; Chen, J.F.; Tang, S.G.; Guo, S.C.; He, C.Q.; Qu, X.Y. Effects of essential oil/palygorskite composite on performance, egg quality, plasma biochemistry, oxidation status, immune response and intestinal morphology of laying hens. Poult. Sci. 2022, 101, 101632. [Google Scholar] [CrossRef]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef]
- Yin, D.; Du, D.; Yuan, J.E.; Gao, J.; Wang, Y.; Aggrey, S.E.; Guo, Y. Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci. Rep. 2017, 7, 7334. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wareth, A.A.A.; Lohakare, J.D. Productive performance, egg quality, nutrients digestibility, and physiological response of bovans brown hens fed various dietary inclusion levels of peppermint oil. Anim. Feed Sci. Technol. 2020, 267, 114554. [Google Scholar] [CrossRef]
- Migliorini, M.J.; Boiago, M.M.; Stefani, L.M.; Zampar, A.; Roza, L.F.; Barreta, M.; Arno, A.; Robazza, W.S.; Giuriatti, J.; Galvão, A.C.; et al. Oregano essential oil in the diet of laying hens in winter reduces lipid peroxidation in yolks and increases shelf life in eggs. J. Therm. Biol. 2019, 85, 102409. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Li, X.; Yang, X.; Long, F.; Yang, X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poult. Sci. 2019, 98, 6751–6760. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yu, L.; Xu, T.; Zhu, N. Microbiota and metabolome responses in the cecum and serum of broiler chickens fed with plant essential oils or virginiamycin. Sci. Rep. 2020, 10, 5382. [Google Scholar] [CrossRef] [Green Version]
- Abdelli, N.; Pérez, J.F.; Vilarrasa, E.; Cabeza Luna, I.; Melo-Duran, D.M.; Angelo, D.; Solà-Oriol, D. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Mandal, G.P.; Patra, A.K.; Kumar, P.; Samanta, I.; Pradhan, S.; Samanta, A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018, 236, 39–47. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimaa, A.A.; Samar, A.T.; Dina, M.M.A.; Doaa, M.A.F.; Aziza, M.H.; Abdallah, E.M. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet. Res. 2021, 17, 312. [Google Scholar]
- Ding, X.; Yu, Y.; Su, Z.; Zhang, K. Effects of essential oils on performance, egg quality, nutrient digestibility and yolk fatty acid profile in laying hens. Anim. Nutr. 2017, 3, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Nadia, R.; Hassan, R.A.; Qota, E.M.; Fayek, H.M. Effect of natural antioxidant on oxidative stability of eggs and productive and reproductive performance of laying hens. Int. J. Poult. Sci. 2008, 7, 134–150. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Wei, J.; Yang, C.; Yang, Z.; Yang, W.; Jiang, S. Effects of star anise (Illicium verum Hook. f.) essential oil on laying performance and antioxidant status of laying hens. Poult. Sci. 2018, 97, 3957–3966. [Google Scholar] [CrossRef]
- Botsoglou, N.; Florou-Paneri, P.; Botsoglou, E.; Dotas, V.; Giannenas, I.; Koidis, A.; Mitrakos, P. The effect of feeding rosemary, oregano, saffron and alpha-tocopheryl acetate on hen performance and oxidative stability of eggs. S. Afr. J. Anim. Sci. 2005, 35, 143–151. [Google Scholar]
- Bozkurt, M.; Kucukyilmaz, K.; Catli, A.U.; Cinar, M.; Bintas, E.; Coven, F. Performance, egg quality, and immune response of laying hens fed diets supplemented with mannan-oligosaccharide or an essential oil mixture under moderate and hot environmental conditions. Poult. Sci. 2012, 91, 1379–1386. [Google Scholar] [CrossRef]
- Bimczok, D.; Rau, H.; Sewekow, E.; Janczyk, P.; Souffrant, W.B.; Rothkotter, H.J. Influence of carvacrol on proliferation and survival of porcine lymphocytes and intestinal epithelial cells in vitro. Toxicol. In Vitro 2008, 22, 652–658. [Google Scholar] [CrossRef]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012, 158, 2870–2877. [Google Scholar] [CrossRef] [Green Version]
- Grobas, S.; Mendez, J.; De Blas, C.; Mateos, G.G. Influence of dietary energy, supplemental fat and linoleic acid concentration on performance of laying hens at two ages. Br. Poult. Sci. 1999, 40, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Skrivan, M.; Marounek, M.; Bubancova, I.; Podsednicek, M. Influence of limestone particle size on performance and egg quality in laying hens aged 24-36 weeks and 56-68 weeks. Anim. Feed Sci. Technol. 2010, 158, 110–114. [Google Scholar] [CrossRef]
- Feng, J.; Lu, M.; Wang, J.; Zhang, H.; Qiu, K.; Qi, G.; Wu, S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pi, G.; Li, F. The role of intestinal flora in the regulation of bone homeostasis. Front. Cell. Infect. Microbiol. 2021, 11, 579323. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Salem, N.J. Egg yolk as a source of long-chain polyunsaturated fatty acids in infant feeding. Am. J. Clin. Nutr. 1992, 55, 411–414. [Google Scholar] [CrossRef]
- Plotz, T.; Krummel, B.; Laporte, A.; Pingitore, A.; Persaud, S.J.; Jorns, A.; Elsner, M.; Mehmeti, I.; Lenzen, S. The monounsaturated fatty acid oleate is the major physiological toxic free fatty acid for human beta cells. Nutr. Diabetes 2017, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef]
- Willett, W.C. The Mediterranean diet: Science and practice. Public Health Nutr. 2006, 9, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Galobart, J.; Barroeta, A.C.; Baucells, M.D.; Codony, R.; Ternes, W. Effect of dietary supplementation with rosemary extract and alpha-tocopheryl acetate on lipid oxidation in eggs enriched with omega 3-fatty acids. Poult. Sci. 2001, 80, 460–467. [Google Scholar] [CrossRef]
- Bolukbasi, S.C.; Urusan, H.; Erhan, M.K.; Kiziltunc, A. Effect of dietary supplementation with bergamot oil (Citrus bergamia) on performance and serum metabolic profile of hens, egg quality and yolk fatty acid composition during the late laying period. Archiv. Fur. Geflugelkunde 2010, 74, 172–177. [Google Scholar]
- Abdel-Wareth, A. Effect of dietary supplementation of thymol, synbiotic and their combination on performance, egg quality and serum metabolic profile of Hy-Line Brown hens. Br. Poult. Sci. 2016, 57, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Sowinska, A.; Bierla, J.B.; Czarnowska, E.; Rybak, A.; Grzybowska-Chlebowczyk, U. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota—Key players in the pathogenesis of celiac disease. World J. Gastroenterol. 2017, 23, 7505–7518. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Q.P.; Hu, K.; He, Y.; Ai, Q.; Hu, L.H.; Yu, J.L. Vitamin A and retinoic acid exhibit protective effects on Necrotizing enterocolitis by regulating intestinal flora and enhancing the intestinal epithelial barrier. Arch. Med. Res. 2018, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.L.; Ji, X.Q.; Liang, H.; Liu, Y.; Wang, B.; Sun, L.L.; Li, W.W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018, 9, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Shang, H.; Zhao, J.; Dong, X.; Guo, Y.; Zhang, H.; Cheng, J.; Zhou, H. Inulin improves the egg production performance and affects the cecum microbiota of laying hens. Int. J. Biol. Macromol. 2020, 155, 1599–1609. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef]
- Li, I.; Wu, S.; Liou, J.; Liu, H.; Chen, J.; Chen, C. Effects of Deinococcus spp. supplement on egg quality traits in laying hens. Poult. Sci. 2018, 97, 319–327. [Google Scholar] [CrossRef]
- Pinnell, L.J.; Dunford, E.; Ronan, P.; Hausner, M.; Neufeld, J.D. Recovering glycoside hydrolase genes from active tundra cellulolytic bacteria. Can. J. Microbiol. 2014, 60, 469–476. [Google Scholar] [CrossRef]
- Oladokun, S.; Koehler, A.; MacIsaac, J.; Ibeagha-Awemu, E.M.; Adewole, D.I. Bacillus subtilis delivery route: Effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2020, 100, 100809. [Google Scholar] [CrossRef] [PubMed]
- Serino, M. SCFAs—The thin microbial metabolic line between good and bad. Nat. Rev. Endocrinol. 2019, 15, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic t-reg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, J.; Redondo-Blanco, S.; Gutierrez-del-Rio, I.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Q.; Huang, Z.; Lv, L.; Liu, X.; Yin, C.; Yan, H.; Yuan, J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016, 120, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.A.; Bedford, M.R.; Penn, C.W.; Pallen, M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 2014, 9, e91941. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; Verstegen, M.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. World Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Hunkapiller, A.A.; Layton, A.C.; Chang, Y.; Robbins, K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog. Dis. 2013, 10, 331–337. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef]

| Item | |
|---|---|
| Wheat | 72.00 |
| Soybean meal (44% CP) | 14.50 |
| Soybean oil | 1.00 |
| Limestone | 10.30 |
| Premix 1 | 2.20 |
| Total | 100.00 |
| Calculated nutrient levels | |
| Crude protein | 16.06 |
| Calcium | 3.86 |
| Metabolizable energy, MJ/kg | 10.96 |
| Available phosphorus | 0.32 |
| SID lysine | 0.66 |
| SID methionine | 0.37 |
| SID threonine | 0.52 |
| SID tryptophan | 0.19 |
| Items | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | AGP | EO1 | EO2 | EO3 | |||
| Egg production, % | |||||||
| Weeks 1–4 | 90.13 a | 91.32 a | 85.36 b | 91.42 a | 86.82 b | 0.89 | 0.001 |
| Weeks 5–8 | 87.46 b | 92.38 a | 85.71 b | 91.95 a | 86.50 b | 0.90 | 0.001 |
| Weeks 1–8 | 88.77 b | 91.86 a | 85.48 c | 91.64 a | 86.67 c | 0.67 | 0.001 |
| Average egg weight, g | |||||||
| Weeks 1–4 | 58.68 b | 59.46 a | 59.62 a | 58.97 b | 59.47 a | 0.19 | 0.001 |
| Weeks 5–8 | 58.98 c | 59.65 ab | 59.96 a | 59.34 b | 59.93 a | 0.18 | 0.001 |
| Weeks 1–8 | 58.81 c | 59.56 a | 59.795 a | 59.18 b | 59.70 a | 0.13 | 0.001 |
| ADFI, g/hen per day | |||||||
| Weeks 1–4 | 115.34 | 120.34 | 115.96 | 116.93 | 118.89 | 3.70 | 0.649 |
| Weeks 5–8 | 109.61 | 116.34 | 109.63 | 111.03 | 114.81 | 3.95 | 0.389 |
| Weeks 1–8 | 112.47 | 118.32 | 112.79 | 113.98 | 116.85 | 2.87 | 0.193 |
| FCR, g/g | |||||||
| Weeks 1–4 | 2.18 | 2.22 | 2.28 | 2.16 | 2.30 | 0.06 | 0.117 |
| Weeks 5–8 | 2.25 | 2.24 | 2.27 | 2.15 | 2.32 | 0.08 | 0.349 |
| Weeks 1–8 | 2.22 ab | 2.23 ab | 2.28 a | 2.16 b | 2.31 a | 0.05 | 0.026 |
| Broken-egg production | |||||||
| Weeks 1–4 | 1.57 | 3.20 | 3.61 | 1.78 | 2.21 | 0.78 | 0.076 |
| Weeks 5–8 | 3.60 | 4.91 | 3.09 | 3.34 | 5.21 | 0.85 | 0.082 |
| Weeks 1–8 | 2.58 | 4.05 | 3.35 | 2.60 | 3.72 | 0.75 | 0.203 |
| Items | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | AGP | EO1 | EO2 | EO3 | |||
| Eggshell thickness, 10−2 mm | |||||||
| Week 4 | 28.78 a | 28.56 a | 28.24 ab | 27.91 b | 27.87 b | 0.27 | 0.005 |
| Week 8 | 28.73 | 28.89 | 29.02 | 29.42 | 29.20 | 0.47 | 0.643 |
| Eggshell strength, kg | |||||||
| Week 4 | 3.55 | 3.45 | 3.45 | 3.46 | 3.57 | 0.24 | 0.971 |
| Week 8 | 3.42 | 3.41 | 3.45 | 361 | 3.55 | 0.26 | 0.918 |
| Shape index | |||||||
| Week 4 | 1.35 | 1.34 | 1.34 | 1.36 | 1.38 | 0.02 | 0.080 |
| Week 8 | 1.34 | 1.35 | 1.34 | 1.34 | 1.37 | 0.02 | 0.160 |
| Albumen height, mm | |||||||
| Week 4 | 6.53 | 6.44 | 6.14 | 6.40 | 5.76 | 0.43 | 0.393 |
| Week 8 | 5.99 | 6.05 | 5.98 | 5.96 | 5.90 | 0.44 | 0.998 |
| Haugh unit | |||||||
| Week 4 | 79.53 | 78.96 | 77.83 | 78.77 | 73.65 | 3.51 | 0.462 |
| Week 8 | 75.70 | 75.81 | 76.21 | 76.70 | 74.28 | 3.66 | 0.974 |
| Fatty Acid | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | AGP | EO1 | EO2 | EO3 | |||
| C4:0 | 0.002 b | 0.003 b | 0.005 b | 0.005 b | 0.008 a | 0.001 | 0.005 |
| C12:0 | 0.005 b | 0.006 a | 0.006 a | 0.006 a | 0.006 a | 0.000 | 0.003 |
| C14:0 | 0.277 | 0.273 | 0.269 | 0.267 | 0.271 | 0.009 | 0.814 |
| C15:0 | 0.044 a | 0.043 a | 0.041 ab | 0.037 bc | 0.034 c | 0.003 | 0.006 |
| C16:0 | 18.656 | 18.581 | 19.411 | 19.050 | 19.220 | 0.554 | 0.520 |
| C18:0 | 6.136 b | 6.565 a | 6.748 a | 6.499 b | 6.624 a | 0.175 | 0.026 |
| C21:0 | 0.047 | 0.057 | 0.060 | 0.059 | 0.057 | 0.004 | 0.056 |
| C24:0 | 0.097 | 0.092 | 0.091 | 0.090 | 0.098 | 0.007 | 0.716 |
| C16:1 | 2.660 | 2.818 | 2.664 | 2.799 | 2.753 | 0.186 | 0.866 |
| C18:1, cis(n-9) | 15.191 c | 15.245 bc | 15.490 bc | 16.069 ab | 16.452 a | 0.379 | 0.012 |
| C20:1 n9 | 0.131 b | 0.148 ab | 0.156 a | 0.158 a | 0.153 a | 0.008 | 0.034 |
| C18:2, cis(n-6) | 9.518 | 9.505 | 9.336 | 9.157 | 9.573 | 0.472 | 0.899 |
| C18:3 n-3 | 0.360 | 0.368 | 0.361 | 0.333 | 0.349 | 0.025 | 0.675 |
| C20:2 | 0.138 | 0.153 | 0.159 | 0.148 | 0.142 | 0.007 | 0.059 |
| C20:3 n-6 | 0.149 | 0.154 | 0.155 | 0.143 | 0.139 | 0.006 | 0.057 |
| C20:4 n-6 | 1.397 | 1.430 | 1.420 | 1.382 | 1.460 | 0.050 | 0.578 |
| C20:5 | 0.010 b | 0.013 a | 0.014 a | 0.013 a | 0.014 a | 0.001 | 0.001 |
| C22:6 | 0.701 | 0.731 | 0.713 | 0.715 | 0.780 | 0.037 | 0.292 |
| SFA | 25.392 | 26.309 | 26.778 | 26.013 | 26.319 | 0.599 | 0.204 |
| UFA | 30.260 | 30.565 | 30.468 | 30.917 | 31.816 | 0.529 | 0.061 |
| MUFA | 17.983 c | 18.212 c | 18.311 bc | 19.025 ab | 19.358 a | 0.349 | 0.003 |
| PUFA | 12.276 | 12.355 | 12.158 | 11.891 | 12.457 | 0.552 | 0.867 |
| n-3 PUFA | 1.062 | 1.100 | 1.074 | 1.048 | 1.129 | 0.056 | 0.627 |
| n-6 PUFA | 11.064 | 11.089 | 10.911 | 10.682 | 11.172 | 0.501 | 0.876 |
| n-3/n-6 | 0.096 | 0.099 | 0.099 | 0.098 | 0.101 | 0.003 | 0.605 |
| UFA/SFA | 1.197 | 1.196 | 1.144 | 1.189 | 1.210 | 0.028 | 0.221 |
| EFA | 11.276 | 11.303 | 11.117 | 10.872 | 11.383 | 0.522 | 0.874 |
| Items | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | AGP | EO2 | |||
| Shannon | 7.94 | 7.84 | 8.18 | 0.32 | 0.579 |
| Simpson | 0.99 | 0.98 | 0.99 | 0.01 | 0.627 |
| Chao1 | 855.41 | 790.28 | 1006.06 | 79.45 | 0.050 |
| ACE | 850.20 | 786.00 | 996.60 | 78.94 | 0.055 |
| Treatments 2 | SEM | p-Value | |||
|---|---|---|---|---|---|
| Items, % | Control 2 | AGP | EO2 | ||
| Phylum | |||||
| Desulfobacterota | 3.72 a | 2.04 b | 2.66 b | 0.45 | 0.010 |
| Actinobacteriota | 1.34 b | 1.51 b | 3.88 a | 0.91 | 0.016 |
| Class | |||||
| Desulfovibrionia | 3.69 a | 2.02 b | 2.61 b | 0.44 | 0.008 |
| Family | |||||
| Lachnospiraceae | 6.79 b | 10.16 a | 6.69 b | 0.85 | 0.002 |
| Desulfovibrionaceae | 3.69 a | 2.02 b | 2.61 b | 0.44 | 0.008 |
| Genus | |||||
| Desulfovibrio | 3.63 a | 2.00 b | 2.56 b | 0.44 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Zhang, L.; Li, H.; Xia, F.; Bai, H.; Piao, X.; Sun, Z.; Cui, H.; Shi, L. Dietary Oregano Essential Oil Supplementation Influences Production Performance and Gut Microbiota in Late-Phase Laying Hens Fed Wheat-Based Diets. Animals 2022, 12, 3007. https://doi.org/10.3390/ani12213007
Gao F, Zhang L, Li H, Xia F, Bai H, Piao X, Sun Z, Cui H, Shi L. Dietary Oregano Essential Oil Supplementation Influences Production Performance and Gut Microbiota in Late-Phase Laying Hens Fed Wheat-Based Diets. Animals. 2022; 12(21):3007. https://doi.org/10.3390/ani12213007
Chicago/Turabian StyleGao, Fei, Lianhua Zhang, Hui Li, Fei Xia, Hongtong Bai, Xiangshu Piao, Zhiying Sun, Hongxia Cui, and Lei Shi. 2022. "Dietary Oregano Essential Oil Supplementation Influences Production Performance and Gut Microbiota in Late-Phase Laying Hens Fed Wheat-Based Diets" Animals 12, no. 21: 3007. https://doi.org/10.3390/ani12213007





