Sentinel Lymph Node Mapping and Biopsy in Cats with Solid Malignancies: An Explorative Study
Abstract
Simple Summary
Abstract
1. Introduction
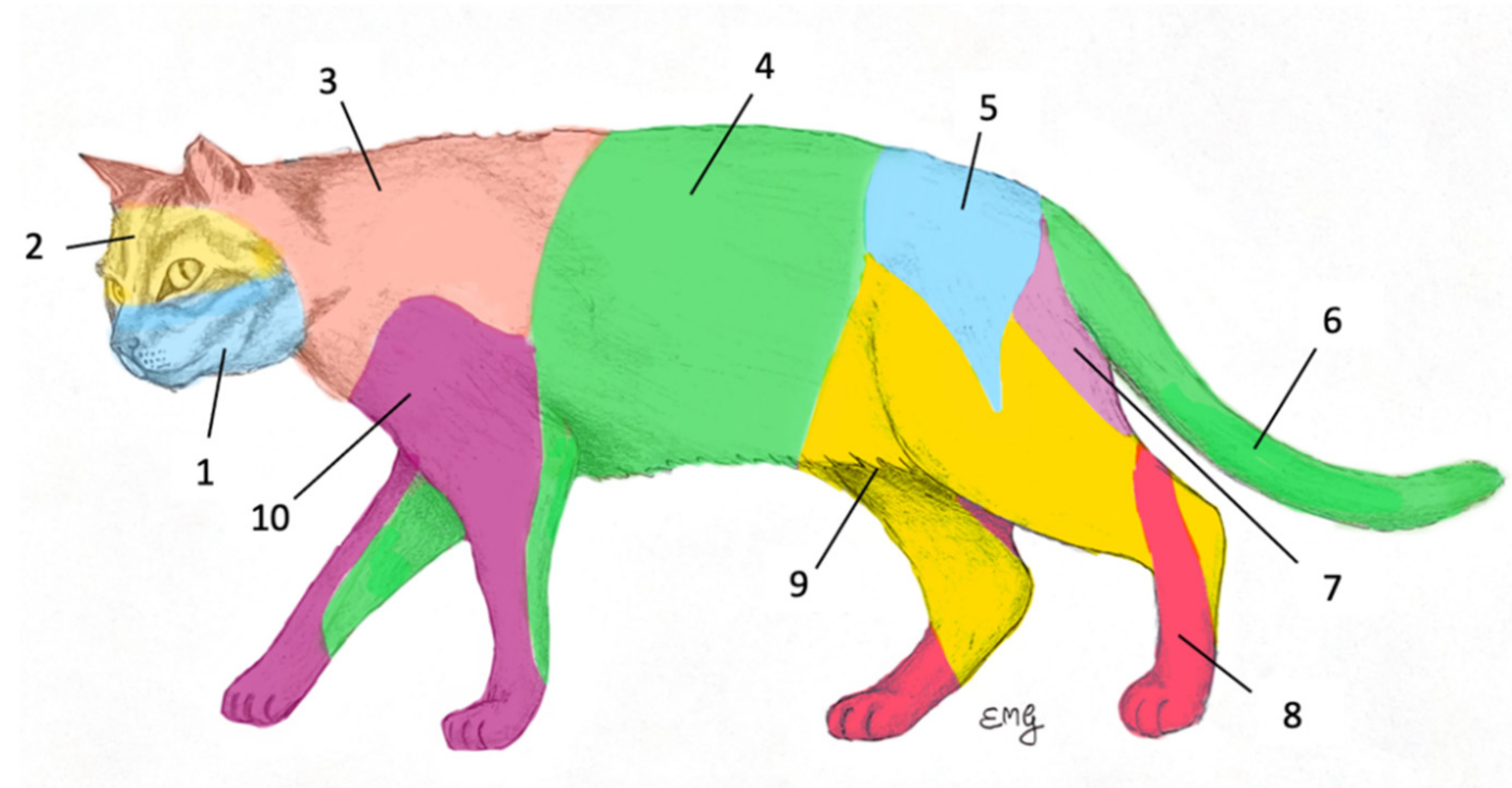
2. Materials and Methods
- Curative-intent surgical excision of the primary tumor; all tumors’ presentation were included: first presentation, recurrence, and T0; single and multiple presentations.
- Concurrent SLN mapping and extirpation, i.e., sentinel lymph node biopsy (SLNB)
- Total correspondence: all excised SLN would have been predicted based on the lymphosomes’ concept.
- Partial correspondence: the mapping procedure guided the extirpation of the RLN and at least one more node at an unpredictable site.
- Non-correspondence: all extirpated SLN occurred at different locations than the RLN.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrari, R.; Chiti, L.E.; Manfredi, M.; Ravasio, G.; De Zani, D.; Zani, D.D.; Giudice, C.; Gambini, M.; Stefanello, D. Biopsy of sentinel lymph nodes after injection of methylene blue and lymphoscintigraphic guidance in 30 dogs with mast cell tumors. Vet. Surg. 2020, 49, 1099–1108. [Google Scholar] [CrossRef]
- Ferrari, R.; Boracchi, P.; Chiti, L.E.; Manfredi, M.; Giudice, C.; de Zani, D.; Spediacci, C.; Recordati, C.; Grieco, V.; Gariboldi, E.M.; et al. Assessing the risk of nodal metastases in canine integumentary mast cell tumors: Is sentinel lymph node biopsy always necessary? Animals 2021, 11, 2373. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, K.M.; Thamm, D.H.; Worley, D.R.; Kamstock, D.A. Correlation of nodal mast cells with clinical outcome in dogs with mast cell tumour and a proposed classification system for the evaluation of node metastasis. J. Comp. Pathol. 2014, 151, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Baginski, H.; Davis, G.; Bastian, R.P. The prognostic value of lymph node metastasis with grade 2 MCTs in dogs: 55 cases (2001–2010). J. Am. Anim Hosp. Assoc. 2014, 50, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Worley, D.R. Incorporation of sentinel lymph node mapping in dogs with mast cell tumours: 20 consecutive procedures. Vet. Comp. Oncol. 2014, 12, 215–226. [Google Scholar] [CrossRef]
- Skinner, O.T.; Boston, S.E.; Souza, C.H.M. Patterns of lymph node metastasis identified following bilateral mandibular and medial retropharyngeal lymphadenectomy in 31 dogs with malignancies of the head. Vet. Comp. Oncol. 2017, 15, 881–889. [Google Scholar] [CrossRef]
- Chiti, L.E.; Stefanello, D.; Manfredi, M.; Zani, D.D.; de Zani, D.; Boracchi, P.; Giudice, C.; Grieco, V.; Di Giancamillo, M.; Ferrari, R. To map or not to map the cN0 neck: Impact of sentinel lymph node biopsy in canine head and neck tumours. Vet. Comp. Oncol. 2021, 19, 661–670. [Google Scholar] [CrossRef]
- Owen, L.N. Classification of Tumours in Domestic Animals; World Health Organization: Geneva, Switzerland, 1980; pp. 48–50.
- Langenbach, A.; McManus, P.M.; Hendrick, M.J.; Shofer, F.S.; Sorenmo, K.U. Sensitivity and specificity of methods of assessing the regional lymph nodes for evidence of metastasis in dogs and cats with solid tumors. J. Am. Vet. Med. Assoc. 2001, 218, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.K.; Kass, P.H.; Christopher, M.M. Cytologic–histologic concordance in the diagnosis of neoplasia in canine and feline lymph nodes: A retrospective study of 367 cases. Vet. Comp. Oncol. 2017, 15, 1206–1217. [Google Scholar] [CrossRef]
- Herring, E.S.; Smith, M.M.; Robertson, J.L. Lymph node staging of oral and maxillofacial neoplasms in 31 dogs and cats. J. Vet. Dent. 2002, 19, 122–126. [Google Scholar] [CrossRef]
- Fournier, Q.; Cazzini, P.; Bavcar, S.; Pecceu, E.; Ballber, C.; Elders, R. Investigation of the utility of lymph node fine-needle aspiration cytology for the staging of malignant solid tumors in dogs. Vet. Clin. Pathol. 2018, 47, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Marconato, L.; Buracco, P.; Boracchi, P.; Giudice, C.; Iussich, S.; Grieco, V.; Chiti, L.E.; Favretto, E.; Stefanello, D. The impact of extirpation of non-palpable/normal-sized regional lymph nodes on staging of canine cutaneous mast cell tumours: A multicentric retrospective study. Vet. Comp. Oncol. 2018, 16, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.; Rohrer-Bley, C.; Nolff, M.C. Near-infrared fluorescent image-guided lymph node dissection compared with locoregional lymphadenectomies in dogs with mast cell tumours. J. Small Anim. Pract. 2022, 63, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Oblak, M.L.; Ram, A.; Singh, A.; Nykamp, S. Determining agreement between preoperative computed tomography lymphography and indocyanine green near infrared fluorescence intraoperative imaging for sentinel lymph node mapping in dogs with oral tumours. Vet. Comp. Oncol. 2021, 19, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Randall, E.K.; Jones, M.D.; Kraft, S.L.; Worley, D.R. The development of an indirect computed tomography lymphography protocol for sentinel lymph node detection in head and neck cancer and comparison to other sentinel lymph node mapping techniques. Vet. Comp. Oncol. 2020, 18, 634–644. [Google Scholar] [CrossRef]
- Motomura, K. Sentinel node biopsy for breast cancer: Past, present, and future. Breast Cancer 2015, 22, 212–220. [Google Scholar] [CrossRef]
- Rossi, F.; Körner, M.; Suárez, J.; Carozzi, G.; Meier, V.S.; Roos, M.; Rohrer Bley, C. Computed tomographic-lymphography as a complementary technique for lymph node staging in dogs with malignant tumors of various sites. Vet. Radiol. Ultrasound 2018, 59, 155–162. [Google Scholar] [CrossRef]
- Manfredi, M.; de Zani, D.; Chiti, L.E.; Ferrari, R.; Stefanello, D.; Giudice, C.; Pettinato, V.; Longo, M.; Di Giancamillo, M.; Zani, D.D. Preoperative planar lymphoscintigraphy allows for sentinel lymph node detection in 51 dogs improving staging accuracy: Feasibility and pitfalls. Vet. Radiol. Ultrasound 2021, 62, 602–609. [Google Scholar] [CrossRef]
- Lapsley, J.; Hayes, G.M.; Janvier, V.; Newman, A.W.; Peters-Kennedy, J.; Balkman, C.; Sumner, J.P.; Johnson, P. Influence of locoregional lymph node aspiration cytology vs. sentinel lymph node mapping and biopsy on disease stage assignment in dogs with integumentary mast cell tumors. Vet. Surg. 2021, 50, 133–141. [Google Scholar] [CrossRef]
- Collivignarelli, F.; Tamburro, R.; Aste, G.; Falerno, I.; Signore, F.; Del Simeoni, F.; Patsikas, M.; Gianfelici, J.; Terragni, R.; Attorri, V.; et al. Lymphatic drainage mapping with indirect lymphography for canine mammary tumors. Animals 2021, 11, 1115. [Google Scholar] [CrossRef]
- Den Toom, I.J.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; de Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; Takes, R.P.; et al. Elective neck dissection or sentinel lymph node biopsy in early stage oral cavity cancer patients: The Dutch experience. Cancers 2020, 12, 1783. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, E.M.; Stefanello, D.; Nolff, M.C.; De Zani, D.; Zani, D.; Grieco, V.; Giudice, C.; Recordati, C.; Ferrari, F.; Ferrari, R.; et al. Sentinel lymph node biopsy is feasible in dogs with scars from prior local excision of solid malignancies. Animals 2022, 12, 2195. [Google Scholar] [CrossRef] [PubMed]
- Chiti, L.E.; Gariboldi, E.M.; Ferrari, R.; Luconi, E.; Boracchi, P.; De Zani, D.; Zani, D.; Manfredi, M.; Spendiacci, C.; Grieco, V.; et al. Surgical complications following sentinel lymph node biopsy guided by γ-probe and methylene blue in 113 tumour-bearing dogs. Vet. Comp. Oncol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Marconato, L.; Polton, G.; Stefanello, D.; Morello, E.; Ferrari, R.; Henriques, J.; Tortorella, G.; Benali, S.L.; Bergottini, R.; Vasconi, M.E.; et al. Therapeutic impact of regional lymphadenectomy in canine stage II cutaneous mast cell tumours. Vet. Comp. Oncol. 2018, 16, 580–589. [Google Scholar] [CrossRef]
- Marconato, L.; Stefanello, D.; Kiupel, M.; Finotello, R.; Polton, G.; Massari, F.; Ferrari, R.; Agnoli, C.; Capitani, O.; Giudice, C.; et al. Adjuvant medical therapy provides no therapeutic benefit in the treatment of dogs with low-grade mast cell tumours and early nodal metastasis undergoing surgery. Vet. Comp. Oncol. 2020, 18, 409–415. [Google Scholar] [CrossRef]
- Chalfon, C.; Sabattini, S.; Finotello, R.; Faroni, E.; Guerra, D.; Pisoni, L.; Ciammaichella, L.; Vasconi, M.E.; Annoni, M.; Marconato, L. Lymphadenectomy improves outcome in dogs with resected Kiupel high-grade cutaneous mast cell tumours and overtly metastatic regional lymph nodes. J. Small Anim. Pract. 2022, 63, 661–669. [Google Scholar] [CrossRef]
- Reck, A.; Kessler, M. Melanocytic tumours of the nasal planum in cats: 10 cases (2004–2019). J. Small Anim. Pract. 2021, 62, 131–136. [Google Scholar] [CrossRef]
- Odenweller, P.H.; Smith, M.M.; Taney, K.G. Validation of Regional Lymph Node Excisional Biopsy for Staging Oral and Maxillofacial Malignant Neoplasms in 97 Dogs and 10 Cats (2006–2016). J. Vet. Dent. 2019, 36, 97–103. [Google Scholar] [CrossRef]
- Arz, R.; Chiti, L.E.; Krudewig, C.; Grieco, V.; Meier, V.; Fejös, C.; Stefanello, D.; Nolff, M.C. Lymph node metastasis in feline cutaneous low-grade mast cell tumor. J. Feline Med. Surg. 2023, 25, 1098612X221138468. [Google Scholar] [CrossRef]
- Patsikas, M.N.; Papadopoulou, P.L.; Charitanti, A.; Kazakos, G.M.; Soultani, C.B.; Tziris, N.E.; Tzegas, S.I.; Jakovljevic, S.; Savas, I.; Stamoulas, K.G. Computed tomography and radiographic indirect lymphography for visualization of mammary lymphatic vessels and the sentinel lymph node in normal cats. Vet. Radiol. Ultrasound 2010, 51, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Pitman, K.T.; Johnson, J.T.; Brown, M.L.; Myers, E.N. Sentinel lymph node biopsy in head and neck squamous cell carcinoma. Laryngoscope 2002, 112, 2101–2113. [Google Scholar] [CrossRef] [PubMed]
- Arz, R.; Seehusen, F.; Meier, V.S.; Nolff, M.C. Indocyanine-based near-infrared lymphography for real-time detection of lymphatics in a cat with multiple mast cell tumours. J. Feline Med. Surg Open Rep. 2022, 27, 20551169221074961. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, D.; Valenti, P.; Faverzani, S.; Bronzo, V.; Fiorbianco, V.; Pinto da Cunha, N.; Romussi, S.; Cantatore, M.; Caniatti, M. Ultrasound-guided cytology of spleen and liver: A prognostic tool in canine cutaneous mast cell tumor. J. Vet. Intern. Med. 2009, 23, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Finora, K.; Leibman, N.F.; Fettman, M.J.; Powers, B.E.; Hackett, T.A.; Withrow, S.J. Cytological comparison of fine-needle aspirates of liver and spleen of normal dogs and of dogs with cutaneous mast cell tumours and an ultrasonographically normal appearing liver and spleen. Vet. Comp. Oncol. 2006, 4, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Congiusta, M.; Lawrence, J.; Rendahl, A.; Goldschmidt, S. Variability in recommendations for cervical lymph node pathology for staging of canine oral neoplasia: A survey study. Front. Vet. Sci. 2020, 7, 506. [Google Scholar] [CrossRef]
- Kraus, K.A.; Clifford, C.A.; Davis, G.J.; Kiefer, K.M.; Drobatz, K.J. Outcome and Prognostic Indicators in Cats Undergoing Splenectomy for Splenic Mast Cell Tumors. J. Am. Anim. Hosp. Assoc. 2015, 51, 231–238. [Google Scholar] [CrossRef]
- Henry, C.; Herrera, C. Mast cell tumors in cats: Clinical update and possible new treatment avenues. J. Feline Med. Surg. 2013, 15, 41–47. [Google Scholar] [CrossRef]
- Suami, H.; Yamashita, S.; Soto-Miranda, M.A.; Chang, D.W. Lymphatic Territories (Lymphosomes) in a Canine: An Animal Model for Investigation of Postoperative Lymphatic Alterations. PLoS ONE 2013, 8, e69222. [Google Scholar] [CrossRef]
- Baum, H. The Lymphatic System of the Dog; Mayer, M.; Bettin, L.; Bellamy, K.; Stamm, I., Translators; University of Saskatchewan: Saskatoon, SK, Canada, 2021; pp. 26–34. [Google Scholar]
- Sabattini, S.; Bettini, G. Grading Cutaneous Mast Cell Tumors in Cats. Vet. Pathol. 2019, 56, 43–49. [Google Scholar] [CrossRef]
- Ciekot, P.A.; Powers, B.E.; Withrow, S.J.; Straw, R.C.; Ogilvie, G.K.; LaRue, S.M. Histologically low-grade, yet biologically high-grade, fibrosarcomas of the mandible and maxilla in dogs: 25 cases (1982–1991). J. Am. Vet. Med. Assoc. 1994, 204, 610–615. [Google Scholar]
- Mendez, S.E.; Drobatz, K.J.; Duda, L.E.; White, P.; Kubicek, L.; Sorenmo, K.U. Treating the locoregional lymph nodes with radiation and/or surgery significantly improves outcome in dogs with high-grade mast cell tumours. Vet. Comp. Oncol. 2020, 18, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Martano, M.; Morello, E.; Buracco, P. Feline injection-site sarcoma: Past, present and future perspectives. Vet. J. 2011, 188, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hershey, A.E.; Sorenmo, K.U.; Hendrick, M.J.; Shofer, F.S.; Vail, D.M. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996). J. Am. Vet. Med. Assoc. 2000, 216, 58–61. [Google Scholar] [CrossRef] [PubMed]
- De Campos, C.B.; Damasceno, K.A.; Gamba, C.O.; Ribeiro, A.M.; Machado, C.J.; Lavalle, G.E.; Cassali, G.D. Evaluation of prognostic factors and survival rates in malignant feline mammary gland neoplasms. J. Feline Med. Surg. 2016, 18, 1003–1012. [Google Scholar] [CrossRef]
- Litster, A.L.; Sorenmo, K.U. Characterisation of the signalment, clinical and survival characteristics of 41 cats with mast cell neoplasia. J. Feline Med. Surg. 2006, 8, 177–183. [Google Scholar] [CrossRef]
- Grimes, J.A.; Mestrinho, L.A.; Berg, J.; Cass, S.; Oblak, M.L.; Murphy, S.; Amsellem, P.M.; Brown, P.; Hamaide, A.; Matz, B.M. Histologic evaluation of mandibular and medial retropharyngeal lymph nodes during staging of oral malignant melanoma and squamous cell carcinoma in dogs. J. Am. Vet. Med. Assoc. 2019, 254, 938–943. [Google Scholar] [CrossRef]
- Liptak, J.M.; Boston, S.E. Nonselective Lymph Node Dissection and Sentinel Lymph Node Mapping and Biopsy. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 793–807. [Google Scholar] [CrossRef]
- Thompson, C.F.; St John, M.A.; Lawson, G.; Grogan, T.; Elashoff, D.; Mendelsohn, A.H. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2013, 270, 2115–2122. [Google Scholar] [CrossRef]
- Renaudeau, C.; Lefebvre-Lacoeuille, C.; Campion, L.; Dravet, F.; Descamps, P.; Ferron, G.; Houvenaeghel, G.; Giard, S.; Tunon de Lara, C.; Dupré, P.F.; et al. Evaluation of sentinel lymph node biopsy after previous breast surgery for breast cancer: GATA study. Breast 2016, 28, 54–59. [Google Scholar] [CrossRef]
- Wong, J.H.; Cagle, L.A.; Morton, D.L. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann. Surg. 1991, 214, 637–641. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, T.S.; Kim, S.K. Current status of optical imaging for evaluating lymph nodes and lymphatic system. Korean J. Radiol. 2015, 16, 21–31. [Google Scholar] [CrossRef] [PubMed]


| Cat Signalment | Tumor Location and Laterality | Tumor Histotype | Regional Lymphocenter | Mapping Technique | Sentinel Lymphocenters | Correspondence Sentinel and Regional Lymphocenter | SLN n | Histological SLN Status |
|---|---|---|---|---|---|---|---|---|
| Cat n.1 DS, SF, 10 years, 9.4 kg | Salivary gland, R | Salivary gland carcinoma | Retropharyngeal R | ICG | Retropharyngeal R *, Superficial cervical R | Partial | 2 | Neg |
| Cat n.2 DS, SF, 10 years, 5 kg | Ear, R | MCT, low grade | Superficial cervical R | ICG | Parotid R Superficial cervical R | Partial | 3 | HN3 |
| Cat n.3 DS, IM, 9 years, 4.7 kg | Shoulder, L | Scar of excised MCT (grade not available) | Superficial cervical L vs. Axillary L | ICG | Superficial cervical L | Unpredictable | 1 | HN3 |
| Cat n.4 DS, SF, 5 years | Tarsus, R | MCT, low grade | Popliteal R | ICG | Popliteal R | Correspondence | 1 | HN2 |
| Cat n.5 DS, NM, 18 years, 3.5 kg | Ear canal, L | Ceruminous gland carcinoma | Retropharyngeal L | ICG | Retropharyngeal L | Correspondence | 1 | Neg |
| Cat n.6 DS, SF, 12 years, 2.4 kg | Hand third digit, R | MCT, low grade | Superficial cervical R | ICG | Axillary R | Non-correspondence | 1 | HN1 |
| Cheek, R | MCT, low grade | Mandibular R | ICG | Superficial cervical R | Non-correspondence | 1 | HN2 | |
| Lip commissure, L | MCT, low grade | Mandibular L | ICG | Mandibular L Superficial cervical L | Partial | 2 | HN0 | |
| Cat n.7 DS, SF, 13 years, 2.4 kg | Eyelid, R | MCT, grade not available | Parotid R | ICG | Mandibular R | Non-correspondence | 2 | HN3 |
| Cat n.8 DS, SF, 13 years, 5 kg | Chin, Median | SCC | Indeterminable R vs. L | Radio + MB | Mandibular L | Unpredictable | 2 | Neg |
| Cat n.9 DS, SF, 15 years, 3.8 kg | Ear, L | Fibrosarcoma | Parotid L | Radio + MB | Parotid L, Retropharyngeal L, Superficial cervical L | Partial | 3 | Neg |
| Cat n.10 DS, SF, 14 years, 4.8 kg | Mandibula, L | SCC | Mandibular L | Radio + MB | Mandibular L | Correspondence | 2 | Neg |
| Cat n.11 DS, SF, 13 years, 5 kg | Ventral to external ear, R | MCT, low grade | Parotid R and Superficial cervical R | Radio + MB | Parotid R Superficial cervical R | Correspondence | 2 | HN1 |
| Cat n.12 DS, SF, 1.5 years, 4 kg | Chin, Median | MCT, low grade | Indeterminable R vs. L | Radio + MB | Mandibular R Mandibular L | Unpredictable | 2 | HN1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiti, L.E.; Gariboldi, E.M.; Stefanello, D.; De Zani, D.; Grieco, V.; Nolff, M.C. Sentinel Lymph Node Mapping and Biopsy in Cats with Solid Malignancies: An Explorative Study. Animals 2022, 12, 3116. https://doi.org/10.3390/ani12223116
Chiti LE, Gariboldi EM, Stefanello D, De Zani D, Grieco V, Nolff MC. Sentinel Lymph Node Mapping and Biopsy in Cats with Solid Malignancies: An Explorative Study. Animals. 2022; 12(22):3116. https://doi.org/10.3390/ani12223116
Chicago/Turabian StyleChiti, Lavinia Elena, Elisa Maria Gariboldi, Damiano Stefanello, Donatella De Zani, Valeria Grieco, and Mirja Christine Nolff. 2022. "Sentinel Lymph Node Mapping and Biopsy in Cats with Solid Malignancies: An Explorative Study" Animals 12, no. 22: 3116. https://doi.org/10.3390/ani12223116
APA StyleChiti, L. E., Gariboldi, E. M., Stefanello, D., De Zani, D., Grieco, V., & Nolff, M. C. (2022). Sentinel Lymph Node Mapping and Biopsy in Cats with Solid Malignancies: An Explorative Study. Animals, 12(22), 3116. https://doi.org/10.3390/ani12223116






