To Save Pangolins: A Nutritional Perspective
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Diet of Pangolins in the Wild
2.1. The Type of Food in the Wild
2.2. Nutrient Composition of the Pangolin Diet in the Wild
3. The Food for Pangolins under Human Care
3.1. Diet Resources under Human Care
3.2. Nutritional Composition Analysis of Food under Human Care
4. Comparison between Diets in the Wild and Diets under Human Care
5. Other Factors Affecting the Nutrition of Pangolins
5.1. Digestibility of Food
5.2. Diet Reflected in Feces
5.3. Intestinal Microbiota
6. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, W.H.; Lin, X.D.; Chen, Y.M.; Xie, C.G.; Tan, Z.Z.; Zhou, J.J.; Chen, S.; Holmes, E.C.; Zhang, Y.Z. Newly identified viral genomes in pangolins with fatal disease. Virus Evol. 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Duan, W.W.; Huang, X.G.; Zhu, K.M.; Li, W.; Yao, Y. The resources status and development prospect of artificial breeding of Manis pentadactyla Linnaeus. Hunan For. Sci. Technol. 2012, 39, 75–77. (In Chinese) [Google Scholar]
- Wu, S.B.; Ma, G.Z.; Tang, M.; Chen, H.; Liu, N.F. The status and conservation strategy of pangolin resource in China. J. Nat. Resour. 2002, 17, 174–180. (In Chinese) [Google Scholar]
- The state forestry administration of the People’s Republic of China. Resource Investigation of Key Terrestrial Wildlife in China, 1st ed.; China Forestry Publishing House: Beijing, China, 2009; pp. 1–348. [Google Scholar]
- National Forestry and Grassland Administration. Announcement of the National Forestry and Grassland Administration. (Pangolin Protection Level Adjusted) [EB/OL]. 2020. Available online: http://www.forestry.gov.cn/main/72/20200605/141226066456941.html (accessed on 27 February 2022). (In Chinese)
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China 2015, 1st ed.; China Medical Science Press: Beijing, China, 2015; p. 268. [Google Scholar]
- Yang, C.W.; Chen, S.; Chang, C.Y.; Lin, M.F.; Block, E.; Lorentsen, R.; Chin, J.S.C.; Dierenfeld, E.S. History and dietary husbandry of pangolins in captivity. Zoo Biol. 2007, 26, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Liu, Q.; Feng, G.X.; Ke, Y.Y. Preliminary study on food nutrient contents of Chinese pangolin (Manis pentadactyla). J. Zhanjiang Norm. Coll. (Nat. Sci.) 1999, 20, 76–78. (In Chinese) [Google Scholar]
- Chen, D.; Xiao, L.C.; Zeng, Z.L. Research status of ecology and captive breeding of Chinese pangolin. South China Agric. 2021, 15, 10. (In Chinese) [Google Scholar]
- Zhang, F.H.; Kong, R.L.; Wu, S.B.; Zhou, C.Y.; Dong, C.H.; Li, S.S. Death causes of three captive pangolins. J. Econ. Anim. 2015, 19, 152–155. (In Chinese) [Google Scholar]
- Tan, L.H.; He, M.H.; Wu, Y.Q.; Li, Y.J.; Zhong, Y.L.; Que, T.C. Etiological analysis and preventive treatment of gastric perforation in Malay pangolin (Manis javanica). Hubei J. Anim. Vet. Sci. 2021, 42, 19–21. (In Chinese) [Google Scholar]
- Cabana, F.; Plowman, A.; Nguyen, T.V.; Chin, S.C.; Wu, S.L.; Lo, H.Y.; Watabe, H.; Yamamoto, F. Feeding Asian pangolins: An assessment of current diets fed in institutions worldwide. Zoo Biol. 2017, 36, 298–305. [Google Scholar] [CrossRef]
- Cheng, D.Y.; Yu, Z.B.; Li, Q.S.; Zhang, Q.; Li, Z.C. Study of food for pangolins in artificial rearing conditions. China J. Chin. Mater. Med. 2000, 25, 23–24. (In Chinese) [Google Scholar]
- Redford, K.H. Feeding and food preference in captive and wild giant anteaters (Myrmecophaga tridactyla). J. Zool. 1985, 205, 559–572. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Wang, Y.G. The ant eating habits of pangolins. Chin. J. Wildl. 1985, 7, 42–43. (In Chinese) [Google Scholar]
- Wang, S.L. A preliminary study on the living habits of scaly anteater cyprinus chinensis in the wild. Straits Sci. 2005, 21, 52–53. (In Chinese) [Google Scholar]
- Swart, J.M.; Richardson, P.R.K.; Ferguson, J.W.H. Ecological factors affecting the feeding behaviour of pangolins (Manis temminckii). J. Zool. 1999, 247, 281–292. [Google Scholar] [CrossRef]
- Wu, S.B.; Liu, N.F.; Li, Y.Y.; Sun, R.Y. Observation on food habits and foraging behavior of Chinese pangolin (Manis pentadactyla). Chin. J. Appl. Environ. Biol. 2005, 11, 337–341. (In Chinese) [Google Scholar]
- Ye, X.Q.; Wang, X.; Hu, C. Nutritional analysis and evaluation of 2 species of ant. J. Zhejiang Agric. Univ. 1995, 21, 303–304. (In Chinese) [Google Scholar]
- Ntukuyoh, A.I.; Udiong, D.S.; Ikpe, E.; Akpakpan, A.E. Evaluation of nutritional value of termites (Macrotermes bellicosus): Soldiers, workers, and queen in the niger delta region of nigeria. Int. J. Food Nutr. Saf. 2012, 1, 60–65. [Google Scholar]
- Simpson, S.J.; Raubenheimer, D.; Behmer, S.T.; Whitworth, A.; Wright, G.A. A Comparison of nutritional regulation in solitarious- and gregarious-phase nymphs of the desert locust Schistocerca gregaria. J. Exp. Biol. 2002, 205, 121–129. [Google Scholar] [CrossRef]
- Ke, Y.Y.; Chang, H.; Wu, S.B.; Liu, Q.; Feng, G.X. A study on Chinese pangolin’s main food nutrition. Zool. Res. 1999, 20, 394–395. (In Chinese) [Google Scholar]
- Wang, H.; Zhou, W.X.; Zhang, Y.Q.; Guan, X.H.; Li, H. Nutrition analysis of Polyhachis vicina Roger in Aer Mountain. J. Inn. Mong. Univ. Natl. 2016, 31, 133–136. (In Chinese) [Google Scholar]
- Chen, D.; Li, D.; Wang, W.F.; Zhong, C.; Liao, C.G. Nutrition analysis of common ants in Lesser Khingan Range. Chin. Agric. Sci. Bull. 2011, 27, 79–82. (In Chinese) [Google Scholar]
- Yang, D.R.; Shu, C.; Li, C.D. Analysis of nutrition components in five species of insects. Acta Nutr. Sin. 1996, 18, 231–234. (In Chinese) [Google Scholar]
- Ma, J.E.; Li, L.M.; Jiang, H.Y.; Zhang, X.J.; Li, J.; Li, G.Y.; Chen, J.P. Acidic mammalian chitinase gene is highly expressed in the special oxyntic glands of Manis javanica. FEBS Open Bio 2018, 8, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Deblauwe, I.; Janssens, G.P.J. New insights in insect prey choice by chimpanzees and gorillas in Southeast Cameroon: The role of nutritional value. Am. J. Phys. Anthropol. 2008, 135, 42–55. [Google Scholar] [CrossRef]
- Brütsch, T.; Jaffuel, G.; Vallat, A.; Turlings, T.C.J.; Chapuisat, M. Wood ants produce a potent antimicrobial agent by applying formic acid on tree-collected resin. Ecol. Evol. 2017, 7, 2249–2254. [Google Scholar] [CrossRef]
- Falótico, T.; Labruna, M.B.; Verderane, M.P.; De resende, B.D.; Izar, P.; Ottoni, E.B. Repellent Efficacy of formic acid and the abdominal secretion of carpenter ants (hymenoptera: Formicidae) against Amblyomma ticks (acari: Ixodidae). J. Med. Entomol. 2007, 44, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Li, X.F.; Huang, C.H.; Guo, C.Q. Preliminary study on the nutritional composition of Coptotermes formosanus Shiraki. J. Hunan Univ. Arts Sci. Nat. Sci. Ed. 2012, 24, 29–32. (In Chinese) [Google Scholar]
- Ji, Y.J.; Kong, X.F.; Zhou, X.L.; Geng, M.M.; Yin, Y.L. Determination of nutrients in three insects. Nat. Prod. Res. Dev. 2013, 25, 1229–1233. (In Chinese) [Google Scholar]
- Tang, H.; Chen, J.; Liao, Y.; Yang, S.Z.; Zhang, L. Review on biological characteristics of Odontotermes formosanus in forest. South China Agric. 2014, 8, 43–48. (In Chinese) [Google Scholar]
- Zhang, Y.X. Analysis of the biological characteristics of red imported fire ants and discussion on comprehensive control measures. J. Agric. Catastrophology 2021, 11, 176–177. (In Chinese) [Google Scholar]
- Wang, C.L.; Wu, J.; Xiao, G.R. A study on the bionomics and predatory effect of Camponotus japonicus to Dendrolimus punctatus. For. Res. 1991, 4, 405–408. (In Chinese) [Google Scholar]
- Wang, H. Preliminary Exploration on the Artificial Breeding of Manis javanica. Master’s Thesis, Zhejiang Normal University, Jinhua, China, 2015. (In Chinese). [Google Scholar]
- Hua, L.; Gong, S.; Wang, F.; Li, W.; Ge, Y.; Li, X.; Hou, F. Captive breeding of pangolins: Current status, problems and future prospects. ZooKeys 2015, 2015, 99–114. [Google Scholar]
- Lu, Q.B.; Hou, X.M.; Wang, Y.J. Optimization and screening of suitable diet formula for pangolin. J. Zhejiang Agric. Sci. 2014, 599, 594–595. (In Chinese) [Google Scholar]
- Gao, H.M.; Peng, J.J.; Yu, J.Y.; Zhu, J.; Ma, X.H. A preliminary study on the adaptation of Manis javanica to artificial substitute for food. J. Chongqing Norm. Univ. (Nat. Sci. Ed.) 2018, 35, 48–53. (In Chinese) [Google Scholar]
- Lin, M.F.; Chang, C.Y.; Yang, C.W.; Dierenfeld, E.S. Aspects of digestive anatomy, feed intake and digestion in the Chinese pangolin (Manis pentadactyla) at Taipei Zoo. Zoo Biol. 2015, 34, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Hu, J.Y.; Wu, Y.J.; Irwin, D.M.; Chen, W.; Zhang, Z.G.; Yu, L. Comparative study of gut microbiota from captive and confiscated-rescued wild pangolins. J. Genet. Genom. 2021, 48, 825–835. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Panda, S.; Sahu, S.K.; Roy, P.K.; Purohit, K.L.; Mishra, C.R. Hand-rearing of rescued Indian pangolin (Manis crassicaudata) at Nandankanan Zoological Park, Odisha. In Indian Zoo Year Book, 1st ed.; Acharjyo, L.N., Panda, S., Eds.; Indian Zoo Directors’ Association and Central Zoo Authority: New Delhi, India, 2013; Volume 7, pp. 17–25. [Google Scholar]
- Yu, J.Y.; Jiang, F.L.; Peng, J.J.; Yin, X.L.; Ma, X.H. The first birth and survival of cub in captivity of critically endangered Malayan pangolin (Manis javanica). Agric. Sci. Technol. 2015, 16, 2322–2330. [Google Scholar]
- Yang, Y.X. China Food Composition Tables 2004, 2nd ed.; Peking University Medical Press: Beijing, China, 2005; pp. 1–351. [Google Scholar]
- Zhang, H.P.; Li, Z.Y.; Li, G.B.; Zhang, X.; Liu, P. Trace elements and amino acids from Yunnan yellow ant. J. Yunnan Norm. Univ. (Nat. Sci. Ed.) 2002, 22, 39–42. (In Chinese) [Google Scholar]
- Zhang, F.H.; Zou, C.Y.; Yu, Y.S.; Wang, W.H.; Xu, N.; Wu, S.B. A preliminary report of B vitamins deficiency and treatment for the captive Sunda pangolin (Manis javanica). Chin. J. Wildl. 2019, 40, 1001–1004. (In Chinese) [Google Scholar]
- Xu, N.; Yu, J.M.; Zhang, F.H.; Wu, S.B.; Zou, C.Y.; Wang, Q.Y.; Wang, Y.F. Colony composition and nutrient analysis of Polyrhachis dives ants, a natural prey of the Chinese pangolin (Manis pentadactyla). Zoo Biol. 2022, 41, 157–165. [Google Scholar] [CrossRef]
- Liu, D.W. Research on the Constituents and Pharmacological Activities of Polyhachis vicina Roger. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2004. (In Chinese). [Google Scholar]
- Xie, G.L.; Liu, M.J.; Yun, J.Y. The analysis of nutritious constituents in Polyrhachis vicina Roger. Chin. J. Public Health 1997, 13, 36–37. (In Chinese) [Google Scholar]
- Zhong, Y.L.; Wei, Y.J.; Shi, G.N.; Chen, P.Y.; Liu, L.L.; Wei, L.; Que, T.C. Analysis and comparison of artificial diet formulations of Pangolin. Guangxi J. Anim. Husb. Vet. Med. 2021, 37, 219–221. (In Chinese) [Google Scholar]
- Imai, M.; Shibata, T.; Mineda, T.; Suga, Y.; Onouchi, T. Histological and histochemical investigations on the stomach in man, Japanese monkey (Macaca fuscata yakui) and some other kinds of animals. Okajimas Folia Anat. Jpn. 1973, 49, 433–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervaet, A. Assessing the Presence of Chitinases in the Digestive Tract and Their Relationship to Diet and Morphology in Freshwater Fish. Master’s Thesis, Ghent University, Ghent, Belgium, 2019. [Google Scholar]
- Cabana, F.; Tay, C. The addition of soil and chitin into Sunda pangolin (Manis Javanica) diets affect digestibility, faecal scoring, mean retention time and body weight. Zoo Biol. 2020, 39, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.K.; Houston, C.S.; Koehler, G.M.; Cadd, G.G.; Fain, S.R. Techniques for application of faecal DNA methods to field studies of ursids. Mol. Ecol. 1997, 6, 1091–1097. [Google Scholar] [CrossRef]
- Cong, R.J.; Wu, X.B.; Li, F.; Zhang, X.Y.; Hou, Y.C.; Zhang, Y.Z.; Hao, Z.; Zhang, X.S. Application of stable isotope analysis in avian ecology. Acta Ecol. Sin. 2015, 35, 4945–4957. (In Chinese) [Google Scholar]
- Heath, M.E. Twenty-Four-Hour variations in activity, core temperature, metabolic rate, and respiratory quotient in captive Chinese pangolins. Zoo Biol. 1987, 6, 1–10. [Google Scholar] [CrossRef]
- Kircher, M.; Kelso, J. High-throughput DNA sequencing-concepts and limitations. Bioessays 2010, 32, 524–536. [Google Scholar] [CrossRef]
- Shao, X.N.; Song, D.Z.; Huang, Q.W.; Li, S.; Yao, M. Fast surveys and molecular diet analysis of carnivores based on fecal DNA and metabarcoding. Biodivers. Sci. 2019, 27, 543–556. (In Chinese) [Google Scholar]
- Sun, N.C.M.; Liang, C.C.; Chen, B.Y.; Lin, C.C.; Pei, K.J.C.; Li, H.F. Comparison of two faecal analysis techniques to assess Formosan pangolin Manis pentadactyla pentadactyla diet. Mammalia 2020, 84, 41–49. [Google Scholar] [CrossRef]
- Nalepa, C.A. Body size and termite evolution. Evol. Biol. 2011, 38, 243–257. [Google Scholar] [CrossRef]
- Bravo, J.A.; Marcela, J.P.; Forsythe, P.; Kunze, W.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Communication between gastrointestinal bacteria and the nervous system. Curr. Opin. Pharmacol. 2012, 12, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Brucker, R.M.; Bordenstein, S.R. Speciation by symbiosis. Trends Ecol. Evol. 2012, 27, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.A.M.J.; Hesta, M.; Hollants, J.; Janssens, G.P.J.; Huys, G. Phylogenetic analysis of faecal microbiota from captive cheetahs reveals underrepresentation of Bacteroidetes and Bifidobacteriaceae. BMC Microbiol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Becker, A.A.M.J.; Luo, Y.; Zhang, W.F.; Ge, B.Q.; Leng, C.Q.; Wang, G.Y.; Ding, L.M.; Wang, J.M.; Fu, X.Y.; et al. The fecal microbiota of dogs switching to a raw diet only partially converges to that of wolves. Front. Microbiol. 2021, 12, 701439. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.C.; Lien, C.Y.; Chan, Y.T.; Chen, C.L.; Yang, Y.C.; Yeh, L.S. Monitoring the gestation period of rescued Formosan pangolin (Manis pentadactyla pentadactyla) with progesterone radioimmunoassay. Zoo Biol. 2012, 31, 479–489. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, N.; Yu, Y.; Wu, S.; Li, S.; Wang, W. Expression profile of the digestive enzymes of Manis javanica reveals its adaptation to diet specialization. ACS Omega 2019, 4, 19925–19933. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Dong, Z.L. Studies on sugar, fat, protein, vitamin, amino acid content in Odontotermes formosanus (Shiraki) and its fungus comb. Bull. Sci. Technol. 1993, 9, 41–43. (In Chinese) [Google Scholar]
- Xue, D.J.; Li, Y.Q. The analysis of nutritious constituents in Odontotermes fornosanus shiraki. Chin. J. Mod. Appl. Pharm. 2002, 15, 472–473. (In Chinese) [Google Scholar]
- Weng, L.L.; Jiang, D.C.; Dong, F.; Song, L.J. Comparison of chemical constituents of Formica rufa L.∙and Polyrhachi vicina Roger. J. Chin. Med. Mater. 2004, 27, 716–718. (In Chinese) [Google Scholar]
- Lu, Y.; Wang, D.R.; Han, D.B.; Zhang, Z.S.; Zhang, C.H. Analysis of protein amino acids and fatty acids of large termites. Acta Nutr. Sin. 1992, 14, 103–106. (In Chinese) [Google Scholar]
- Wang, J.; Liu, T.X. Analysis of physico-chemical properties of Polyrhachis vicina Roger. Mod. Food Sci. Technol. 2010, 26, 1092–1163. (In Chinese) [Google Scholar]
- Wu, L.Z.; Chen, Y.; Zhang, J. Determination of total amino acid and iron content in Macrotermes annandadei and their fungus beds. Chin. Tradit. Herb. Drugs 1986, 17, 27. (In Chinese) [Google Scholar]
- Li, S.L.; Ren, Y.J.; Cui, X.; Qian, Z.S.; Dong, Q. Comparative analysis of nutrients between Formica sanguinea Latr and Polyrhachis vicina Roger. Chin. Pharm. J. 1994, 42, 521–523. (In Chinese) [Google Scholar]
- Shi, Y.Q.; Yang, M.F.; Tang, M.L.; Zhao, X.H.; Pan, X.F. Study on nutrient composition of ant in the northern forest region. J. Northeast For. Univ. (Chin. Ed.) 1995, 23, 122–126. (In Chinese) [Google Scholar]
- Yin, G.S.; Feng, R.Q.; Wang, G.X.; Zhao, J.S. Analysis of nutritional components of Polyrhachis vicina Roger and preparation of its Royal Jelly. Sci. Technol. Food Ind. 1996, 1, 5–7. (In Chinese) [Google Scholar]
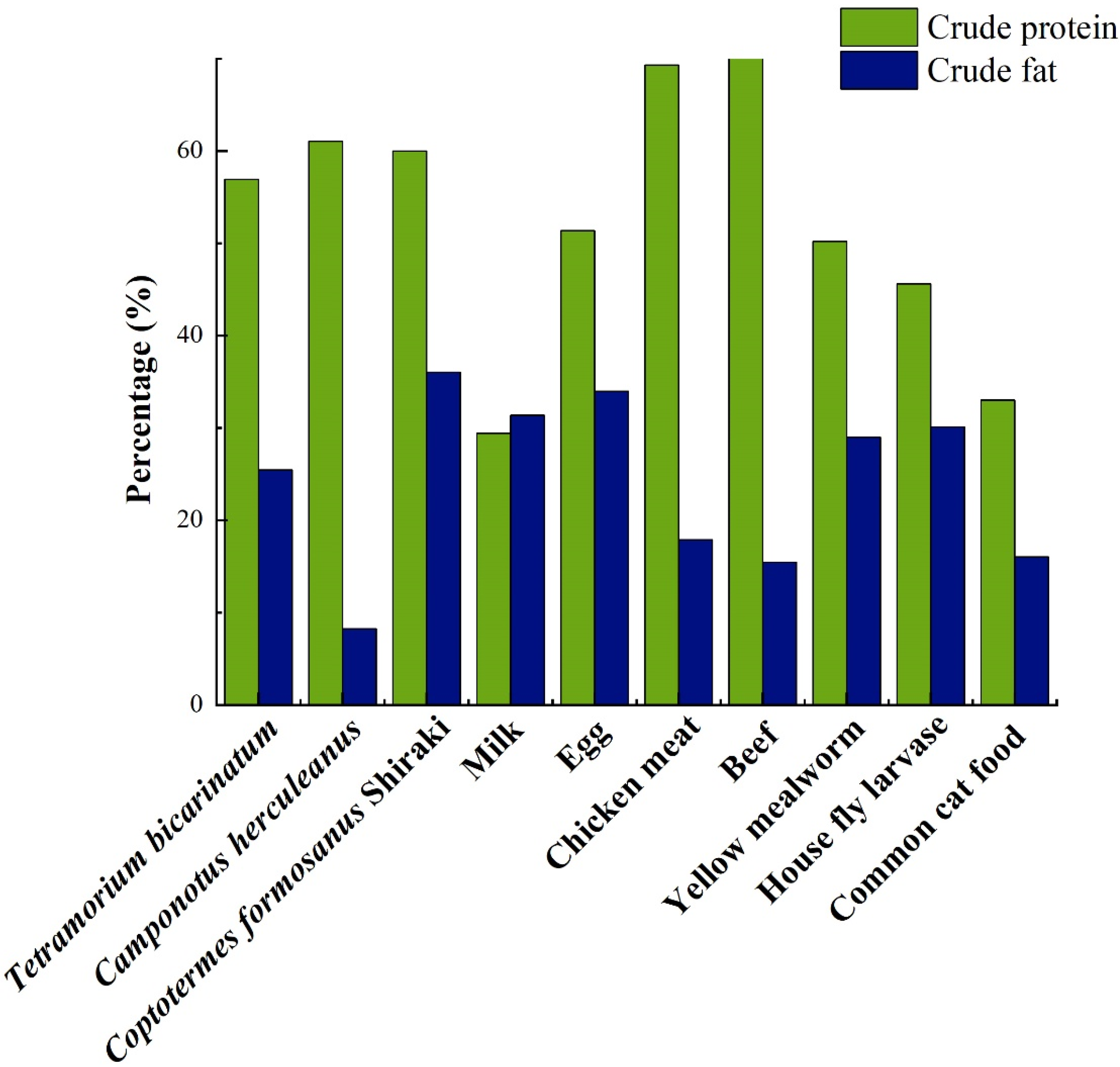

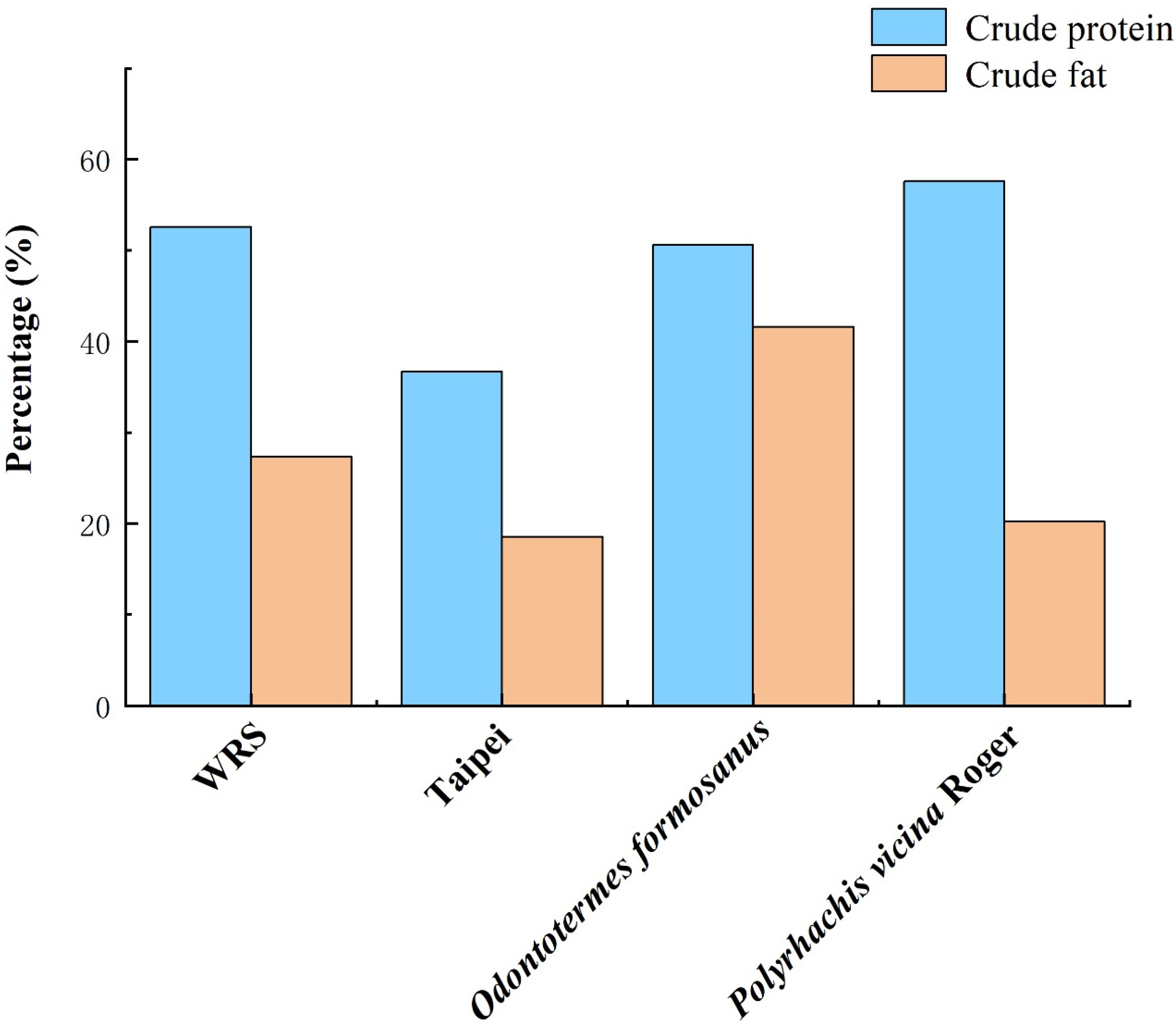
| Species | Crude Protein | Crude Fat | Carbohydrate | Moisture | Crude Ash | References |
|---|---|---|---|---|---|---|
| Tetramorium bicarinatum | 56.91 | 25.44 | 8.42 | / | / | [22] |
| Camponotus herculeanus | 61.03 | 8.25 | 15.78 | / | / | [22] |
| Coptotermes formosanus | 50.62 | 41.60 | 0.48 | / | / | [22] |
| Odontotermes formosanus | 50.62 | 41.60 | 0.42 | / | / | [8] |
| Myrmica rubra | 56.81 | 25.45 | 7.90 | / | / | [8] |
| Polyrhachis dives | 56.15 | 25.10 | 7.20 | / | / | [8] |
| Polyrhachis vicina Roger | 57.60 | 20.20 | / | 7.10 | 11.10 | [23] |
| Formica truncicola Forel | 56.60 | 29.10 | 3.50 | 7.30 | 3.50 | [24] |
| Oecophylla smaragdina | 57.89 | 16.62 | 10.46 | 8.94 | 4.19 | [25] |
| Macrotermes denticulatus | 49.92 | 14.21 | 9.89 | 11.20 | 3.60 | [25] |
| Institution | Crude Protein | Crude Fat | ADF | NDF | Ca | P | WSC | References |
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | ||
| WRS | 52.58 | 27.33 | 10.17 | 12.09 | 0.22 | 0.25 | 1.58 | [12] |
| Ragunan | 50.86 | 24.63 | 9.76 | 15.80 | 0.15 | 0.83 | 3.06 | [12] |
| SVW | 53.68 | 31.27 | 8.86 | 9.12 | 0.25 | 0.67 | 1.60 | [12] |
| Taipei 1 | 36.70 | 18.56 | 14.63 | 15.49 | 0.84 | 0.84 | 24.19 | [12] |
| Taipei 2 | 40.00 | / | / | / | 2.50 | 0.50 | / | [39] |
| Taipei 3 | 32.20 | / | / | / | 2.00 | 0.40 | / | [39] |
| Shenzhen | 33.18 | 3.45 | 13.06 | 25 | 0.21 | 0.34 | 2.11 | [40] |
| Guangzhou | 47.22 | 14.23 | 9.17 | 23.33 | 1.73 | 0.76 | 19.84 | [40] |
| Ueno | 32.41 | 27.51 | 4.61 | 9.44 | 0.94 | 0.67 | 25.84 | [12] |
| Leipzig | 32.69 | 23.82 | 9.14 | 15.34 | 1.27 | 0.37 | 21.59 | [12] |
| Nandakannan | 55.11 | 20.13 | / | / | / | / | / | [41] |
| Chongqing | 37.11 | 28.65 | 16.01 | 18.94 | / | / | 12.29 | [42] |
| Species | Crude Protein (%) | Crude Fat (%) | References |
|---|---|---|---|
| Milk (dry matter basis) | 29.41 | 31.37 | [43] |
| Egg (dry matter basis) | 51.35 | 33.98 | [43] |
| Chicken meat (dry matter basis) | 69.29 | 17.86 | [43] |
| Beef (dry matter basis) | 73.16 | 15.44 | [43] |
| Egg (fresh matter basis) | 13.30 | 9.40 | [22] |
| Milk (fresh matter basis) | 3.00 | 2.90 | [22] |
| Chicken meat (fresh matter basis) | 18.50 | 11.20 | [30] |
| Beef (fresh matter basis) | 19.90 | 13.10 | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-M.; Janssens, G.P.J.; Xie, C.-G.; Xie, B.-W.; Xie, Z.-G.; He, H.-J.; Wang, Y.-N.; Xu, J. To Save Pangolins: A Nutritional Perspective. Animals 2022, 12, 3137. https://doi.org/10.3390/ani12223137
Wang X-M, Janssens GPJ, Xie C-G, Xie B-W, Xie Z-G, He H-J, Wang Y-N, Xu J. To Save Pangolins: A Nutritional Perspective. Animals. 2022; 12(22):3137. https://doi.org/10.3390/ani12223137
Chicago/Turabian StyleWang, Xin-Mei, Geert P. J. Janssens, Chun-Gang Xie, Bo-Wen Xie, Zhi-Gang Xie, Hai-Jian He, Yan-Ni Wang, and Jia Xu. 2022. "To Save Pangolins: A Nutritional Perspective" Animals 12, no. 22: 3137. https://doi.org/10.3390/ani12223137






