Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Therapeutic Ultrasound
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Topic
2.2. General Inclusion and Exclusion Criteria
2.3. Study Selection and Categorization
3. Results
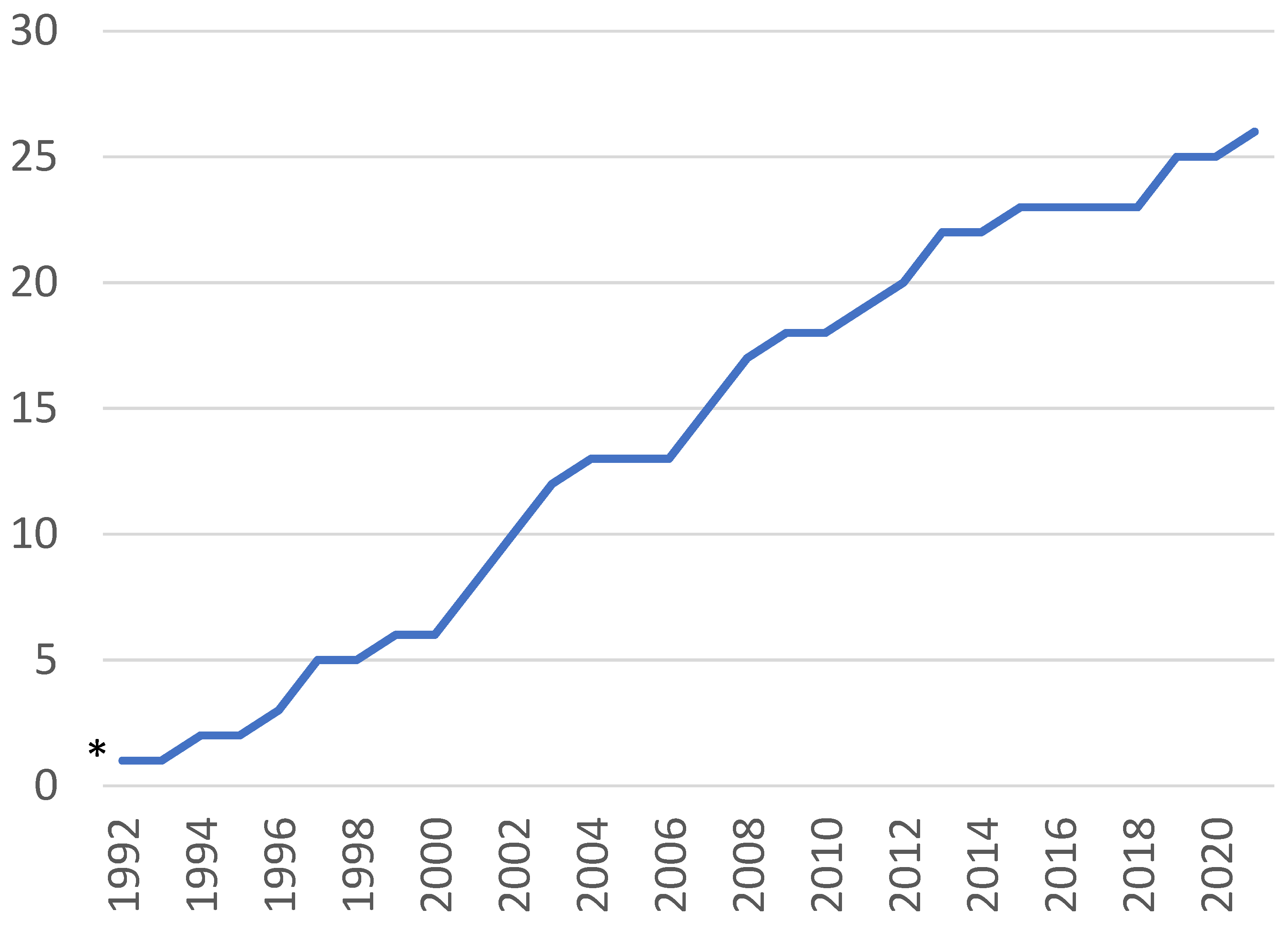
3.1. Characteristics of the Literature
3.2. Musculoskeletal Conditions in Dogs
3.2.1. Tissue Temperature
3.2.2. Tendon Injury
3.2.3. Osteoarthritis
3.2.4. Other Limb Joint Lesions
3.2.5. Paraparesis, Hindquarter Weakness, and Disc Lesions
3.2.6. Bone Tissue and Bone Healing
| Main Author [Ref.] Publication Year Country | Study Design | Study Population | Therapeutic Ultrasound: No. of Sessions and Duration Mode US Frequency Intensity | Controls | Outcome Variables | Main Results | Study Risk of Bias |
| Surface, muscle, and tendon temperature | |||||||
| Steiss [23] 1999 USA | Experimental, before-after design | 9 healthy dogs | One 10-min session Continuous mode TU frequency NI Intensity 0.5–2.0 W/cm2 Recordings with and without coat | Status before treatment | Temperature in biceps femoris recorded by thermistor | Temperature increase of >1.6 °C at 5 cm depth was obtained only when the coat was clipped and with intensity 2.0 W/cm2. No deep muscle temperature change with an unclipped coat | Low |
| Levine [24] 2001 USA | Experimental, treatments administered in random order | 10 adult male and female dogs | One 10-min session Mode and TU frequency NI 1.0–1.5 W/cm2 | Status before treatment | Caudal thigh muscle temperature at 1, 2, and 3 cm depth | 1.0 MHz: temperature rise 3.0 °C at 1 cm, 1.6 °C at 3 cm. 1.5 MHz: temperature rise 4.6 °C at 1 cm, 2.4 °C at 3 cm | Low |
| Acevedo [25] 2019 USA | Experimental Prospective cross-over design without controls | 10 adult dogs | One 10-min session Testing continuous vs. pulsed mode 3.3 MHz 1.0–1.5 W/cm2 | Status before treatment | Calcaneus tendon temperature Tarsal flexion | Greatest increase in tendon temperature (mean 3.5 °C) with continuous TU at 1.5 W/cm2. Much smaller heating effect of pulsed TU (mean 1.5 °C). Tarsal flexion increased during treatment, returning to baseline within 5 min of discontinuation | Low |
| Albuquerque [22] 2021 Brazil | Experimental Before-after design | 5 healthy adult dogs | One 10-min session Continuous mode 3.3 MHz 1.5 W/cm2 | Status before treatment | Surface temperature monitored by thermography | Mean increase in surface temperature at end of the 10-min treatment was 3.8 °C, returning to baseline about 10 min after cessation of TU | Low |
| Tendon injury | |||||||
| Saini [26] 2002 India | Experimental, non-randomized controls | 5 healthy mongrel dogs with surgically severed Achilles tendons, all immobilized | Daily 10-min sessions for 10 days Mode NI TU frequency NI Intensity 1.5 W/cm2 | Non-treated dogs | Follow-up for 120 days Clinical assessment Weight-bearing score Ultrasonography Tendon biopsy with histomorphology | Duration of lameness somewhat shorter in the TU group than in the controls. More rapid healing shown by ultrasonography and tendon histology | Moderate (only three treated and two non-treated dogs) |
| Osteoarthritis | |||||||
| Muste [27] 2015 Romania | Clinical cohort without controls | 8 dogs with hind limb knee osteoarthritis | 10 daily sessions (with a 2-day break) Pulsed mode Frequency NI Intensity 0.5 W/cm2 | None | 10- to 12-month follow-up Degree of lameness Pain score Joint motion measured by goniometer | Improvement of joint mobility in all dogs, accompanied by reduced pain and lameness | High (no control, unclear and inconsistent information in the article) |
| Limb joint lesions | |||||||
| Lang [16] 1980 USA | Clinical cohort without controls | 45 dogs with various types of joint lesions | 1–8 sessions, duration and intervals not specified. Pulsed mode Frequency NI Intensity 3 W/cm2 | None | Clinical examinations with an assessment of swelling, pain, activity, and function at an unspecified time point. Radiography as needed | 35/45 dogs’ symptoms fully resolved at end of treatment | High (heterogeneous cohort and TU treatments, follow-up not systematic, no controls) |
| Paraparesis, hindquarter weakness, and spinal lesions | |||||||
| Lang [16] 1980 USA | Clinical cohort without controls | 65 dogs with various types and locations of spinal lesions, mainly diagnosed as disc lesions | 1–8 sessions, duration and intervals not specified Pulsed mode Frequency NI Intensity 3 W/cm2 | None | Clinical examinations with an assessment of swelling, pain, activity and function at an unspecified time point.Radiography as needed | 48/65 dogs’ symptoms fully resolved at end of treatment | High (heterogeneous cohort and TU treatments, follow-up not systematic, no controls) |
| Maiti [30] 2007 India | Clinical cohort, controlled, non-randomized | 15 adult dogs with hindquarter weakness | Three 10-min sessions per week until discharge Pulsed mode 1 MHz 2 W/cm2 | Conventional therapy and US (n = 5), conventional therapy and interferential therapy (n = 5), conventional therapy only (n = 5) | Clinical neurological examination with the grading of motor function on days 3, 7, 10, and 14 and thereafter once a week. Biochemical measurements Radiographs of the spine | Less pain in TU in comparison with the control group receiving only conventional therapy | High (small sample, insufficient details on clinical assessments) |
| Sharma [31] 2011 India | Clinical cohort without controls | 16 dogs with different grades of paraparesis, all receiving medical treatment ambulatory or non-ambulatory | 5- to 10-min sessions twice weekly until discharge Pulsed mode Frequency NI Intensity 1.5–2.0 W/cm2 | None | Clinical, neurological examination, restoration of activity and function. Blood samples, heart rate, respiratory rate, rectal temperature. Duration of follow-up not specified. | Symptoms fully resolved in 10/16 dogs after 2–10 treatment sessions | High (heterogeneous sample, no controls) |
| Ansari [28] 2012 Zama [29] 2013 India | Randomized controlled trial | 16 dogs diagnosed with hindquarter weakness (HQW) 8 healthy age-matched control dogs | Daily 5-min sessions for 14 days Pulsed mode 1.0 MHz 0.5 W/cm2 | Conventional drug therapy | Clinical assessment Postural reactions Erythrocyte oxidant-antioxidant balance followed for 28 days by lipid peroxidation, reduced glutathione, superoxide dismutase and catalase | All dogs had improved postural reactions, except for the hopping reaction, by day 14. At day 28, hopping was improved in 4/8 dogs in the TU group vs. 2/8 in the control group. Clinical improvement was reported (without details) in all dogs in both groups. TU with conventional drug therapy seemed to counter oxidative stress associated with HQW. The degree of improvement differed little from drug effects alone | Moderate |
| Bone tissue and bone healing | |||||||
| Silveira [32] 2008 Brazil | Clinical cohort, controlled | 6 healthy dogs | TU to distal-skull area of radius and ulna Daily 5-min sessions for 20 days Continuous mode 1.0 MHz 0.5 W/cm2 | Non-treated contralateral radius and ulna | Bone density by radiography | No effects of TU on bone density at 20-day follow-up | Moderate (lower US dosage than in most other studies) |
| Singh [17] 1994 India | Experimental, randomized controlled trial | 8 healthy mongrel dogs with experimental femoral fracture | Ten 5-min sessions, alternate days, Pulsed mode Frequency NA Intensity 0.5 W/cm2 | Non-treated dogs | Histomorphology, calcium deposits, muscle fibre striation after euthanasia at day 40 postoperatively | Callus formation was firmer, inflammatory reactions milder in the TU group than in controls. | Moderate (small number of animals, no quantitative data provided) |
| Singh [18] 1994 Singh [21] 1997 India | Experimental, randomized controlled trial | 8 healthy mongrel dogs with experimental humerus fracture | Ten 5-min sessions, alternate days, Mode NI Frequency NI Intensity 1.0 W/cm2 | Non-treated dogs | Histomorphology at an unspecified time point. Clinical observations, angiography, radiography | Less inflammatory reaction, better tissue differentiation, signs of better callus strength and less hyaline muscle degeneration in TU-treated dogs. Faster healing detected in radiographs in TU-treated dogs. | Moderate (small number of animals, no quantitative data provided) |
| Cook [33] 2001 USA | Experimental Canine spinal fusion model, controlled | 14 adult male dogs with posterior spinal fusions at two sites (L2–L3 and L5–L6) | 6–7 daily 20-min sessions for 6 or 12 weeks Pulsed mode 1.5 MHz 30 mW/cm2 | Placebo-treated spinal fusion site in the same dog | Evaluation at 6 and 12 weeks Palpation Torsional stiffness Radiographic grading CT and MRI Histology | Complete radiographic and histological fusion at 12 weeks in 100% of sites receiving TU. Of non-treated control sites, 78% had complete radiographic fusion and 44% had complete histological fusion. Mechanical stiffness improved in the treated group. Differences were statistically significant. | Low |
| Rawool [34] 2003 USA | Experimental, controlled | 6 dogs with ulnar osteotomies | One 20-min session Pulsed mode 1.5 MHz 30 mW/cm2 | Placebo-treated dogs (device turned off) | Vascularity around osteotomy measured by power Doppler sonography for 11 days | Increased vascularity in TU-treated dogs at 7 and 11 days compared with placebo-treated dogs | Moderate (small number of TU-treated and control animals) |
| Kaur [35] 2004 India | Experimental, controlled | 10 adult healthy dogs with experimental radial diaphyseal defect | Daily 10-min sessions for 10 days Pulsed mode US frequency NI Comparison of 0.5 and 1.0 W/cm2 | None | Clinical examinations, radiography, and angiography before and up to 60 days after surgery | Earlier bone healing and weight bearing at 0.5 W/cm2 compared with 1.0 W/cm2 | High (no non-treatment controls) |
| Ikai [36] 2008 Japan | Experimental, controlled | 4 healthy beagle dogs with surgically induced bone defects in the mandibular bone bilaterally | Daily 20-min sessions for 4 weeks. Pulsed mode Burst 1.5 MHz, repeated treatments at 1.0 kHz 30 mW/mm2 | Non-treated contralateral side | Histology and immunohistochemistry 4 weeks after surgery | Accelerated regeneration of cementum and mandibular bone in the TU group. Expression of heat shock protein higher in gingival epithelial cells of the US-treated tooth | Low |
3.3. Musculoskeletal Conditions in Horses and Donkeys
3.3.1. Tissue Temperature
3.3.2. Tendon Injury and Inflammation
3.3.3. Acute Aseptic Arthritis
3.3.4. Back Muscle Pain
3.3.5. Spinal and Other Bone and Joint Lesions
| Main Author [Ref.] Publication Year Country | Study Design | Study Population | Therapeutic Ultrasound: No. of Sessions and Duration Mode TU Frequency Intensity | Controls | Outcome Variables | Main Results | Study Risk of Bias |
| Muscle and tendon temperature | |||||||
| Adair [37] 2019 USA | Experimental, before-after design | 10 healthy mares | One 10-min session Mode NI 1 MHz 1–2 W/cm2 | Baseline temperature | Temperature measured by a thermistor at different depths in epaxial muscles | Muscle temperature rise at the end of treatment 1.2–2.5 °C, highest at 1 and 5 cm depth | Low |
| Montgomery [38] 2011 USA | Experimental, before-after design | 10 healthy horses | Tendons: One 10-min session Continuous mode 3.3 MHz Comparing 1.0 with 1.5 W/cm2 Epaxial muscles: One 20-min session Continuous mode 3.3 MHz 1.5 W/cm2 | Baseline temperature | Temperature monitored by a thermistor in the superficial and deep flexor tendons of the thoracic limb and in epaxial muscles | The temperature rises at the end of treatment 2.5–5.2 °C in tendons (therapeutic temperature rise according to authors) and 0.7–1.3 °C in epaxial muscles (non-therapeutic temperature rise) | Low |
| Tendon and ligament injury and bursitis | |||||||
| Lang [16] 1980 USA | Clinical cohort without controls | 10 horses with synovitis and bursitis | 1–8 sessions, duration and intervals not specified. Pulsed mode TU frequency NI Intensity 3 W/cm2 | None | Clinical examinations with an assessment of swelling, pain, activity, and function at an unspecified time point. Radiography as needed | 5/10 horses fully resolved at end of treatment | High (heterogeneous cohort, follow-up not systematic) |
| Fernandes [39] 2003 Brazil | Experimental, randomized controlled trial | 18 horses with collagen-induced injuries of superficial digital flexor tendon | 8 sessions 2 intervention groups, one with continuous and one with pulsed mode 3 MHz 1 W/cm2 | Device switched off | 40 days’ follow-up Clinical examinations UltrasonographyHistology | Regression of clinical signs was detected on average in 9 days in horses receiving continuous US, 12 days in horses receiving pulsed mode US, and 21 days in controls. Decreases in clinical severity by ultrasonography after 40 days were 42.5%, 57.7%, and 34.1% in the three groups, respectively. Intense neovascularization and fibroblastic activity in US-treated groups compared with controls | Low |
| Sharifi [40] 2007 Iran | Experimental, non-randomized controls | 8 castrated horses with experimentally induced lacerations of the superficial digital flexor tendon of the right hind limb | Daily 10-min sessions for 14 days 3 MHz Intensity 1 W/Esq | Non-treated horses | Clinical assessment and hydroxyproline content in the lacerated tendon at 60 days | Local tenderness and swelling were reported as less severe in treated limbs. Significantly less decline in tendon hydroxyproline tendon content in the treated limb, indicating improved tendon regeneration | Moderate |
| Carrozzo [41] 2019 Italy | Clinical cohort without controls | 23 client-owned sport horses with injuries to the suspensory ligament | 6 sessions 1st week, thereafter 3–6 sessions per week for 2–3 weeks 6 min 38 kHz Intensity NI | None | Clinical evaluation Time to healing by diagnostic ultrasound Return to competition status | 20/23 horses showed healing by diagnostic ultrasound and returned to competition status | High |
| Acute aseptic arthritis | |||||||
| Singh [19] 1996 Singh [20] 1997 India | Experimental, randomized controlled trial | 8 donkeys with aseptic arthritis induced in the left carpal joint | Daily 10-min sessions for 7 days Pulsed mode 1 MHz 1.0–1.5 W/cm2 | Untreated donkeys | 30-day follow-up Rectal temperature, respiratory rate, pulse rate, and joint circumference. Synovial biopsies. Clinical assessments Histopathology and histochemistry | Earlier improvement of lameness, faster reduction of joint swelling, and less pain on flexion in TU-treated donkeys than in non-treated donkeys. Synovial cytological and biochemical parameters indicate less inflammation in the TU group. Histomorphology: Improved healing and fewer signs of cartilage degeneration in TU-treated donkeys | Moderate (only four donkeys in each group) |
| Back muscle pain [myositis] | |||||||
| Mercado [42] 2002 Argentina | Clinical cohort, non-randomized controls | 63 showjumpers with back pain (longissimus dorsi muscle) | Daily 20-min sessions for 30 days Pulsed mode TU frequency NI 3.5 W/cm2 | TU alone compared with (a) TENS and (b) TU plus TENS | Weekly clinical assessments and ultrasonography, 28-day follow-up | Nearly total recovery at 28 days in the TU alone group, faster than with TENS treatment alone, but slower than in the group with TU combined with TENS | Moderate (no non-treated controls) |
| Spinal and other bone and joint lesions | |||||||
| Lang [16] 1980 USA | Clinical cohort without controls | 30 horses with various types and locations of spinal and other bone and joint lesions | 1–8 sessions, duration and intervals not specified. Pulsed mode TU frequency NI 3 W/cm2 | None | Clinical examinations with an assessment of swelling, pain, activity, and function at an unspecified time point. Radiography as needed | 24/30 horses fully resolved at end of treatment (unspecified time point) | High (heterogeneous cohort, follow-up not systematic, no controls) |
3.4. TU for Contraception in Dogs and Cats
| Main Author [Ref.] Publication Year Country | Study Design | Study Population | Therapeutic Ultrasound: No. of Sessions and Duration Mode TU Frequency Intensity | Controls | Outcome Variables | Main Results | Study Risk of Bias |
| Dogs | |||||||
| Fahim [15] 1977 USA | Experimental intervention study, before-after design | Experiment 1: 24 adult healthy dogs Experiment 2: 6 adult healthy dogs | Experiment 1: 1-3 15-min sessions, weekly intervals Mode NI TU frequency NI Intensity 1 W/cm2 Experiment 2: One 15-min session Intensity 2 W/cm2 | None (comparisons between different ultrasound treatments) | Follow-up at 60 days Semen analyses (sperm count) Blood testosterone | Zero sperms after 60 days with three treatments at 1 W/cm2, 70% reduction with two treatments, 40% with one treatment. After one treatment with 2 W/cm2, spermatogenesis ceased after 14 days. Sperm stem cells and Sertoli cells were not affected. No changes in blood testosterone levels | Low |
| Leoci [43] 2009 Italy | Experimental intervention study, before-after design | 5 healthy mixed-breed male dogs | Three 5-min sessions with 2-day intervals Mode NI US frequency NI Intensity 1.5 W/cm2 | Status before TU | 14-day follow-up Testicular size and tenderness Semen analysis (sperm count) | Testicular tenderness and reduced testicular volume. All dogs azoospermic at 14 days’ post-treatment | Low |
| Leoci [44] 2015 Italy | Randomized controlled trial | 100 mature healthy mixed-breed maledogs | Five intervention groups: One to three 5-min sessions with 5-min to 48-h intervals and different areas of testicles treated Mode and frequency NA Intensity 1.5 W/cm2 | Non-treated (device switched off) | On day 30: Testicular size, sperm evaluation, blood testosterone.At days 40–47: Histological evaluation of the testicles | Reduced testicular size, azoospermia, and testicular tissue degeneration in dogs receiving three sessions of TU over the entire testicles at 48-h intervals, 1.5 W/cm2. No major effects of other regimens. No changes in testosterone levels in any experimental group | Low |
| Khanbazi [45] 2020 Iran | Experimental randomized study | 10 mixed-breed adult fertile dogs | Three 5-min sessions, thereafter one session every 48 h (NI on for how long) 1.0 MHz 1.5 W/cm2 | Placebo-treated (device turned off) | 63-day follow-up Ultrasound of testis Semen evaluation Serum testosterone and other biomarkers Oxidative stress index Histology | No difference in semen quality or testosterone levels Temporary increases in inflammatory and oxidative stress biomarkers Histological signs of late testicular necrosis and degeneration | Moderate (some information on the TU regime lacking) |
| Cats | |||||||
| Fahim [15] 1977 USA | Intervention study, before-after design | 30 male cats | Group A: One 10-min session Mode NI US frequency NI Intensity 1 W/cm2 Group B: same plus repeated same treatment at 48 h | Untreated cats | Follow-up at 60 days Histology in testicular biopsies Blood testosterone | Suppression of spermatogenesis at 60 days, more pronounced after two treatments. Unchanged testosterone levels | Low |
4. Discussion
Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, D.; Watson, T. Therapeutic ultrasound. In Canine Rehabilitation and Physical Therapy, 2nd ed.; Millis, D.L., Levine, D., Eds.; Saunders: Philadelphia, PA, USA, 2014; pp. 328–334. [Google Scholar]
- Jalal, J.; Leong, T.S.H. Microstreaming and its role in applications: A mini-review. Fluids 2018, 3, 93. [Google Scholar] [CrossRef]
- Tezel, A.; Mitragotri, S. Interactions of Inertial Cavitation Bubbles with Stratum Corneum Lipid Bilayers during Low-Frequency Sonophoresis. Biophys. J. 2003, 85, 3502–3512. [Google Scholar] [CrossRef]
- Tsai, W.C.; Pang, J.H.; Hsu, C.C.; Chu, N.K.; Lin, M.S.; Hu, C.F. Ultrasound stimulation of types I and III collagen expression of tendon cell and upregulation of transforming growth factor beta. J. Orthop. Res. 2006, 24, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.D. Nonthermal effects of therapeutic ultrasound: The frequency resonance hypothesis. J. Athlet. Train. 2002, 37, 293–299. [Google Scholar]
- Sakamoto, M. Effects of physical agents on muscle healing with a focus on animal model research. Phys. Ther. Res. 2021, 24, 1–8. [Google Scholar] [CrossRef]
- Schandelmaier, S.; Kaushal, A.; Lytvyn, L.; Heels-Ansdell, D.; Siemieniuk, R.A.; Agoritsas, T.; Guyatt, G.H.; Vandvik, P.O.; Couban, R.; Mollon, B.; et al. Low intensity pulsed ultrasound for bone healing: Systematic review of randomized controlled trials. BMJ 2017, 356, j656. [Google Scholar] [CrossRef]
- Robertson, V.J.; Baker, K.G. A review of therapeutic ultrasound: Effectiveness studies. Phys. Ther. 2001, 81, 1339–1350. [Google Scholar] [CrossRef]
- Van der Windt, D.A.W.M.; van der Heijden, G.J.M.G.; van den Berg, S.G.M.; Ter Riet, G.; de Winter, A.F.; Bouter, L.M. Ultrasound therapy for musculoskeletal disorders: A systematic review. Pain 1999, 81, 257–271. [Google Scholar] [CrossRef]
- Van der Worp, H.; van den Akker-Scheek, I.; van Schie, H.; Zwerver, J. ESWT for tendinopathy: Technology and clinical implications. Knee Surg Sports Traumatol Arthrosc. 2013, 21, 1451–1458. [Google Scholar] [CrossRef]
- Bergh, A.; Lund, I.; Bostrom, A.; Hyytiainen, H.; Asplund, K. Systematic review of complementary and alternative veterinary medicine: “Miscellaneous therapies”. Animals 2021, 12, 3356. [Google Scholar] [CrossRef]
- Statens Beredning för Medicinsk och Social Utvärdering [SBU]. SBU:s Metodbok 2022. Available online: https://www.sbu.se/sv/metod/sbus-metodbok/?pub=48286 (accessed on 8 April 2022).
- Higgins, J.; Thomas, J. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2. Available online: https://training.cochrane.org/handbook/current2021 (accessed on 8 April 2022).
- BMJ Best Practice. What Is GRADE? 2022. Available online: https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade (accessed on 8 April 2022).
- Fahim, M.S.; Fahim, Z.; Harman, J.; Thompson, I.; Montie, J.; Hall, D.G. Ultrasound as a new method of male contraception. Fertil. Steril. 1977, 28, 823–831. [Google Scholar] [CrossRef]
- Lang, D.C. Ultrasonic treatment of musculoskeletal conditions in the horse, dog and cat. Vet. Record 1980, 106, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sobti, V.K.; Singh, K.I. Clinical, haematological and radiological effects if therapeutic ultrasound on the healing of femur fracture in dogs. Indian Vet. J. 1994, 1117–1119. [Google Scholar]
- Singh, M.; Sobti, V.K.; Singh, K.I. Clinical, haematological and radiological observations on therapeutic ultrasound in humerus fracture healing in dogs. Indian J. of Anim. Sci. 1994, 64, 22–24. [Google Scholar]
- Singh, K.I.; Sobti, V.K.; Arora, A.K.; Bhatia, R. Therapeutic ultrasound [1 Watt/cm2] in experimental acute traumatic arhtrits in the equines. Indian Vet. J. Surg. 1996, 17, 81–92. [Google Scholar]
- Singh, K.I.; Sobti, V.K.; Roy, K.S. Gross and histomorphological effects of therapeutic ultrasound [1 Watt/cm2] in experimental acute traumatic arthritis in donkeys. J. Equine Vet. Sci. 1997, 17, 150–155. [Google Scholar] [CrossRef]
- Singh, M.; Sobti, M.; Roy, K.S. Effects of therapeutic ultrasound in healing of humerus fracture in dogs. Indian Vet. J. 1997, 74, 151–154. [Google Scholar]
- Albuquerque, S.A.; Martins, O.; Aguiars, A.; Silva, L.O.; Pacheco, A.D.; Pessoa, L.M.B.; Maggi, L.E.; de Souza, S.F. Pelvic limb thermography in dogs submitted to different thermotherapy modalities. Turk. J. Vet. Anim. Sci. 2021, 45, 37–43. [Google Scholar] [CrossRef]
- Steiss, J.E.; Adams, C.C. Effect of coat on rate of temperature increase in muscle during ultrasound treatment of dogs. Am. J. Vet. Res. 1999, 60, 76–80. [Google Scholar]
- Levine, D.; Millis, D.L.; Mynatt, T. Effects of 3.3-MHz ultrasound on caudal thigh muscle temperature in dogs. Vet. Surg. 2001, 30, 170–174. [Google Scholar] [CrossRef]
- Acevedo, B.; Millis, D.L.; Levine, D.; Guevara, J.L. Effect of therapeutic ultrasound on calcaneal tendon heating and extensibility in dogs. Front. Vet. Sci. 2019, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.S.; Roy, K.S.; Bansal, P.S.; Singh, B.; Simran, P.S. A preliminary study on the effect of ultrasound therapy on the healing of surgically severed achilles tendons in five dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Muste, A.; Muste, M.; Scurtu, L.; Tanase, A.; Ilie, I.I.; Hodis, L.; Beteg, F. The improvement of the joint inflammatory status in dogs through ultrasound therapy. Sci. Work. Ser. C Vet. Med. 2015, 61, 220–223. [Google Scholar]
- Ansari, M.M.; Zama, M.M.S.; Saxena, A.C.; Gugjoo, M.B. Clinical studies on therapeutic ultrasound and diathermy in dogs with hind quarter weakness. Indian J. Vet. Surg. 2012, 33, 136–139. [Google Scholar]
- Zama, M.M.S.; Ansari, M.M.; Dimri, U.; Hoque, M.; Maiti, S.K.; Kinjadevkar, P. Effect of therapeutic ultrasound and diathermy on oxidant-antioxidant balance in dogs suffering from hind quarter weakness. J. App. Anim. Res. 2013, 41, 82–86. [Google Scholar] [CrossRef][Green Version]
- Maiti, S.K.; Sharma, A.K.; Kumar, N.; Gupta, G.P.; Sharma, A.K. Effects of ultrasound and interferential therapies on hindquarter weakness in dogs. Indian J. Anim. Sci. 2007, 77, 1273–1276. [Google Scholar]
- Sharma, A.K.; Gupta, O.P.; Singh, G.R.; Maiti, S.K.; Pawde, A.M. Effectiveness of TU in the treatment of paraparesis in the dog. Indian J. Vet. Surg. 2011, 32, 135–137. [Google Scholar]
- Silveira, D.S.; Pippi, N.L.; Costa, F.S.; Vescovi, L.A.; Castro, L.M.; Weiss, C.A.; da Silva, G.F.; Júnior, R.R.A.; Braga, F.d.A.; Vulcano, L.C.; et al. O ultra-som terapêutico de 1 MHz, na dose de 0,5 W cm-2, sobre o tecido ósseo de cães avaliado por densitometria óptica em imagens radiográficas [The 1 MHz therapeutic ultrasound, in doses of 0.5 W cm2;, on dog’s bone tissue evaluated through optic densitometry in radiographic images]. Ciência Rural 2008, 38, 2225–2231. [Google Scholar]
- Cook, S.D.; Salkeld, S.L.; Patron, L.P.; Ryaby, J.P.; Whitecloud, T.S. Low-intensity pulsed ultrasound improves spinal fusion. Spine J. 2001, 1, 246–254. [Google Scholar] [CrossRef]
- Rawool, N.M.; Goldberg, B.B.; Forsberg, F.; Winder, A.A.; Hume, E. Power Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound. J. Ultrasound Med. 2003, 22, 145–153. [Google Scholar] [CrossRef]
- Kaur, A.; Sobti, V.K.; Mohindroo, J. Evaluation of therapeutic ultrasound [0.5 Watt/cm2 vs 1.0 Watt/cm2] in bone healing in dogs: Clinical and radiographic findings. Indian J. Vet. Surg. 2004, 25, 83–85. [Google Scholar]
- Ikai, H.; Tamura, T.; Watanabe, T.; Itou, M.; Sugaya, A.; Iwabuchi, S.; Mikuni-Takagaki, Y.; Deguchi, S. Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J. Periodontal Res. 2008, 4, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Adair, H.S.; Levine, D. Effects of 1-MHz ultrasound on epaxial muscle temperature in horses. Front Vet. Sci. 2019, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, L.; Elliott, S.B.; Adair, H.S. Muscle and tendon heating rates with therapeutic ultrasound in horses. Vet. Surg. 2013, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.A.L.; Alves, G.E.S.; Souza, J.C.A. Efeito do ultra-som terapêutico em tendinite experimental de eqüinos: Estudo clínico, ultra-sonográfico e histopatológico de dois protocolos (Clinic, ultrasonographic and histopatological studies of two protocols of ultrasonic therapy on experimental tendonitis in horses). Arq. Bras. Med. Vet. Zootec. 2003, 55, 27–34. [Google Scholar]
- Sharifi, D.; Mohitmafi, S.; Rassouli, A.; Shams, G.R. Effect of ultrasound therapy on the hydroxyproline content in experimental tendon injuries in horses. Iran. J. Vet. Surg. 2007, 2, 36–41. [Google Scholar]
- Carrozzo, U.; Toniato, M.; Harrison, A. Assessment of noninvasive low-frequency ultrasound as a means of treating injuries to suspensory ligaments in horses: A research paper. J. Equine Vet. Sci. 2019, 80, 80–89. [Google Scholar] [CrossRef]
- Mercado, M.C.; Liñeiro, J.A.G.; Lightowler, C.H. Asociación de electroanalgesia [TENS] y ultrasonoterapia en el trataniento de lesiones inflamatorias del m. longissimus dorsi en el equino [Electroanalgesia and ultrasonotherapy relationship on the treatment of inflammatory lesions of longissimus dorsi muscle in the horse]. Rev. Cient. 2002, 12, 127–132. [Google Scholar]
- Leoci, R.; Aiudi, G.; De Sandro Salvati, A.; Silvestre, F.; Binetti, F.; Lacalandra, G.M. Ultrasound as a mechanical method for male dog contraception. Reprod. Domest. Anim. 2009, 44 (Suppl. 2), 326–328. [Google Scholar] [CrossRef]
- Leoci, R.; Aiudi, G.; Silvestre, F.; Lissner, E.A.; Marino, F.; Lacalandra, G.M. Therapeutic ultrasound as a potential male dog contraceptive: Determination of the most effective application protocol. Reprod. Domest. Anim. 2015, 50, 712–718. [Google Scholar] [CrossRef]
- Khanbazi, M.H.; Mogheiseh, A.; AhrariKhafi, M.S.; Nazifi, S.; Ahmadi, N.; Khazaei, M. The effects of therapeutic ultrasound waves on testicular tissue, echogenicity, semen quality, oxidative stress, and acute-phase proteins in dogs. Theriogenology 2020, 153, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Loghlin, M.; Lewith, G.; Falkenberg, T. Science, practice and mythology: A definition and examination of the implications of scientism in medicine. Health Care Anal. 2013, 21, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, P.; Alam, M.; Li, S.; Chow, S.K.H.; Zheng, Y.P. Low-intensity pulsed ultrasound stimulation for bone fractures healing: A review. J. Ultrasound Med. 2022, 41, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Parekh, S.; Hooper, L.; Loke, Y.K.; Ryder, J.; Sutton, A.J.; Hing, C.; Kwok, C.S.; Pang, C.; Harvey, I. Dissemination and publication of research findings: An updated review of related biases. Health Technol. Assess. 2010, 14, 1–193. [Google Scholar] [CrossRef] [PubMed]
- Rücker, G.; Carpenter, J.R.; Schwarzer, G. Detecting and adjusting for small-study effects in meta-analysis. Biometr. J. 2011, 53, 351–368. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boström, A.; Asplund, K.; Bergh, A.; Hyytiäinen, H. Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Therapeutic Ultrasound. Animals 2022, 12, 3144. https://doi.org/10.3390/ani12223144
Boström A, Asplund K, Bergh A, Hyytiäinen H. Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Therapeutic Ultrasound. Animals. 2022; 12(22):3144. https://doi.org/10.3390/ani12223144
Chicago/Turabian StyleBoström, Anna, Kjell Asplund, Anna Bergh, and Heli Hyytiäinen. 2022. "Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Therapeutic Ultrasound" Animals 12, no. 22: 3144. https://doi.org/10.3390/ani12223144
APA StyleBoström, A., Asplund, K., Bergh, A., & Hyytiäinen, H. (2022). Systematic Review of Complementary and Alternative Veterinary Medicine in Sport and Companion Animals: Therapeutic Ultrasound. Animals, 12(22), 3144. https://doi.org/10.3390/ani12223144





