Effect of Epidermal Growth Factor (EGF) on Cryopreserved Piedmontese Bull Semen Characteristics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Collection and Extension
2.2. Thawed Semen Evaluation
2.2.1. Sperm Concentration and Kinematic
2.2.2. Sperm Viability, Acrosomal Integrity, and Plasma Membrane Integrity
2.2.3. Flow Cytometric Analysis
DNA Integrity
Mitochondrial Activity
Apoptosis (Annexin-V/PI-Binding Assay)
2.2.4. Sperm Mucus Penetration Test
2.2.5. Antioxidant Activity (Superoxide Dismutase, SOD)
2.3. Fertilizing Capability
2.4. Statistical Analysis
3. Results
3.1. Motility and Velocity Parameters
3.2. Sperm Viability and Integrity of Acrosomes, Plasma Membrane, and DNA
3.3. Apoptosis
3.4. Sperm Mitochondrial Membrane Potential, Mucus Penetration Distance, and SOD Activity
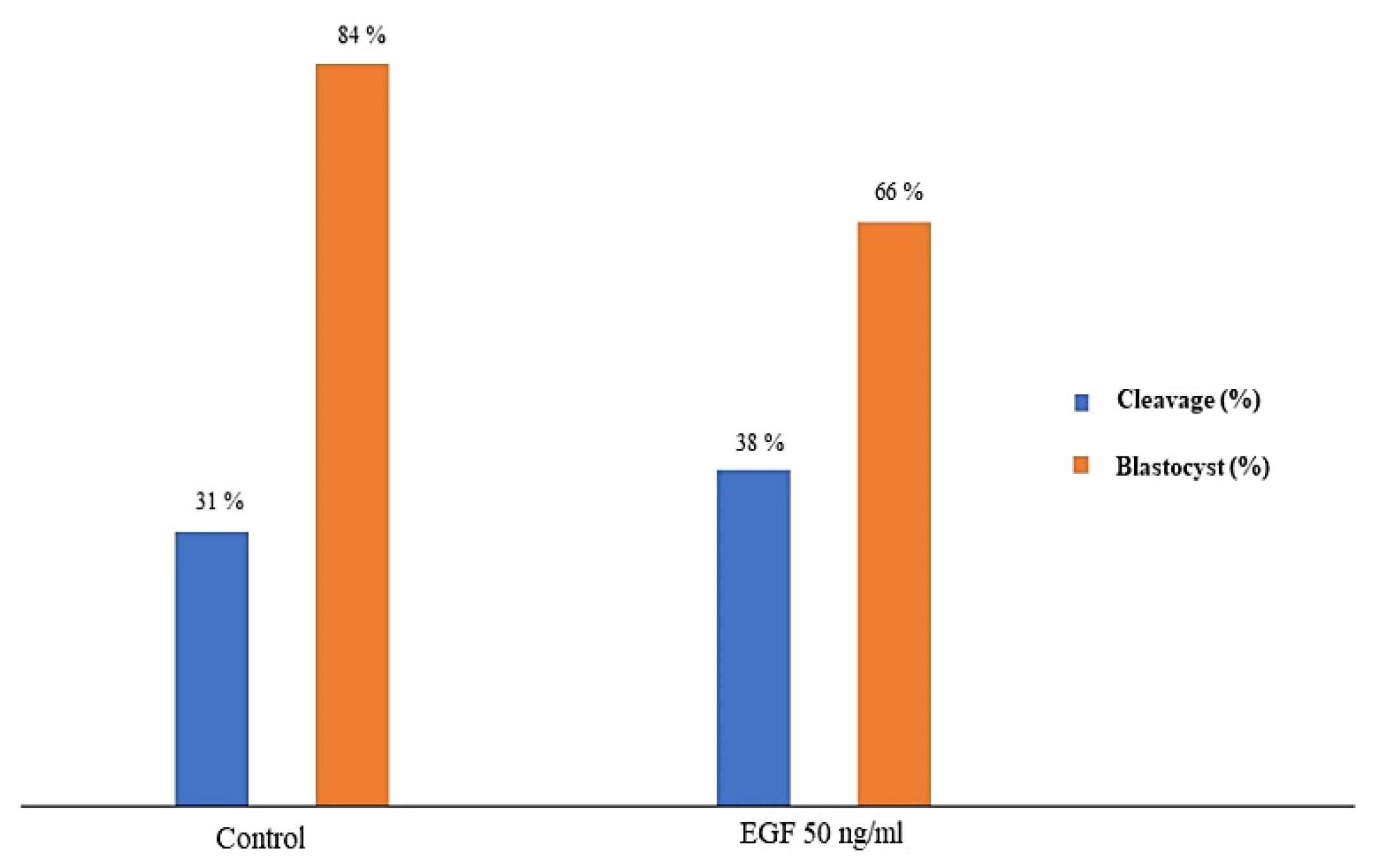
3.5. Fertilizing Potential
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bucak, M.N.; Tuncer, P.B.; Sarıözkan, S.; Başpınar, N.; Taşpınar, M.; Çoyan, K.; Bilgili, A.; Akalın, P.P.; Büyükleblebici, S.; Aydos, S.; et al. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: Antioxidants protect DNA integrity against cryodamage. Cryobiology 2010, 61, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Bailey, J.L.; Blodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon mini review. J. Androl. 2000, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Talukdar, D.J.; Ahmed, K.; Deka, B.C.; Sinha, S.; Deori, S.; Das, G.C. Cryo-capacitation changes during cryopreservation of swamp buffalo spermatozoa. Indian J. Anim. Sci. 2016, 86, 397–400. [Google Scholar]
- Gürler, H.; Malama, E.; Heppelmann, M.; Calisici, O.; Leiding, C.; Kastelic, J.P.; Bollwein, H. Effects of Cryopreservation on Sperm Viability, Synthesis of Reactive Oxygen Species, and DNA Damage of Bovine Sperm. Theriogenology 2016, 86, 562–571. [Google Scholar] [CrossRef]
- Amirat, L.; Anton, M.; Tainturier, D.; Chatagnon, G.; Battut, I.; Courtens, J.L. Modifications of bull spermatozoa induced by three extenders: Biociphos, low density lipoprotein and Triladyl, before, during and after freezing and thawing. Reproduction 2005, 129, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Elkhawagah, A.R.; Longobardi, V.; Neglia, G.; Salzano, A.; Zullo, G.; Sosa, G.A.; Campanile, G.; Gasparrini, B. Effect of relaxin on fertility parameters of frozen-thawed buffalo (Bubalusbubalis) sperm. Reprod. Domest. Anim. 2015, 50, 756–762. [Google Scholar] [CrossRef]
- Adamson, E.D. EGF receptor activities in mammalian development. Mol. Reprod. Dev. 1990, 27, 16–22. [Google Scholar] [CrossRef]
- Ahmad, K.; Naz, R.K. Role of epidermal growth factor in reproduction. Assist. Reprod. Tech. Androl. 1993, 4, 85–92. [Google Scholar]
- Yan, Y.C.; Sun, Y.P.; Zhang, M.L. Testis epidermal growth factor and spermatogenesis. Arch. Androl. 1998, 40, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Endo, Y.; Oba, M.; Suzuki, S.; Nozawa, S. Effect of epidermal growth factor on human sperm capacitation. Fertil. Steril. 1993, 60, 905–910. [Google Scholar] [CrossRef]
- Naz, R.K.; Kaplan, P. Effects of epidermal growth factor on human sperm cell function. J. Androl. 1993, 14, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Lax, Y.; Rubinstein, S.; Breitbart, H. Epidermal growth factor induces acrosomal exocytosis in bovine sperm. FEBS Lett. 1994, 339, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Pastor, F.; Johannisson, A.; Gil, J.; Kaabi, M.; Anel, L.; Paz, P.; Rodriguez-Martinez, H. Use of chromatin stability assay, mitochondrial stain JC-1, and fluorometric assessment of plasma membrane to evaluate frozen-thawed ram semen. Anim. Reprod. Sci. 2004, 84, 121–133. [Google Scholar] [CrossRef]
- Harper, M.K. Gamete and zygote transport. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neill, I.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 123–188. [Google Scholar]
- Shiva Shankar Reddy, N.; Jagan Mohanarao, G.; Atreja, S. Effects of adding taurine and trehalose to a tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation. Anim. Reprod. Sci. 2010, 119, 183–190. [Google Scholar] [CrossRef]
- Naz, R.K.; Ahmad, K. Presence of expression products of c-erbB-1 and c-erbB-2/HER2 genes on mammalian sperm cell, and effects of their regulation on fertilization. J. Reprod. Immunol. 1992, 21, 223–239. [Google Scholar] [CrossRef]
- Naz, R.K.; Minhas, B.S. Enhancement of sperm function for treatment of male infertility. J. Androl. 1995, 16, 384–388. [Google Scholar]
- Oliva-Hernández, J.; P´erez-Gutiérrez, J.F. Localization of the epidermal growth factor (EGF) in the epididymis and accessory genital glands of the boar and functional effects on spermatozoa. Theriogenology 2008, 70, 1159–1169. [Google Scholar] [CrossRef]
- Kowsar, R.; Ronasi, S.; Sadeghi, N.; Sadeghi, K.; Miyamoto, A. Epidermal growth factor alleviates the negative impact of urea on frozen-thawed bovine sperm, but the subsequent developmental competence is compromised. Sci. Rep. 2021, 11, 4687. [Google Scholar] [CrossRef]
- Sundararaman, M.N.; Kalatharan, J.; Thilak Pon Jawahar, K. Computer assisted semen analysis for quantification of motion characteristics of bull sperm during cryopreservation cycle. Vet. World 2012, 5, 723–726. [Google Scholar] [CrossRef]
- Boccia, L.; Di Palo, R.; De Rosa, A.; Attanasio, L.; Mariotti, E.; Gasparrini, B. Evaluation of buffalo semen by Trypan blue/Giemsa staining and related fertility in vitro. Ital. J. Anim. Sci. 2007, 6, 739–742. [Google Scholar] [CrossRef]
- Akhter, S.; Ansari, M.S.; Andrabi, S.M.H.; Ullah, N.; Qayyum, M. Effect of antibiotics in extender on bacterial control and spermatozoal quality of cooled buffalo (Bubalus bubalis) bull semen. Reprod. Domes. Anim. 2008, 43, 272–278. [Google Scholar] [CrossRef]
- Evenson, D.; Jost, L. Sperm chromatin structure assay is useful for fertility assessment. Method Cell Sci. 2000, 22, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Anzar, M.; He, L.; Buhr, M.M.; Kroetsch, T.G.; Pauls, K.P. Sperm apoptosis in fresh and cryopreserved bull semen detected by flow cytometry and its relationship with fertility. Biol. Reprod. 2002, 66, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.M.; Barik, S.; Rizk, B.; Kulkarni, P.M.; Thorneycroft, I.H. Analysis of in Vitro Migration Patterns of Human Spermatozoa by a Petri Dish-Based Horizontal Column. Biol. Reprod. 1999, 61, 406–410. [Google Scholar] [CrossRef]
- Hamano, K.; Tanaka, S.; Kawana, Y.; Tsujiih, H.; Sasada, H.; Sato, E.; Takahashi, T.; Miyawaki, K.; Arima, H.E. Evaluation of Bull Fertility by Migration of Frozen-Thawed and Washed Sperm in Medium Containing Cervical Mucus. J. Reprod. Dev. 2011, 47, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Sariozkan, S.; Bucak, M.N.; Tuncer, P.B.; Ulutas, P.A.; Bilgen, A. The influence of cysteine and taurine on microscopic-oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology 2009, 58, 134–138. [Google Scholar] [CrossRef]
- Elkhawagah, A.R.; Martino, N.A.; Ricci, A.; Storti, V.; Rumbolo, F.; Lange-Consiglio, A.; Vincenti, L. Effect of relaxin on cryopreserved beef bull semen characteristics. Cryobiology 2020, 95, 51–59. [Google Scholar] [CrossRef]
- Holm, P.; Booth, P.J.; Schmidt, M.H.; Greve, T.; Callesen, H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serumproteins. Theriogenology 1999, 52, 683–700. [Google Scholar] [CrossRef]
- Bucak, M.N.; Atessahin, A.; Yuce, A. Effect of antioxidants and oxidative stress parameters on ram semen after the freeze–thawing process. Small Rumin. Res. 2008, 75, 128–134. [Google Scholar] [CrossRef]
- Makarevich, A.V.; Spalekova, E.; Olexikova, L.; Lukac, N.; Kubovicova, E.; Hegedusova, Z. Functional characteristics of ram cooling-stored spermatozoa under the influence of epidermal growth factor. Gen. Physiol. Biophys. 2011, 30, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandiel, M.M.M.; El-Khawagah, A.R.M.; Mahmoud, K.G. Effect of epidermal growth factor on buffalo frozen spermatozoa biometry and metabolic activity. Asian Pac. J. Reprod. 2017, 6, 43–48. [Google Scholar] [CrossRef]
- NagDas, S.K.; Winfrey, V.P.; Olson, G.E. Identification of Ras and its downstream signaling elements and their potential role in hamster sperm motility. Biol. Reprod. 2002, 67, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Fujita, A.; Nakamura, K.; Kato, T.; Watanabe, N.; Ishizaki, T.; Kimura, K.; Mizoguchi, A.; Naramiya, S. Ropporin, a sperm-specific binding protein of rophilin that is localized in the fibrous sheet of the sperm flagella. J. Cell Sci. 2000, 113, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, R.; Shannon, P. Do sperm cells age? A review of the physiological change in sperm during storage at ambient temperature. Reprod. Fertil. Dev. 1997, 9, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.B.; Presicce, G.A.; Brockett, C.C.; Foote, R.H. Quantification of bull sperm characteristics measured by computer assisted sperm analysis (CASA) and their relationship to fertility. Theriogenology 1998, 49, 871–879. [Google Scholar] [CrossRef]
- Trzcińska, M.; Bryła, M.; Smorąg, Z. Effect of liquid storage on membrane integrity and mitochondrial activity: A new diagnostic method of evaluating boar sperm quality. J. Anim. Feed Sci. 2008, 17, 372–380. [Google Scholar] [CrossRef]
- Casey, P.J.; Hillman, R.B.; Robertson, K.R.; Yudin, A.I.; Liu, I.K.; Drobnis, W.Z. Validation of an acrosomal stain for equine sperm that differentiates between living and dead sperm. J. Androl. 1993, 14, 289–297. [Google Scholar] [CrossRef]
- Kasai, T.; Ogawa, K.; Mizuno, K.; Nagai, S.; Uchida, Y.; Ohta, S.; Fujie, M.; Suzuki, K.; Hirata, S.; Hoshi, K. Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian J. Androl. 2002, 4, 97–103. [Google Scholar]
- Selvaraju, S.; Ravindra, J.P.; Ghosh, J.; Gupta, P.S.P.; Suresh, K.P. Evaluation of sperm functional attributes in relation to in vitro sperm–zona pellucida binding ability and cleavage rate in assessing frozen-thawed buffalo (Bubalus bubalis) semen quality. Anim. Reprod. Sci. 2008, 106, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Andrabi, S.M.H.; Jahan, S. Semen quality parameters as fertility predictors of water buffalo bull spermatozoa during low-breeding season. Theriogenology 2016, 86, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F. Human fertilization in vivo, with special reference to progression, storage and release of competent spermatozoa. Hum. Reprod. 1987, 2, 329–332. [Google Scholar] [CrossRef]
- Savage, C.R., Jr.; Inagami, T.; Cohen, S. The Primary Structure of Epidermal Growth Factor. J. Biol. Chem. 1972, 247, 7612–7621. [Google Scholar] [CrossRef]
- Coyan, K.; Baspınar, N.; Bucak, M.N.; Akalın, P.P. Effects of cysteine and ergothioneine on post-thawed Merino ram sperm and biochemical parameters. Cryobiology 2011, 63, 1–6. [Google Scholar] [CrossRef]
- Lonergan, P.; Carolan, C.; Van Langendonckt, A.; Donnay, I.; Khatir, H.; Mermillod, P. Role of Epidermal Growth Factor in Bovine Oocyte Maturation and Preimplantation Embryo Development In Vitro. Biol. Reprod. 1996, 54, 1420–1429. [Google Scholar] [CrossRef] [Green Version]
- Ríos, G.L.; Buschiazzo, J.; Mucci, N.C.; Kaiser, G.G.; Cesari, A.; Alberio, R.H. Combined epidermal growth factor and hyaluronic acid supplementation of in vitro maturation medium and its impact on bovine oocyte proteome and competence. Theriogenology 2015, 83, 874–880. [Google Scholar] [CrossRef]
| Temperature Interval | Rate |
|---|---|
| +4 °C to −9 °C | −4 °C/min |
| −9 °C to −25 °C | −50 °C/min |
| −25 °C to −100 °C | −35 °C/min |
| −100 °C to −144 °C | −20 °C/min |
| −144 °C to −150 °C | −4 °C/min |
| Parameter | Group | Post-Thaw (Mean ± SEM) | 1 h (Mean ± SEM) | 2 h (Mean ± SEM) | 3 h (Mean ± SEM) | 4 h (Mean ± SEM) |
|---|---|---|---|---|---|---|
| Total motility (%) | control | 90.36 ± 0.58 | 83.38 ± 0.87 a | 67.78 ± 1.67 | 50.31 ± 1.41 a | 43.05 ± 1.21 a |
| EGF 50 ng/mL | 89.71 ± 1.00 | 89.97 ± 0.83 b | 69.00 ± 1.38 | 53.91 ± 1.10 | 44.91 ± 1.14 | |
| EGF 100 ng/mL | 91.00 ± 0.33 | 85.53 ± 0.69 | 67.97 ± 1.19 | 56.39 ± 1.17 b | 46.42 ± 1.41 | |
| EGF 200 ng/mL | 91.98 ± 0.51 | 85.61 ± 0.04 | 70.17 ± 1.34 | 52.67 ± 1.19 | 48.58 ± 1.08 b | |
| EGF 400 ng/mL | 88.88 ± 1.18 | 86.34 ± 0.56 b | 71.23 ± 1.36 | 52.44 ± 0.85 | 45.09 ± 1.55 | |
| Significance | NS | 0.001 | NS | 0.001 | 0.01 | |
| Progressive motility (%) | control | 53.19 ± 0.66 | 43.56 ± 0.70 a | 26.63 ± 1.30 a | 6.16 ± 1.40 | 4.02 ± 1.25 |
| EGF 50 ng/mL | 53.03 ± 0.85 | 44.56 ± 1.00 | 30.78 ± 1.14 b | 9.28 ± 1.26 | 3.84 ± 0.99 | |
| EGF 100 ng/mL | 54.12 ± 0.57 | 45.58 ± 0.64 | 27.20 ± 0.98 | 8.38 ± 1.29 | 4.09 ± 1.16 | |
| EGF 200 ng/mL | 54.69 ± 0.58 | 48.09 ± 0.71 b | 32.77 ± 1.01 b | 9.89 ± 1.45 | 3.66 ± 0.96 | |
| EGF 400 ng/mL | 54.44 ± 0.97 | 44.89 ± 0.60 | 32.47 ± 1.39 b | 8.06 ± 1.00 | 4.75 ± 1.34 | |
| Significance | NS | 0.001 | 0.001 | NS | NS | |
| Rapid motility (%) | control | 72.09 ± 0.54 | 61.94 ± 0.88 a | 41.77 ± 1.56 a | 18.84 ± 2.20 | 10.34 ± 2.10 |
| EGF 50 ng/mL | 71.56 ± 1.00 | 66.77 ± 1.33 b | 46.72 ± 1.16 b | 23.91 ± 0.88 | 11.83 ± 1.59 | |
| EGF 100 ng/mL | 71.25 ± 0.60 | 64.53 ± 0.83 | 44.33 ± 1.15 | 24.95 ± 1.98 | 11.89 ± 2.25 | |
| EGF 200 ng/mL | 73.23 ± 0.60 | 67.02 ± 1.06 b | 48.20 ± 0.99 b | 25.08 ± 1.98 | 14.08 ± 1.98 | |
| EGF 400 ng/mL | 71.44 ± 1.25 | 63.31 ± 0.69 | 49.31 ± 1.69 b | 24.53 ± 1.56 | 13.41 ± 2.10 | |
| Significance | NS | 0.001 | 0.001 | NS | NS |
| Group | Post-Thawing (Mean ± SEM) | 1 h (Mean ± SEM) | 2 h (Mean ± SEM) | 3 h (Mean ± SEM) | 4 h (Mean ± SEM) | |
|---|---|---|---|---|---|---|
| VAP (μm/s) | control | 87.01 ± 0.38 a | 80.01 ± 0.433 a | 60.52 ± 1.06 a | 50.55 ± 1.22 a | 49.33 ± 1.33 |
| EGF 50 ng/mL | 86.49 ± 0.42 | 80.65 ± 0.42 | 63.13 ± 0.78 | 54.10 ± 1.31 | 49.18 ± 1.19 | |
| EGF 100 ng/mL | 87.32 ± 0.28 | 80.81 ± 0.38 | 63.38 ± 1.09 | 53.16 ± 0.12 | 47.99 ± 1.27 | |
| EGF 200 ng/mL | 87.69 ± 0.21 | 81.78 ± 0.42 b | 64.56 ± 0.92 b | 54.35 ± 0.15 | 49.67 ± 1.05 | |
| EGF 400 ng/mL | 89.56 ± 0.22 b | 82.34 ± 0.39 b | 63.64 ± 1.13 | 55.33 ± 1.14 b | 50.14 ± 1.35 | |
| Significance | 0.001 | 0.001 | 0.01 | 0.01 | NS | |
| VSL (μm/s) | control | 72.26 ± 0.24 a | 65.65 ± 0.43 a | 49.10 ± 0.85 a | 35.50 ± 1.201 a | 32.00 ± 1.35 |
| EGF 50 ng/mL | 71.99 ± 0.24 | 65.02 ± 0.34 | 51.34 ± 0.69 | 39.72 ± 1.32 b | 32.09 ± 1.31 | |
| EGF 100 ng/mL | 73.48 ± 0.16 b | 66.22 ± 0.22 | 51.16 ± 0.85 | 37.87 ± 1.18 | 31.71 ± 1.19 | |
| EGF 200 ng/mL | 73.32 ± 0.20 b | 67.31 ± 0.36 b | 52.80 ± 0.77 b | 39.58 ± 1.19 | 32.60 ± 1.10 | |
| EGF 400 ng/mL | 75.51 ± 0.27 b | 67.45 ± 0.23 b | 52.08 ± 0.87 b | 39.81 ± 1.28 b | 32.73 ± 1.44 | |
| Significance | 0.001 | 0.001 | 0.01 | 0.05 | NS | |
| VCL (μm/s) | control | 143.20 ± 1.07 | 137.54 ± 0.77 a | 102.98 ± 1.65 a | 89.11 ± 2.05 a | 81.28 ± 2.41 |
| EGF 50 ng/mL | 141.92 ± 1.20 | 137.86 ± 0.88 | 107.82 ± 1.35 | 95.52 ± 2.09 | 80.91 ± 2.22 | |
| EGF 100 ng/mL | 141.82 ± 0.85 | 139.58 ± 0.89 | 108.64 ± 1.82 | 93.24 ± 1.84 | 80.47 ± 2.44 | |
| EGF 200 ng/mL | 143.76 ± 0.68 | 141.47 ± 0.94 b | 110.18 ± 1.71 b | 96.63 ± 1.66 b | 84.40 ± 2.02 | |
| EGF 400 ng/mL | 144.73 ± 0.62 | 141.71 ± 0.81 b | 108.52 ± 1.96 | 98.24 ± 1.97 b | 85.01 ± 2.55 | |
| Significance | NS | 0.001 | 0.01 | 0.01 | NS | |
| ALH (μm) | control | 5.98 ± 0.05 | 6.10 ± 0.02 a | 5.54 ± 0.06 | 5.09 ± 0.35 | 2.40 ± 0.28 a |
| EGF 50 ng/mL | 5.90 ± 0.06 | 6.14 ± 0.03 | 5.70 ± 0.08 | 5.47 ± 0.27 | 4.29 ± 0.34 b | |
| EGF 100 ng/mL | 5.84 ± 0.05 | 6.13 ± 0.03 | 5.64 ± 0.05 | 5.61 ± 0.30 | 3.83 ± 0.41 b | |
| EGF 200 ng/mL | 5.96 ± 0.03 | 6.19 ± 0.03 | 5.57 ± 0.45 | 5.47 ± 0.22 | 4.31 ± 0.43 b | |
| EGF 400 ng/mL | 5.93 ± 0.03 | 6.23 ± 0.04 b | 5.37 ± 0.05 | 5.54 ± 0.25 | 4.28 ± 0.38 b | |
| Significance | NS | 0.01 | NS | NS | 0.001 | |
| BCF (Hz) | control | 27.95 ± 0.22 | 22.31 ± 0.13 a | 18.50 ± 0.21 a | 14.12 ± 0.34 a | 14.03 ± 0.33 |
| EGF 50 ng/mL | 27.48 ± 0.18 | 22.26 ± 0.13 | 19.07 ± 0.23 | 14.95 ± 0.37 | 14.80 ± 0.43 | |
| EGF 100 ng/mL | 28.04 ± 0.23 | 22.90 ± 0.13 b | 18.89 ± 0.20 | 15.14 ± 0.40 | 13.12 ± 0.35 | |
| EGF 200 ng/mL | 28.14 ± 0.14 | 22.72 ± 0.16 | 19.63 ± 0.17 b | 15.61 ± 0.42 b | 13.97 ± 0.37 | |
| EGF 400 ng/mL | 28.56 ± 0.23 | 23.01 ± 0.15 b | 19.73 ± 0.26 b | 15.45 ± 0.34 b | 14.75 ± 0.32 | |
| Significance | NS | 0.01 | 0.001 | 0.05 | NS | |
| STR (%) | control | 83.42 ± 0.21 a | 82.30 ± 0.41 a | 82.13 ± 0.35 | 70.64 ± 0.62 a | 64.75 ± 0.74 |
| EGF 50 ng/mL | 83.69 ± 0.28 | 80.88 ± 0.17 b | 82.02 ± 0.28 | 73.52 ± 0.73 b | 64.92 ± 0.84 | |
| EGF 100 ng/mL | 84.38 ± 0.21 b | 82.53 ± 0.19 | 81.64 ± 0.21 | 71.75 ± 0.82 | 66.48 ± 0.62 | |
| EGF 200 ng/mL | 83.98 ± 0.28 | 82.72 ± 0.14 | 82.48 ± 0.21 | 73.25 ± 0.76 b | 65.70 ± 0.74 | |
| EGF 400 ng/mL | 84.56 ± 0.38 b | 82.11 ± 0.19 | 82.77 ± 0.27 | 72.03 ± −0.91 | 64.91 ± 0.85 | |
| Significance | 0.05 | 0.001 | NS | 0.05 | NS | |
| LIN (%) | control | 52.59 ± 0.29 a | 48.95 ± 0.24 a | 49.14 ± 0.30 | 40.33 ± 0.44 | 40.03 ± 0.45 |
| EGF 50 ng/mL | 52.80 ± 0.38 | 48.33 ± 0.15 b | 49.11 ± 0.32 | 42.06 ± 0.55 | 39.73 ± 0.46 | |
| EGF 100 ng/mL | 53.63 ± 0.31 | 48.94 ± 0.19 | 48.53 ± 0.21 | 41.30 ± 0.57 | 40.19 ± 0.42 | |
| EGF 200 ng/mL | 53.09 ± 0.32 | 48.94 ± 0.16 | 49.45 ± 0.22 | 41.70 ± 0.63 | 39.25 ± 0.40 | |
| EGF 400 ng/mL | 54.05 ± 0.34 b | 48.94 ± 0.16 | 49.55 ± 0.30 | 41.08 ± 0.60 | 38.86 ± 0.47 | |
| Significance | 0.01 | 0.05 | NS | NS | NS |
| Group | Sperm Vitality (%) (Mean ± SEM) | Acrosome Integrity (%) (Mean ± SEM) | Plasma Membrane Integrity (%) (Mean ± SEM) | DNA Integrity (%) (Mean ± SEM) |
|---|---|---|---|---|
| Control | 68.56 ± 1.32 | 90.94 ± 1.75 | 60.94 ± 1.65 | 94.02 ± 0.35 |
| EGF 50 ng/mL | 65.50 ± 1.10 | 90.56 ± 1.71 | 61.94 ± 1.95 | 93.85 ± 0.70 |
| EGF 100 ng/mL | 70.06 ± 1.34 | 92.25 ± 1.52 | 64.63 ± 1.74 | 94.02 ± 0.66 |
| EGF 200 ng/mL | 72.38 ± 1.50 | 92.06 ± 1.78 | 63.00 ± 2.35 | 94.25 ± 0.63 |
| EGF 400 ng/mL | 71.06 ± 1.50 | 93.56 ± 1.36 | 62.88 ± 1.95 | 93.43 ± 0.73 |
| Significance | NS | NS | NS | NS |
| Group | Viable Sperm (%) (Mean ± SEM) | Necrotic Sperm (%) (Mean ± SEM) | Apoptotic Sperm (%) (Mean ± SEM) |
|---|---|---|---|
| Control | 45.95 ± 10.65 | 45.90 ± 5.61 | 8.15 ± 5.41 |
| EGF 50 ng/mL | 50.41 ± 5.43 | 47.78 ± 5.28 | 1.80 ± 0.34 |
| EGF 100 ng/mL | 52.69 ± 3.64 | 46.29 ± 3.70 | 1.02 ± 0.23 |
| EGF 200 ng/mL | 51.63 ± 3.64 | 47.36 ± 3.88 | 1.02 ± 0.31 |
| EGF 400 ng/mL | 50.54 ± 2.85 | 48.48 ± 2.81 | 1.15 ± 0.23 |
| Significance | NS | NS | NS |
| Group | HMMP (%) (Mean ± SEM) | Mucus Penetration Distance (cm) (Mean ± SEM) | SOD Activity (u/mL) (Mean ± SEM) |
|---|---|---|---|
| Control | 12.44 ± 1.68 | 8.75 ± 1.93 | 1.68 ± 0.02 |
| EGF 50 ng/mL | 24.15 ± 12.44 | 10.00 ± 1.78 | 1.67 ± 0.04 |
| EGF 100 ng/mL | 30.67 ± 9.39 | 9.73 ± 1.98 | 1.72 ± 0.03 |
| EGF 200 ng/mL | 25.73 ± 10.35 | 10.38 ± 1.14 | 1.69 ± 0.03 |
| EGF 400 ng/mL | 22.61 ± 10.30 | 9.70 ± 0.83 | 1.64 ± 0.04 |
| Significance | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhawagah, A.R.; Ricci, A.; Banchi, P.; Martino, N.A.; Poletto, M.L.; Donato, G.G.; Nervo, T.; Vincenti, L. Effect of Epidermal Growth Factor (EGF) on Cryopreserved Piedmontese Bull Semen Characteristics. Animals 2022, 12, 3179. https://doi.org/10.3390/ani12223179
Alkhawagah AR, Ricci A, Banchi P, Martino NA, Poletto ML, Donato GG, Nervo T, Vincenti L. Effect of Epidermal Growth Factor (EGF) on Cryopreserved Piedmontese Bull Semen Characteristics. Animals. 2022; 12(22):3179. https://doi.org/10.3390/ani12223179
Chicago/Turabian StyleAlkhawagah, Ahmed R., Alessandro Ricci, Penelope Banchi, Nicola A. Martino, Mariagrazia Lucia Poletto, Gian Guido Donato, Tiziana Nervo, and Leila Vincenti. 2022. "Effect of Epidermal Growth Factor (EGF) on Cryopreserved Piedmontese Bull Semen Characteristics" Animals 12, no. 22: 3179. https://doi.org/10.3390/ani12223179
APA StyleAlkhawagah, A. R., Ricci, A., Banchi, P., Martino, N. A., Poletto, M. L., Donato, G. G., Nervo, T., & Vincenti, L. (2022). Effect of Epidermal Growth Factor (EGF) on Cryopreserved Piedmontese Bull Semen Characteristics. Animals, 12(22), 3179. https://doi.org/10.3390/ani12223179







