Comparative Effect of Allicin and Alcoholic Garlic Extract on the Morphology and Infectivity of Eimeria tenella Oocysts in Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Allicin and KOH 5%
2.1.2. Alcoholic Garlic Extraction
2.1.3. Eimeria Oocysts
2.2. Experimental Design
2.2.1. Ethical Approval
2.2.2. Evaluation of the In Vitro Effect of Different Allicin and Alcoholic Garlic Extract Concentrations on the Number and Sporulation Dynamics of E. tenella Oocysts
2.2.3. Evaluating the Effect of Allicin and Alcoholic Garlic Extract on the Morphology of E. tenella Oocysts
2.2.4. In Vivo Infectivity of E. tenella Sporulated Oocysts Treated with Allicin and Alcoholic Garlic Extract
Oocyst Shedding
Cecal Lesion Scoring
Histopathological Examination and Scoring
2.3. Statistical Analysis
3. Results
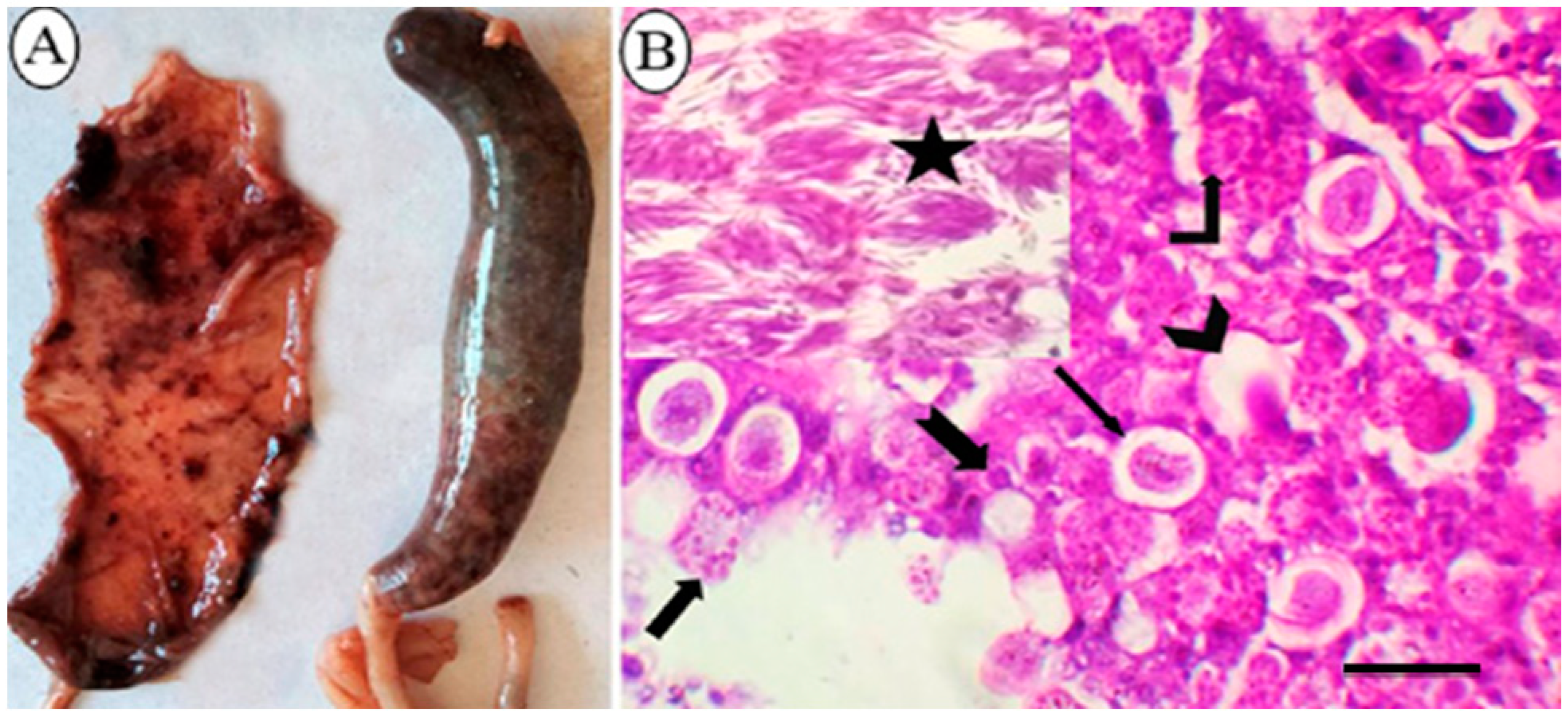
3.1. Macroscopic and Microscopic Lesions of Naturally Infected Broiler Chickens
3.2. In Vitro Effect of Different Concentrations of Allicin and Alcoholic Garlic Extract on the Number and Sporulation Dynamics of E. tenella Oocysts
3.3. Evaluating the Effect of Allicin and Alcoholic Garlic Extract on the Morphology of E. tenella Oocysts
3.4. In Vivo Infectivity of E. tenella Sporulated Oocysts Treated with the Allicin and Alcoholic Garlic Extract
3.4.1. Reduction of Oocysts Shedding
3.4.2. Gross Lesion Scoring
3.4.3. Histopathological Findings and Scoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojimelukwe, A.E.; Emedhem, D.E.; Agu, G.O.; Nduka, F.O.; Abah, A.E. Populations of Eimeria tenella express resistance to commonly used anticoccidial drugs in southern Nigeria. Int. J. Vet. Sci. Med. 2018, 6, 192–200. [Google Scholar] [CrossRef] [Green Version]
- El-Khtam, A.; Abd El Latif, A.; El-Hewaity, M. Efficacy of turmeric (Curcuma longa) and garlic (Allium sativum) on Eimeria species in broilers. Int. J. Basic Appl. Sci. 2014, 3, 349. [Google Scholar]
- Burrell, A.; Tomley, F.M.; Vaughan, S.; Marugan-Hernandez, V. Life cycle stages, specific organelles and invasion mechanisms of Eimeria species. Parasitology 2020, 147, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Fatoba, A.J.; Adeleke, M.A. Diagnosis and control of chicken coccidiosis: A recent update. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2018, 42, 483–493. [Google Scholar] [CrossRef]
- Namratha, M.L.; Reddy, K.; Kuman, Y.; Sandyarani, K.; Laksman, M. Incidence of caecal coccidiosis in adult layer chickens. Curr. Microbiol. App. Sci 2019, 8, 577–580. [Google Scholar] [CrossRef]
- Fernando, M.; Lawn, A.; Rose, M.E.; Al-Attar, M. Invasion of chicken caecal and intestinal lamina propria by crypt epithelial cells infected with coccidia. Parasitology 1983, 86, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Tyzzer, E.E. Coccidiosis in gallinaceous birds. Am. J. Epidemiol. 1929, 10, 269–383. [Google Scholar] [CrossRef]
- Shivaramaiah, C.; Barta, J.R.; Hernandez-Velasco, X.; Téllez, G.; Hargis, B.M. Coccidiosis: Recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet. Med. Res. Rep. 2014, 5, 23. [Google Scholar]
- Jatau, I.D.; Aremu, S.F.; Magaji, Y.; Babashani, M. Oocysticidal effects of some commercial poultry house disinfectants against sporulated Eimeria tenella oocysts. J. Anim. Prod. Res. 2017, 29, 281–288. [Google Scholar]
- Jatau, I.D.; Idah, S.; Okubanjo, O.O.; Natala, A.J.; Aliyu, H.B.; Adamu, J.; Magaji, Y. Evaluation of In vitro disinfection efficacies of sodium hypoclorite (JikR) and cresol (MoriguardR) on oocysts of Eimeria tenella. In Proceedings of the 4th Nigeria International Poultry Summit, Abeokuta, Nigeria, 17–21 February 2013; pp. 120–124. [Google Scholar]
- You, M.-J. Suppression of Eimeria tenella sporulation by disinfectants. Korean J. Parasitol. 2014, 52, 435. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Fehaid, A.; El-Shewehy, D.M.M.; Ramez, A.M.; Alkhaldi, A.A.M.; Mady, R.; Nasr, N.E.; Arafat, N.; Hassanen, E.A.A.; Alsharif, K.F.; et al. S-Methylcysteine Ameliorates the Intestinal Damage Induced by Eimeria tenella Infection via Targeting Oxidative Stress and Inflammatory Modulators. Front. Vet. Sci. 2021, 8, 754991. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Aleem, M.T. Medicinal Plants as an Alternative to Control Poultry Parasitic Diseases. Life 2022, 12, 449. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Oosthuizen, C.B.; Reid, A.-M.; Lall, N. Garlic (Allium sativum) and its associated molecules, as medicine. In Medicinal Plants for Holistic Health and Well-Being; Elsevier: Amsterdam, The Netherlands, 2018; pp. 277–295. [Google Scholar]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1463, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Talukder, S. Histopathology techniques: Tissue processing and staining. Mymensingh Med. Coll. Dep. Pathol. Mymensingh 2007, 11. [Google Scholar]
- Rashid, M.H.; Stevenson, M.A.; Waenga, S.; Mirams, G.; Campbell, A.J.; Vaughan, J.L.; Jabbar, A. Comparison of McMaster and FECPAKG2 methods for counting nematode eggs in the faeces of alpacas. Parasites Vectors 2018, 11, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.; Bushell, A.; Reperant, J.; Doy, T.; Morgan, J.; Shirley, M.; Yvore, P.; Carr, M.M.; Fremont, Y. A survey of Eimeria species in commercially-reared chickens in France during 1994. Avian Pathol. 1996, 25, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Gadelhaq, S.M.; Arafa, W.M.; Abolhadid, S.M. In vitro activity of natural and chemical products on sporulation of Eimeria species oocysts of chickens. Vet. Parasitol. 2018, 251, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.K.; Khan, J.A. In-vitro anticoccidial potential of Saccharum officinarum extract against Eimeria oocysts. Bol. Latinoam. Caribe Plantas Med. Aromat. 2015, 14, 456–461. [Google Scholar]
- Conway, D.P.; McKenzie, M.E. Poultry Coccidiosis: Diagnostic and Testing Procedures, 3rd ed.; Blackwell Publishing: Ames, IA, USA; John Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Alnassan, A.A.; Thabet, A.; Daugschies, A.; Bangoura, B. In vitro efficacy of allicin on chicken Eimeria tenella sporozoites. Parasitol. Res. 2015, 114, 3913–3915. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.H.; Soad, M.; Mervat, A.A.; Gehan, N.A.; Rania, A.A. Comparative biochemical and pathological studies between Toltrazuril and garlic supplementation in chickens infected with coccidiosis. Egypt. J. Anim. Health 2021, 1, 65–80. [Google Scholar] [CrossRef]
- Bukhari, Z.; Marshall, M.M.; Korich, D.G.; Fricker, C.R.; Smith, H.V.; Rosen, J.; Clancy, J.L. Comparison of Cryptosporidium parvum viability and infectivity assays following ozone treatment of oocysts. Appl. Environ. Microbiol. 2000, 66, 2972–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Haug, A.; Williams, R.B.; Larsen, S. Counting coccidial oocysts in chicken faeces: A comparative study of a standard McMaster technique and a new rapid method. Vet. Parasitol. 2006, 136, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Banga, H.; Brar, R.; Singh, N.; Sodhi, S.; Leishangthem, G. Histopathological and immunohistochemical diagnosis of infectious bursal disease in poultry birds. Vet. World 2015, 8, 1331. [Google Scholar] [CrossRef] [Green Version]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone Pubication: Edinburgh, UK, 2002; Volume 172, pp. 593–620. [Google Scholar]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shaibani, I.; Al-Khadher, A.; AlHibah, A. Anticoccidial activity of Allium sativum and Punica granatum against experimentally induced Eimeria tenella infection in broiler chickens. Asian J. Anim. Vet. Sci. 2020, 5, 20–29. [Google Scholar]
- Fleischauer, A.T.; Poole, C.; Arab, L. Garlic consumption and cancer prevention: Meta-analyses of colorectal and stomach cancers. Am. J. Clin. Nutr. 2000, 72, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Mohi-Eldin, M.M.; Haridy, M.A.; Hussein Khalil, A.M.; Abdelnaeim, K. Immunomodulatory and antiparasitic effects of garlic extract loaded on zinc oxide nanoparticles compared with pure garlic extract on Eimeriastiedae-infected rabbits. Benha Vet. Med. J. 2018, 35, 94–105. [Google Scholar] [CrossRef]
- Dar, S.A.; Verma, P.; Ashfaque, M.; Zargar, A.A.; Mir, I.A. Effect of garlic extract on haematobiochemical changes in Eimeria tenella infected broiler chicken. Natl. Acad. Sci. Lett. 2014, 37, 311–316. [Google Scholar] [CrossRef]
- Veerakumari, L.; Chitra, N. Effect of Allium sativum on the carbohydrate metabolism of Haemonchus contortus. Int. J. Sci. Res. 2015, 5, 780–785. [Google Scholar]
- Sadek, H.A.; Abdel-Rahman, S.M.; Bakir, H.Y.; Arafa, M.I.; Ahmed, A.A.; Gaber, M.M. The potential convention of garlic and black seed different extracts as an effective treatment of Cryptosporidium spp.: An experimental study. J. Egypt. Soc. Parasitol. 2020, 50, 613–621. [Google Scholar] [CrossRef]
- Ali, M.; Chand, N.; Khan, R.U.; Naz, S.; Gul, S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar] [CrossRef] [Green Version]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.; A. Al-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; M. Abd-Elhakim, Y.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [Green Version]
- Arczewska-Wlosek, A.; Swiatkiewicz, S. The effect of a dietary herbal extract blend on the performance of broilers challenged with Eimeria oocysts. J. Anim. Feed Sci 2012, 21, 133–142. [Google Scholar] [CrossRef]
- Khan, R.; Nikousefat, Z.; Tufarelli, V.; Naz, S.; Javdani, M.; Laudadio, V. Garlic (Allium sativum) supplementation in poultry diets: Effect on production and physiology. World’s Poult. Sci. J. 2012, 68, 417–424. [Google Scholar] [CrossRef]
- Molan, A.-L.; Faraj, A.M. Effect of selenium-rich green tea extract on the course of sporulation of Eimeria oocysts. J. Dent. Med. Sci. 2015, 14, 68–74. [Google Scholar]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial Properties of Organosulfur Compounds of Garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I.O. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef]






| Compound | Conc. (mg/mL) | Oocysts Reduction % | Sporulation Dynamic | |
|---|---|---|---|---|
| S% | SI% | |||
| Control (K2Cr2O7) | 25 | 00.0 ± 0.0 | 51.2 ± 3.6 a | 00.0 ± 0.0 |
| Allicin | 45 | 56.6 ± 2.1 c | 27.4 ± 8.7 bc | 46.5 ± 1.4 b |
| 90 | 72.7 ± 1.7 b | 26.1 ± 1.2 c | 49.0 ± 2.1 b | |
| 180 | 88.3 ± 1.2 a | 15.9 ± 0.9 d | 68.9 ± 2.1 a | |
| Garlic | 90 | 26.8 ± 0.9 d | 28.2 ± 1.7 bc | 44.9 ± 3.6 b |
| 180 | 38.4 ± 1.2 d | 27.2 ± 1.6 bc | 46.9 ± 1.0 b | |
| 360 | 73.5 ± 2.2 b | 13.6 ± 1.6 d | 73.4 ± 2.8 a | |
| KOH | 50 | 67.4 ± 1.5 b | 35.6 ± 1.5 b | 30.5 ± 2.4 c |
| Compound | Conc (mg/mL) | Oocysts Deformity % | |||
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| Non-Sporulated | Sporulated | Non-Sporulated | Sporulated | ||
| Control (K2Cr2O7) | 25 | 1 | 0 | 0 | 0 |
| Allicin | 45 | 35.1 | 0.90 | 32.3 | 21 |
| 90 | 46.1 | 2.10 | 34.8 | 26.7 | |
| 180 | 51.9 | 5.70 | 41 | 32.3 | |
| Garlic | 90 | 3.4 | 0.20 | 21.7 | 13.6 |
| 180 | 6 | 2.40 | 28.3 | 22.8 | |
| 360 | 14.3 | 3.9 | 44 | 24 | |
| KOH | 50 | 9.3 | 0.00 | 11.5 | 11.5 |
| Lesions | Negative Control | Infected with Non-Treated Oocysts | Infected with Allicin-Treated Oocysts | Infected with Garlic Extract-Treated Oocysts | Infected with KOH-Treated Oocysts |
|---|---|---|---|---|---|
| Epithelial necrosis | 0 | 5 | 1 | 3 | 4 |
| Hemorrhages | 0 | 5 | 0 | 0 | 0 |
| Eosinophils infiltration | 0 | 5 | 0 | 0 | 0 |
| Mononuclear cells infiltration | 0 | 5 | 2 | 3 | 3 |
| Coccidial stages | 0 | 5 | 1 | 3 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ELrahman, S.M.; Mohamed, S.A.-A.; Mohamed, S.E.; El-Khadragy, M.F.; Dyab, A.K.; Hamad, N.; Safwat, M.M.; Nasr, A.A.E.; Alkhaldi, A.A.M.; Gareh, A.; et al. Comparative Effect of Allicin and Alcoholic Garlic Extract on the Morphology and Infectivity of Eimeria tenella Oocysts in Chickens. Animals 2022, 12, 3185. https://doi.org/10.3390/ani12223185
Abd-ELrahman SM, Mohamed SA-A, Mohamed SE, El-Khadragy MF, Dyab AK, Hamad N, Safwat MM, Nasr AAE, Alkhaldi AAM, Gareh A, et al. Comparative Effect of Allicin and Alcoholic Garlic Extract on the Morphology and Infectivity of Eimeria tenella Oocysts in Chickens. Animals. 2022; 12(22):3185. https://doi.org/10.3390/ani12223185
Chicago/Turabian StyleAbd-ELrahman, Salwa Mahmoud, Sara Abdel-Aal Mohamed, Samar Elsayed Mohamed, Manal F. El-Khadragy, Ahmed Kamal Dyab, Nashwa Hamad, Marwa M. Safwat, Asmaa A. E. Nasr, Abdulsalam A. M. Alkhaldi, Ahmed Gareh, and et al. 2022. "Comparative Effect of Allicin and Alcoholic Garlic Extract on the Morphology and Infectivity of Eimeria tenella Oocysts in Chickens" Animals 12, no. 22: 3185. https://doi.org/10.3390/ani12223185






