Effects of Dietary Oregano Essential Oil on Cecal Microorganisms and Muscle Fatty Acids of Luhua Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Moral Statement
2.2. Experimental Design
2.3. Experimental Diet and Feeding Management
2.3.1. Diet Composition and Nutrient Level
2.3.2. Experimental Animal Feeding and Management
2.4. Collection and Treatment of Test Samples
2.4.1. Collection and Processing of Muscle Tissue Samples
2.4.2. Collection of Cecal Microbial Samples
2.5. Experimental Methods
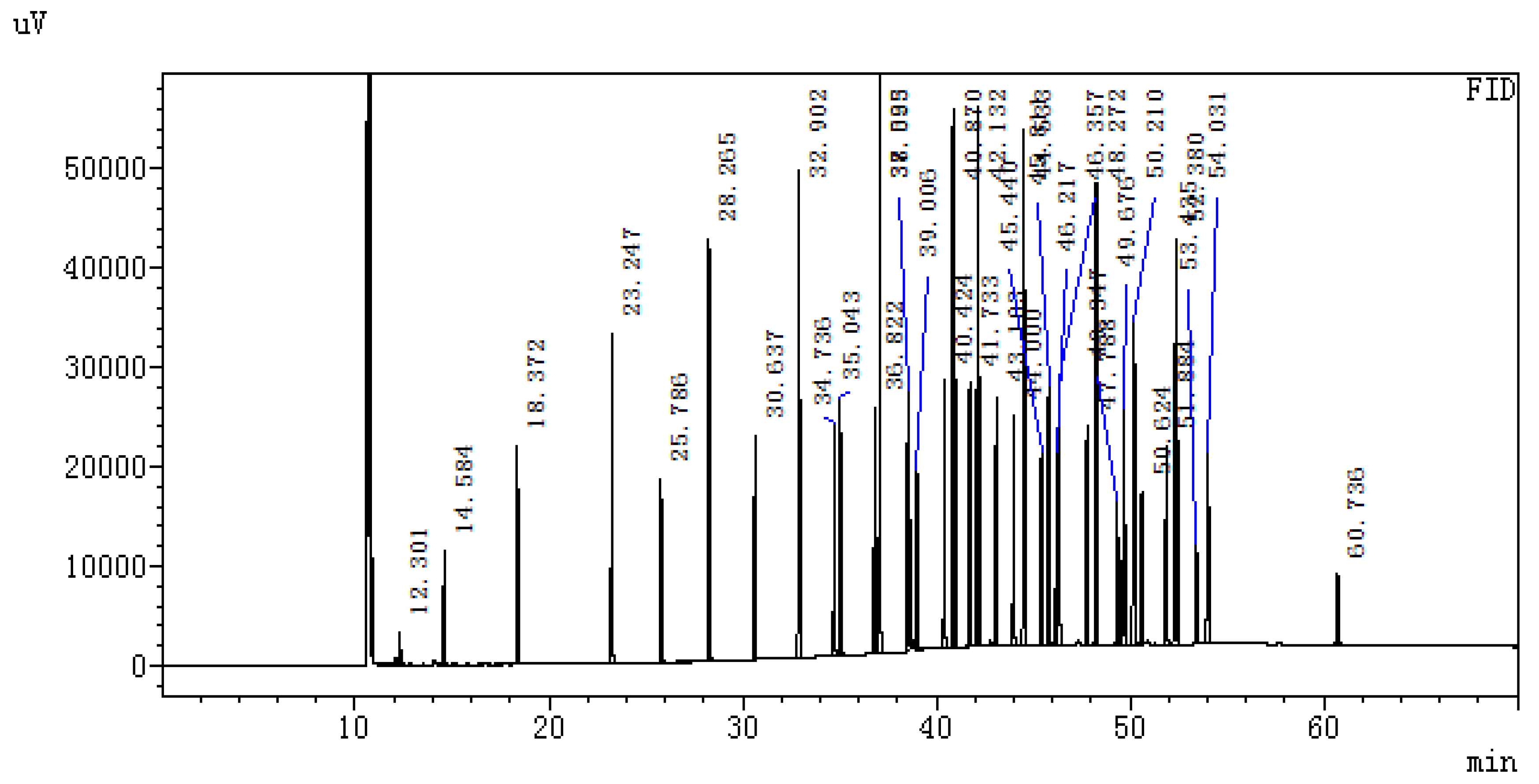
2.5.1. Determination of Fatty Acid Content in Muscle
Chromatographic Condition
2.5.2. Total DNA Extraction, PCR Amplification and 16S rRNA High−Throughput Sequencing
Extraction of Total DNA
PCR Amplification
Mixing and Purification of PCR Products
Library Construction and Computer Sequencing
2.5.3. Bioinformatics Analysis
2.5.4. Statistical Analysis
3. Results
3.1. Effects of Dietary Oregano Essential Oil on Fatty Acids in Breast Muscle of Luhua Chickens
3.2. Effects of Dietary Oregano Essential Oil on Muscle Fatty Acids of Luhua Chicken Legs
3.3. Cecal Microbial Diversity
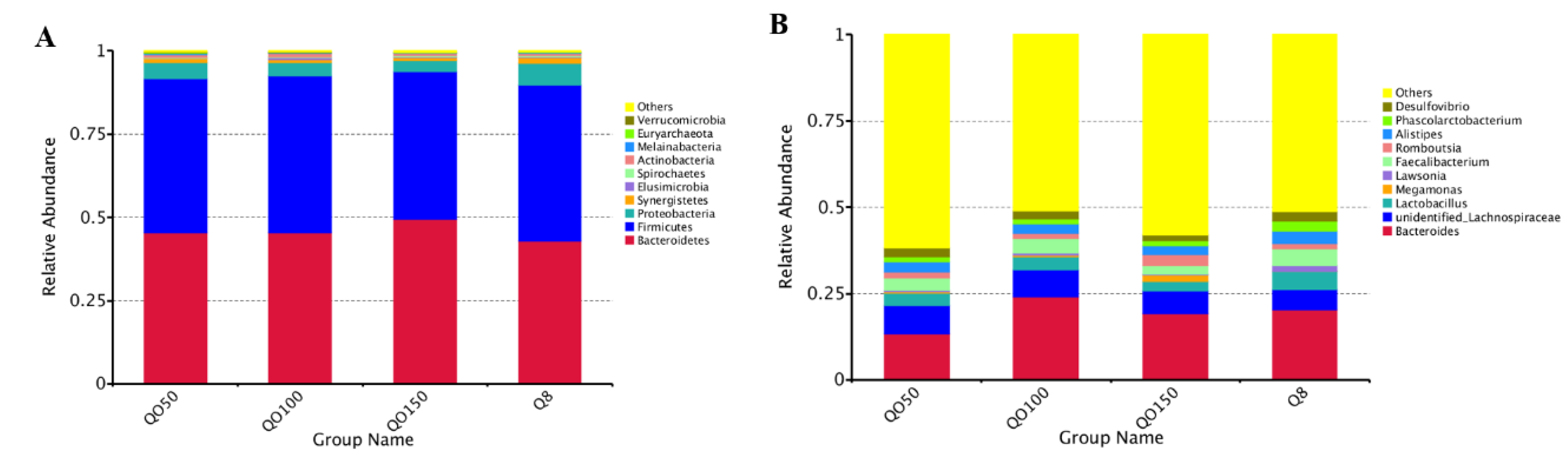
3.4. Cecal Microbial Species Composition and Abundance
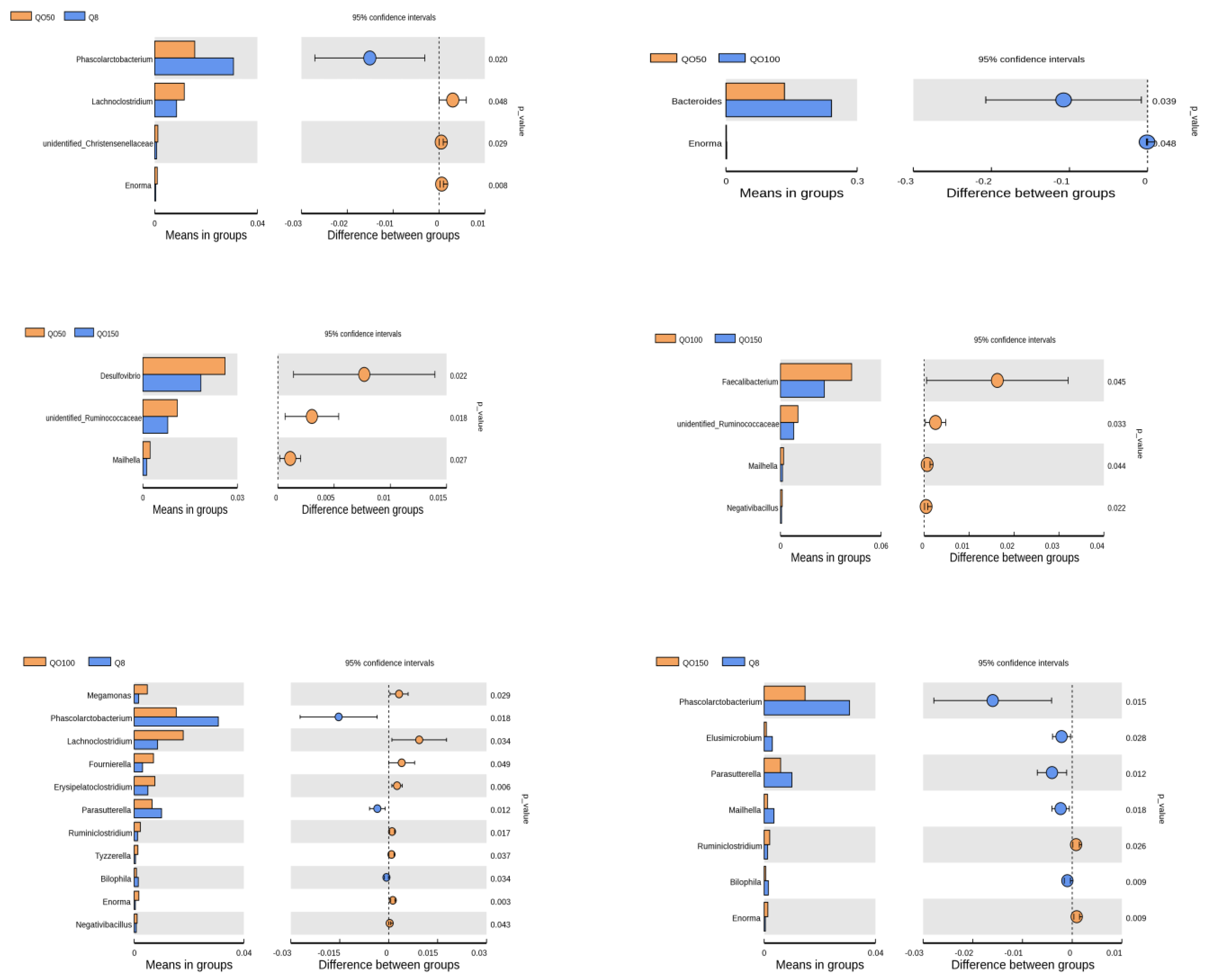
3.5. Analysis of Significant Differences among Species
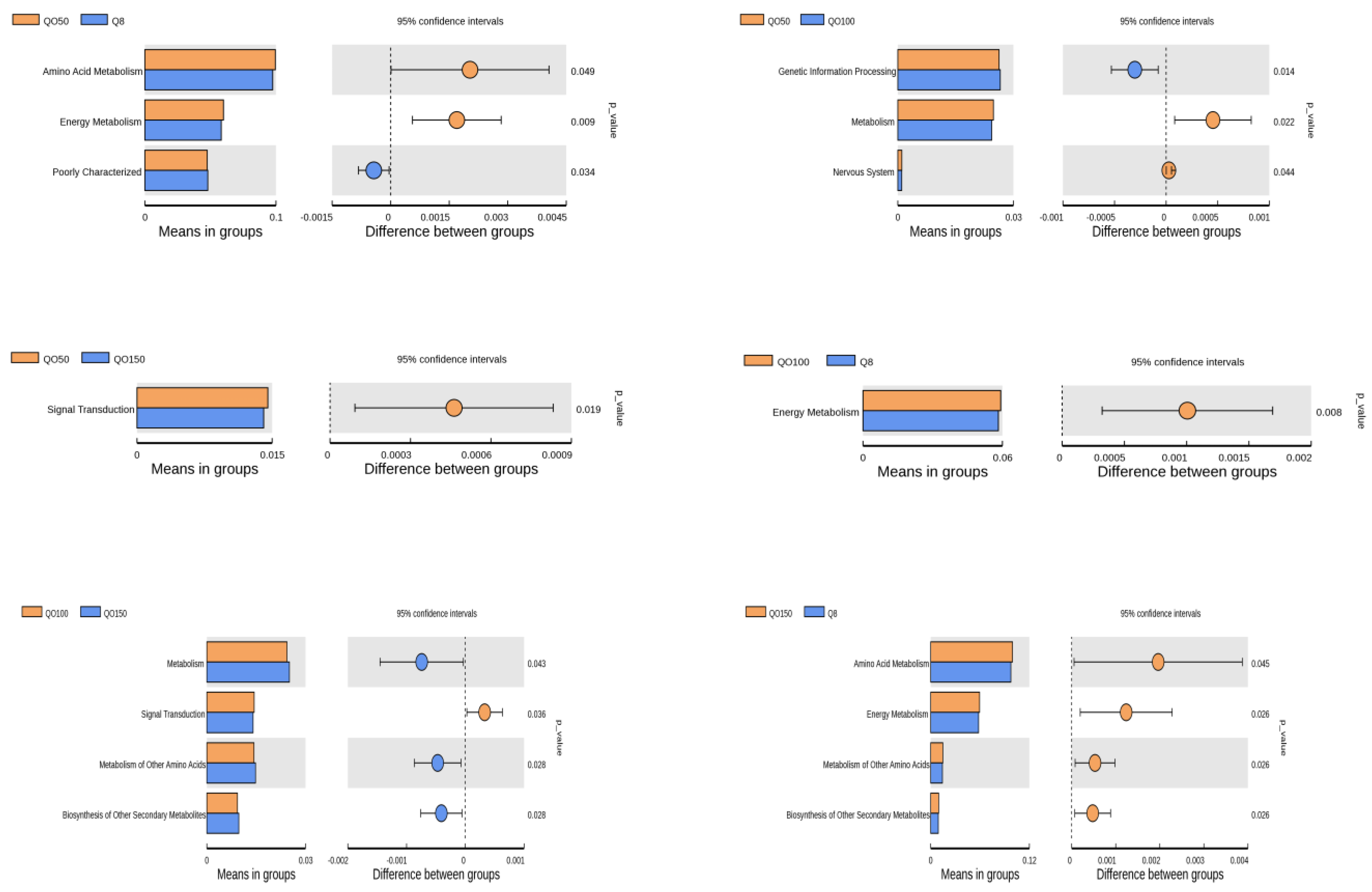
3.6. Prediction of Cecal Microbial Function
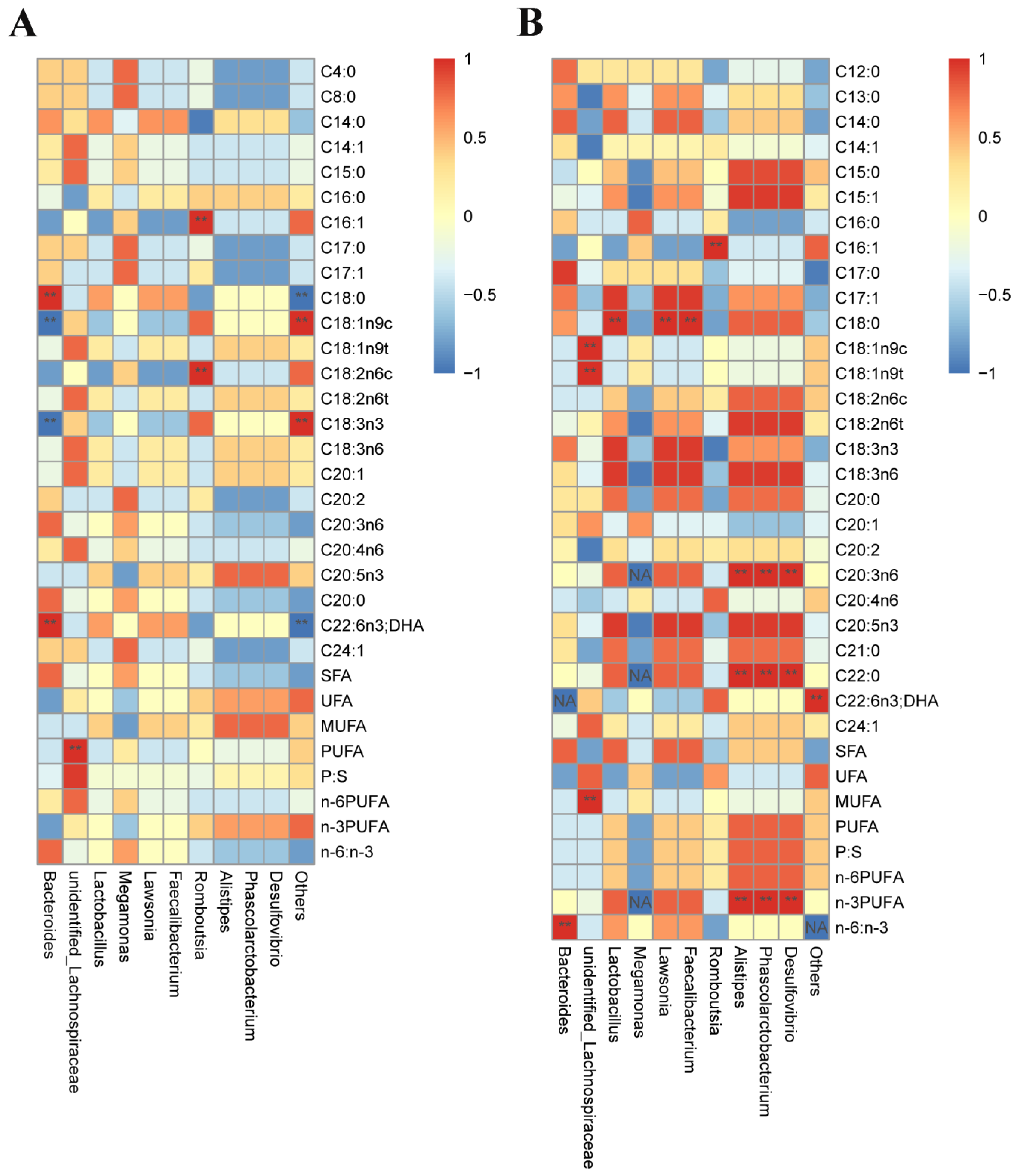
3.7. Correlation Analysis
4. Discussion
4.1. Effects of Oregano Essential Oil on Muscle Fatty Acids of Luhua Chicken
4.2. Effects of Oregano Essential Oil on Cecal Microflora of Luhua Chickens
4.3. Correlation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-Paneri, P. Oregano: A Feed Additive With Functional Properties-ScienceDirect. Therapeutic Foods 2018, 179–208. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch Tierernahr. 2003, 57, 99–106. [Google Scholar] [PubMed]
- Skoufos, I.; Giannenas, I.; Tontis, D.; Bartzanas, T.; Kittas, C.; Panagakis, P.; Tzora, A. Effects of oregano essential oil and attapulgite on growth performance, intestinal microbiota and morphometry in broilers. S. Afr. J. Anim. Sci. 2016, 46, 77–88. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Florou-Paneri, P.; Palatos, G.; Govaris, A.; Botsoglou, D.; Giannenas, I.; Ambrosiadis, I. Oregano herb versus oregano essential oil as feed supplements to increase the oxidative stability of turkey. Int. J. Poult. Sci. 2005, 4, 866–871. [Google Scholar]
- Giannenas, I.; Florou-Paneri, P.; Botsoglou, N.A.; Christaki, E.; Spais, A.B. Effect of supplementing feed with oregano and/or α-tocopheryl acetate on growth of broiler chickens and oxidative stability of meat. J. Anim. Feed Sci. 2005, 14, 521–535. [Google Scholar] [CrossRef]
- Tsinas, A.; Giannenas, I.; Voidarou, C.; Tzora, A.; Skoufos, I. Effects of an oregano based dietary supplement on performance of broiler chickens experimentally infected with Eimeria acervulina and Eimeria maxima. J. Poult. Sci. 2011, 48, 194–200. [Google Scholar] [CrossRef]
- Falcinelli, S.; Picchietti, S.; Rodiles, A.; Cossignani, L.; Merrifield, D.L.; Taddei, A.R.; Maradonna, F.; Olivotto, I.; Gioacchini, G.; Carnevali, O. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 2015, 5, 9336. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Ruan, D.; Fan, Q.; Fouad, A.M.; Sun, Y.; Huang, S.; Wu, A.; Lin, C.; Kuang, Z.; Zhang, C.; Jiang, S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021, 99, skab033. [Google Scholar] [CrossRef]
- Bauer, B.W.; Radovanovic, A.; Willson, N.L.; Bajagai, Y.S.; Hao, V.T.T.; Moore, R.J.; Stanley, D. Oregano: A potential prophylactic treatment for the intestinal microbiota. Heliyon 2019, 5, e02625. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D. Diversity and function of the avian gut microbiota. J. Comp. Physiol. B 2012, 182, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.A.; Bedford, M.R.; Penn, C.W.; Pallen, M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 2014, 9, e91941. [Google Scholar] [CrossRef] [PubMed]
- Clench, M.; Mathias, J. The avian cecum: A review. Wilson Bull. 1995, 107, 93–121. [Google Scholar]
- Chaplin, S.B. Effect of cecectomy on water and nutrient absorption of birds. J. Exp. Zool. Suppl. 1989, 3, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, A.; Horgan, K.; Clipson, N.; Murphy, R.A. Effect of dietary supplementation with a Saccharomyces cerevisiae mannan oligosaccharide on the bacterial community structure of broiler cecal contents. Appl. Environ. Microbiol. 2011, 77, 6653–6662. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Pan, X.; Xu, T.; Zhang, Z.; Zi, X.; Jiang, Y. Cassava foliage affects the microbial diversity of Chinese indigenous geese caecum using 16S rRNA sequencing. Sci. Rep. 2017, 7, 45697. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Li, N.; Zhong, H.; Xu, H.; Zhu, Q.; Liu, Y. Comparative Analysis of the Gut Microbial Composition and Meat Flavor of Two Chicken Breeds in Different Rearing Patterns. Biomed Res. Int. 2018, 2018, 4343196. [Google Scholar] [CrossRef]
- ESPGHAN Committee on Nutrition; Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; Szajewska, H.; et al. Supplementation of N-3 LCPUFA to the diet of children older than 2 years: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 2–10. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.; Czauderna, M.; Smulikowska, S. The enrichment of chicken meat with omega-3 fatty acids by dietary fish oil or its mixture with rapeseed or flaxseed-Effect of feeding duration: Dietary fish oil, flaxseed, and rapeseed and n-3 enriched broiler meat. Anim. Feed Sci. Technol. 2017, 223, 42–52. [Google Scholar] [CrossRef]
- Kırkpınar, F.; Ünlü, H.B.; Özdemir, G. Effects of oregano and garlic essential oils on performance, carcase, organ and blood characteristics and intestinal microflora of broilers. Livest. Sci. 2011, 137, 219–225. [Google Scholar] [CrossRef]
- Nowers, N. The Effect of an Oregano Oil Extract in a Lactating Dairy Cow Diet on Production Responses of Holstein Cows; Stellenbosch Stellenbosch University: Stellenbosch, South Africa, 2016; pp. 13–17. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Monk, J.M.; Mcmurray, D.N.; Chapkin, R.S. 2-clinical effects of n-3 PUFA supplementation in human health and inflammatory diseases. In Omega-3 Oils; AOCS Press: Urbana, IL, USA, 2011; pp. 31–60. [Google Scholar]
- Saleh, A.A.; Eid, Y.Z.; Ebeid, T.A.; Ohtsuka, A.; Hioki, K.; Yamamoto, M.; Hayashi, K. The modification of the muscle fatty acid profile by dietary supplementation with Aspergillus awamori in broiler chickens. Br. J. Nutr. 2012, 108, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Scaife, J.R.; Moyo, J.; Galbraith, H.; Michie, W.; Campbell, V. Effect of different dietary supplemental fats and oils on the tissue fatty acid composition and growth of female broilers. Br. Poult. Sci. 2007, 35, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Dong, C.; Xiaoyan, W.; Qian, G.; Huifen, D.; Jie, L.; Kangle, Y.; Ao, S.; Kun, C.; Qingwu, S. Muscle Fatty Acids, Meat Flavor Compounds and Sensory Characteristics of Xiangxi Yellow Cattle in Comparison to Aberdeen Angus. Animals 2022, 12, 1161. [Google Scholar]
- Liu, L.S.; Liu, T.; Zhou, R.; Wu, J.P. Effects of adding oregano oil to ration on beef fatty acid content and oxidative stability of the beef. J. Gansu Agric. Univ. 2017, 52, 23–29. [Google Scholar]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 545, 556–568. [Google Scholar] [CrossRef]
- Wood, J.D.; Lambe, N.R.; Walling, G.A.; Whitney, H.; Jagger, S.; Fullarton, P.J.; Bayntun, J.; Hallett, K.; Bünger, L. Effects of low protein diets on pigs with a lean genotype. 1. Carcass composition measured by dissection and muscle fatty acid composition. Meat Sci. 2013, 95, 123–128. [Google Scholar] [CrossRef]
- James, L. Chapter 1 Quinoa (Chenopodium quinoa Willd.): Composition, Chemistry, Nutritional, and Functional Properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Simopoulos A, P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Smet, S.D.; Raes, K.; Demeyer, D. Meat fatty acid composition as affected by fatness and genetic factors: A review. Anim. Res. 2004, 53, 81–98. [Google Scholar] [CrossRef]
- Zotte, A.D.; Szendro, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.Y.; He, Y.N.; Wang, Z.H.; Yu, W.T.; Yang, X.G. The status and trends of dietary nutrients intake of Chinese population. Acta Nutr. Sin. 2005, 8, 215–222. [Google Scholar]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Degolier, T.; Mahoney, S.; Duke, G. Relationships of Avian Cecal Lengths to Food Habits, Taxonomic Position, and Intestinal Lengths. Condor 1999, 101, 622–634. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Shaufi, M.A.M.; Sieo, C.C.; Chong, C.W.; Gan, H.M.; Ho, Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Shabbir, M.Z.; Ijaz, A.; Rehman, H. Molecular analysis of the caecal and tracheal microbiome of heat-stressed broilers supplemented with prebiotic and probiotic. Avian Pathol. 2015, 44, 67–74. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef]
- Delport, T.C.; Power, M.L.; Harcourt, R.G.; Webster, K.N.; Tetu, S.G. Colony Location and Captivity Influence the Gut Microbial Community Composition of the Australian Sea Lion (Neophoca cinerea). Appl. Environ. Microbiol. 2016, 82, 3440–3449. [Google Scholar] [CrossRef]
- Dong, X.Y.; Azzam, M.M.M.; Zou, X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017, 96, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Ahir, V.B.; Koringa, P.G.; Bhatt, V.D.; Ramani, U.V.; Tripathi, A.K.; Singh, K.M.; Dhagat, U.M.; Patel, J.S.; Patel, M.M.; Katudia, K.H.; et al. Metagenomic analysis of poultry gut microbes. Indian J. Poult. Sci. 2010, 45, 111–114. [Google Scholar]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.R.; Sarles, W.B.; Shapiro, S.K. The Intestinal Microflora of Hens as Influenced by Various Carbohydrates in a Biotin-deficient Ration. J. Bacteriol. 1948, 56, 619–634. [Google Scholar] [CrossRef]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE 2017, 9, e85423. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Verstegen, M.; Tamminga, S.; Williams, B.A.; Boer, H. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Sodhi, N.; Chenu, J.W.; Cox, J.M.; Riordan, S.M.; Mitchell, H.M. The interplay between Campylobacter and Helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog. 2014, 6, 18. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Filippidou, S.; Wunderlin, T.; Junier, T.; Junier, T.; Jeanneret, N.; Dorador, C.; Molina, V.; Johnson, D.; Junier, P. A combination of extreme environmental conditions favor the prevalence of Endospore-forming Firmicutes. Front. Microbiol. 2016, 7, 1707. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Lee, T.K.; Sul, W.J. Metagenomic Analysis of Chicken Gut Microbiota for Improving Metabolism and Health of Chickens—A Review. Asian-Australas J. Anim. Sci. 2015, 28, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xiong, X.; Su, Y.; Huang, L.; Chen, C. 16S rRNA gene-based association study identified microbial taxa associated with pork intramuscular fat content in feces and cecum lumen. BMC Microbiol. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.; Martinko, J. (Eds.) Brock Biology of Microorganisms, 11th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Wang, B.H.; Wang, Y.; Rui, D.U.; Luo, Y.L.; Liu, C.; Yao, D.; Jin, Y. Research of Fatty Acid Deposition Mechanism in Adipose Tissue of Sunit Sheep. Sci. Technol. Food Ind. 2019, 40, 11–16. [Google Scholar]


| Items | Diets (%) | |||
|---|---|---|---|---|
| Q8 | QO50 | QO100 | QO150 | |
| Corn | 64.00 | 64.00 | 64.00 | 64.00 |
| Wheat middling | 4.00 | 4.00 | 4.00 | 4.00 |
| Quinoa seeds | 8.00 | 8.00 | 8.00 | 8.00 |
| Soybean meal | 20.00 | 20.00 | 20.00 | 20.00 |
| Limestone | 1.20 | 1.20 | 1.20 | 1.20 |
| CaHPO4 | 1.20 | 1.20 | 1.20 | 1.20 |
| NaCl | 0.30 | 0.30 | 0.30 | 0.30 |
| 1% Premix (1) | 1.00 | 1.00 | 1.00 | 1.00 |
| Oregano oil | 0.005 | 0.01 | 0.015 | |
| zeolite powder | 0.30 | 0.295 | 0.29 | 0.285 |
| Total | 100 | 100 | 100 | 100 |
| Nutrient levels | ||||
| Metabolic energy, ME (MJ/Kg) | 12.40 | 12.40 | 12.40 | 12.40 |
| Dry matter, DM | 84.66 | 84.66 | 84.66 | 84.66 |
| Crude protein, CP | 16.25 | 16.25 | 16.25 | 16.25 |
| Crude fibre, CF | 2.74 | 2.74 | 2.74 | 2.74 |
| Ether extract, EE | 3.10 | 3.10 | 3.10 | 3.10 |
| Calcium, Ca | 0.88 | 0.88 | 0.88 | 0.88 |
| Total phosphorus, TP | 0.58 | 0.58 | 0.58 | 0.58 |
| Available phosphorus, AP | 0.20 | 0.20 | 0.20 | 0.20 |
| Lysine, Lys | 0.84 | 0.84 | 0.84 | 0.84 |
| Methionine, Met | 0.33 | 0.33 | 0.33 | 0.33 |
| Methionine + Cystine, Met + Cys | 0.61 | 0.61 | 0.61 | 0.61 |
| Threonine, Thr | 0.62 | 0.62 | 0.62 | 0.62 |
| Tryptophan, Trp | 0.18 | 0.18 | 0.18 | 0.18 |
| Items | Group | p-Values | |||
|---|---|---|---|---|---|
| Q8 | QO50 | QO100 | QO150 | ||
| Butyric acid (C4:0) | 0.18 ± 0.06 b | 0.32 ± 0.06 a | 0.31 ± 0.07 a | 0.32 ± 0.09 a | <0.001 |
| Octanoic acid (C8:0) | 0.16 ± 0.03 | 0.21 ± 0.07 | 0.22 ± 0.06 | 0.20 ± 0.05 | 0.277 |
| Myristic acid (C14:0) | 0.64 ± 0.09 | 0.64 ± 0.06 | 0.58 ± 0.11 | 0.60 ± 0.06 | 0.152 |
| Myristoleic acid (C14:1) | 0.21 ± 0.03 c | 0.23 ± 0.03 b | 0.35 ± 0.04 a | 0.22 ± 0.03 bc | <0.001 |
| Pentadecanoic acid (C15:0) | 2.83 ± 0.62 | 3.06 ± 0.56 | 2.87 ± 0.88 | 2.67 ± 0.82 | 0.602 |
| Palmitic acid (C16:0) | 24.88 ± 3.03 b | 24.34 ± 0.87 b | 24.43 ± 0.79 b | 26.27 ± 1.24 a | 0.004 |
| Palmitoleic acid (C16:1) | 5.46 ± 0.92 | 6.11 ± 1.45 | 5.47 ± 1.02 | 5.56 ± 0.63 | 0.182 |
| Heptadecanoic acid (C17:0) | 0.41 ± 0.10 | 0.37 ± 0.09 | 0.37 ± 0.10 | 0.31 ± 0.10 | 0.810 |
| Heptadecenoic acid (C17:1) | 0.69 ± 0.06 | 0.70 ± 0.24 | 0.71 ± 0.17 | 0.80 ± 0.17 | 0.460 |
| Stearic acid (C18:0) | 8.40 ± 1.21 | 8.01 ± 1.00 | 8.24 ± 0.79 | 8.00 ± 0.88 | 0.543 |
| Oleic acid (C18:1 n9c) | 31.70 ± 4.22 | 32.35 ± 3.83 | 31.25 ± 3.70 | 31.63 ± 3.28 | 0.839 |
| Elaidic acid (C18:1n9t) | 0.49 ± 0.07 d | 1.74 ± 0.08 a | 1.53 ± 0.08 c | 1.67 ± 0.09 b | <0.001 |
| Linoleic acid (C18:2n6c) | 12.23 ± 1.39 b | 12.35 ± 0.78 ab | 12.68 ± 0.85 ab | 13.02 ± 1.21 a | 0.032 |
| Linolelaidic acid (C18:2n6t) | 0.13 ± 0.01 b | 0.16 ± 0.01 a | 0.14 ± 0.01 b | 0.09 ± 0.002 c | <0.001 |
| Linolenic acid (C18:3n3) | 0.69 ± 0.08 | 0.74 ± 0.17 | 0.69 ± 0.06 | 0.75 ± 0.08 | 0.164 |
| γ-linoleic acid (C18:3n6) | 0.16 ± 0.03 | 0.18 ± 0.08 | 0.17 ± 0.02 | 0.14 ± 0.02 | 0.699 |
| Eicosenoic acid (C20:1) | 0.42 ± 0.04 | 0.43 ± 0.09 | 0.36 ± 0.12 | 0.37 ± 0.09 | 0.113 |
| Eicosadienoic acid (C20:2) | 0.45 ± 0.04 b | 0.56 ± 0.04 a | 0.38 ± 0.09 c | 0.41 ± 0.07 bc | <0.001 |
| Eicostrienoic acid (C20:3n6) | 0.56 ± 0.13 | 0.64 ± 0.11 | 0.53 ± 0.14 | 0.52 ± 0.16 | 0.302 |
| Arachidonic acid (AA,C20:4n6) | 4.76 ± 1.53 | 5.84 ± 1.52 | 5.65 ± 1.65 | 4.79 ± 1.52 | 0.133 |
| Eicosapentaenoic acid(EPA,C20:5n3) | 1.07 ± 0.12 b | 1.19 ± 0.23 a | 1.18 ± 0.09 a | 1.16 ± 0.12 a | 0.039 |
| Behenic acid (C22:0) | 0.36 ± 0.07 a | 0.30 ± 0.07 b | 0.36 ± 0.08 ab | 0.36 ± 0.08 ab | 0.045 |
| Docosahexaenoic acid (DHA, C22:6n3) | 0.67 ± 0.14 | 0.69 ± 0.22 | 0.67 ± 0.14 | 0.70 ± 0.14 | 0.939 |
| Nervonic acid (C24:1) | 1.55 ± 0.26 | 1.71 ± 0.39 | 1.66 ± 0.30 | 1.75 ± 0.35 | 0.466 |
| Saturated fatty acid (SFA) | 38.55 ± 1.62 a | 36.75 ± 2.05 c | 36.92 ± 1.80 bc | 38.13 ± 1.82 ab | 0.009 |
| Unsaturated fatty acid (UFA) | 62.50 ± 4.65 | 63.35 ± 2.32 | 63.24 ± 2.14 | 61.15 ± 3.28 | 0.153 |
| Monounsaturated fatty acids (MUFAs) | 42.47 ± 6.69 | 43.07 ± 4.61 | 41.31 ± 4.44 | 40.56 ± 4.15 | 0.429 |
| Polyunsaturated fatty acids (PUFAs) | 20.03 ± 2.48 b | 20.28 ± 2.40 ab | 21.93 ± 2.39 a | 20.58 ± 3.18 ab | 0.041 |
| n-3 Polyunsaturated fatty acids (n-3 PUFAs) | 2.45 ± 0.24 | 2.51 ± 0.49 | 2.53 ± 0.14 | 2.54 ± 0.44 | 0.876 |
| n-6 Polyunsaturated fatty acids (n-6 PUFAs) | 17.44 ± 1.93 | 17.44 ± 2.47 | 19.01 ± 2.37 | 17.92 ± 2.48 | 0.150 |
| n-6PUFA/n-3PUFA | 7.12 ± 0.45 ab | 6.67 ± 1.08 b | 7.50 ± 0.79 a | 7.04 ± 0.50 ab | 0.019 |
| PUFA/SFA | 0.54 ± 0.06 b | 0.55 ± 0.04 b | 0.59 ± 0.04 a | 0.53 ± 0.09 b | 0.015 |
| Items | Group | p-Values | |||
|---|---|---|---|---|---|
| Q8 | QO50 | QO100 | QO150 | ||
| Lauric acid (C12:0) | 0.04 ± 0.003 | 0.10 ± 0.11 | 0.05 ± 0.02 | 0.04 ± 0.003 | 0.246 |
| Tridecylic acid (C13:0) | 0.37 ± 0.02 b | 0.58 ± 0.08 a | 0.52 ± 0.07 a | 0.41 ± 0.12 b | <0.001 |
| Myristic acid (C14:0) | 0.63 ± 0.11 b | 0.67 ± 0.06 ab | 0.74 ± 0.06 a | 0.72 ± 0.09 a | 0.002 |
| Tetradecenoic acid (C14:1) | 0.44 ± 0.03 a | 0.32 ± 0.04 b | 0.20 ± 0.03 d | 0.24 ± 0.06 c | <0.001 |
| Pentadecanoic acid (C15:0) | 0.10 ± 0.01 a | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.08 ± 0.01 b | 0.002 |
| Pentadecenic acid (C15:1) | 0.35 ± 0.05 a | 0.27 ± 0.04 b | 0.12 ± 0.02 c | 0.15 ± 0.05 c | <0.001 |
| Palmitic acid (C16:0) | 26.06 ± 1.29 b | 25.78 ± 2.14 b | 27.20 ± 0.92 a | 27.43 ± 1.48 a | 0.002 |
| Palmitoleic acid (C16:1) | 7.63 ± 1.02 a | 6.56 ± 1.46 b | 6.47 ± 0.55 b | 7.18 ± 0.99 ab | 0.004 |
| Heptadecanoic acid (C17:0) | 0.12 ± 0.02 ab | 0.11 ± 0.02 b | 0.13 ± 0.03 a | 0.12 ± 0.01 ab | 0.023 |
| Heptadecenoic acid (C17:1) | 0.28 ± 0.02 a | 0.15 ± 0.03 b | 0.16 ± 0.02 b | 0.15 ± 0.02 b | <0.001 |
| Stearic acid (C18:0) | 7.19 ± 1.02 | 7.44 ± 0.90 | 7.54 ± 0.48 | 7.16 ± 1.12 | 0.598 |
| Oleic acid (C18:1n9c) | 33.12 ± 2.80 | 35.02 ± 2.70 | 34.51 ± 1.01 | 33.63 ± 3.07 | 0.166 |
| Elaidic acid (C18:1n9t) | 0.42 ± 0.03 c | 0.61 ± 0.09 a | 0.56 ± 0.1 b | 0.44 ± 0.04 c | <0.001 |
| Linoleic acid (C18:2n6c) | 14.93 ± 1.13 | 14.75 ± 1.12 | 14.77 ± 0.67 | 15.27 ± 1.33 | 0.477 |
| Linolelaidic acid (C18:2n6t) | 0.09 ± 0.02 | 0.09 ± 0.03 | 0.06 ± 0.01 | 0.09 ± 0.14 | 0.933 |
| Linolenic acid (C18:3n3) | 1.38 ± 0.07 a | 1.37 ± 0.11 a | 1.28 ± 0.05 b | 1.20 ± 0.08 c | <0.001 |
| γ-linoleic acid (C18:3n6) | 0.19 ± 0.04 a | 0.17 ± 0.03 a | 0.17 ± 0.02 ab | 0.15 ± 0.03 b | 0.003 |
| Arachidic acid (C20:0) | 0.05 ± 0.01 | 0.05 ± 0.004 | 0.08 ± 0.02 | 0.04 ± 0.009 | 0.173 |
| Eicosenoic acid (C20:1) | 0.41 ± 0.05 a | 0.22 ± 0.03 b | 0.26 ± 0.09 b | 0.22 ± 0.03 b | <0.001 |
| Eicosadienoic acid (C20:2) | 0.16 ± 0.03 a | 0.12 ± 0.03 b | 0.12 ± 0.02 b | 0.11 ± 0.03 b | <0.001 |
| Eicostrienoic acid (C20:3n6) | 0.36 ± 0.1 | 0.34 ± 0.12 | 0.30 ± 0.04 | 0.34 ± 0.14 | 0.567 |
| Arachidonic acid (AA, C20:4n6) | 2.18 ± 0.46 | 2.16 ± 0.58 | 2.43 ± 0.55 | 2.14 ± 0.30 | 0.876 |
| Eicosapentaenoic acid (EPA, C20:5n3) | 0.92 ± 0.07 a | 0.90 ± 0.09 a | 0.80 ± 0.13 b | 0.74 ± 0.09 b | <0.001 |
| Heneicosan oic acid (C21:0) | 0.04 ± 0.002 | 0.04 ± 0.003 | 0.04 ± 0.004 | 0.04 ± 0.005 | 0.434 |
| Behenic acid (C22:0) | 0.15 ± 0.05 | 0.17 ± 0.06 | 0.16 ± 0.04 | 0.15 ± 0.04 | 0.913 |
| Docosahexaenoic acid (C22:6n3, DHA) | 0.84 ± 0.03 a | 0.85 ± 0.1 a | 0.58 ± 0.05 b | 0.57 ± 0.08 b | <0.001 |
| Nervonic acid (C24:1) | 0.60 ± 0.12 a | 0.64 ± 0.18 a | 0.54 ± 0.09 ab | 0.45 ± 0.17 b | 0.001 |
| Saturated fatty acid (SFA) | 34.76 ± 1.43 b | 34.93 ± 2.38 b | 36.43 ± 0.90 a | 36.34 ± 1.31 a | 0.001 |
| Unsaturated fattyacid (UFA) | 65.22 ± 1.44 a | 65.07 ± 2.38 a | 63.57 ± 0.90 b | 63.70 ± 1.33 b | 0.001 |
| Monounsaturated fatty acids (MUFA) | 43.24 ± 2.93 b | 65.07 ± 2.38 a | 42.83 ± 0.94 b | 42.44 ± 3.79 b | <0.001 |
| Polyunsaturated fatty acids (PUFA) | 21.98 ± 2.38 | 21.35 ± 1.83 | 20.74 ± 0.88 | 21.26 ± 3.50 | 0.550 |
| n-3 polyunsaturated fatty acids (n-3 PUFAs) | 3.14 ± 0.13 a | 3.12 ± 0.21 a | 2.51 ± 0.27 b | 2.51 ± 0.18 b | <0.001 |
| n-6 polyunsaturated fatty acids (n-6 PUFAs) | 18.69 ± 2.35 | 18.13 ± 1.73 | 18.05 ± 0.83 | 18.67 ± 3.35 | 0.811 |
| n-6 PUFA/n-3 PUFA | 5.96 ± 0.75 b | 5.82 ± 0.50 b | 6.86 ± 0.27 a | 6.71 ± 0.33 a | <0.001 |
| PUFA/SFA | 0.63 ± 0.07 a | 0.61 ± 0.03 ab | 0.57 ± 0.03 b | 0.59 ± 0.1 ab | 0.044 |
| Items | Sample Group | p-Values | |||
|---|---|---|---|---|---|
| Q8 | QO50 | QO100 | QO150 | ||
| Raw PE | 91,771 ± 6326 | 91,706 ± 4162 | 86,699 ± 5442 | 90,030 ± 5086 | 0.335 |
| Raw Tags | 88,060 ± 6492 | 86,349 ± 5064 | 81,734 ± 5208 | 84,817 ± 4592 | 0.247 |
| Clean Tags | 86,507 ± 6438 | 84,766 ± 4912 | 80,095 ± 5134 | 83,171 ± 4487 | 0.223 |
| Effective Tags | 67,843 ± 4736 | 68,198 ± 3673 | 62,426 ± 3300 | 67,831 ± 4192 | 0.063 |
| Base | 28,334,530 ± 1,964,222 | 28,425,931 ± 1,558,632 | 26,017,239 ± 1,374,162 | 28,328,611 ± 1,802,962 | 0.061 |
| AvgLen | 417.5 ± 1.05 | 416.83 ± 0.41 | 416.67 ± 1.03 | 417.67 ± 1.21 | 0.240 |
| Q20 | 98.45 ± 0.09 | 98.405 ± 0.14 | 98.35 ± 0.06 | 98.35 ± 0.04 | 0.191 |
| Q30 | 94.94 ± 0.22 | 94.84 ± 0.35 | 94.72 ± 0.12 | 94.71 ± 0.08 | 0.276 |
| GC (%) | 53.22 ± 0.41 | 53.26 ± 0.15 | 52.94 ± 0.24 | 53.40 ± 0.35 | 0.100 |
| Effective (%) | 73.98 ± 3.37 | 74.375 ± 2.47 | 72.085 ± 2.94 | 75.35 ± 2.15 | 0.256 |
| Items | Group | p-Values | |||
|---|---|---|---|---|---|
| Q8 | QO50 | QO100 | QO150 | ||
| observed_species | 609.67 ± 42.41 | 594.17 ± 21.57 | 597.50 ± 36.73 | 581.00 ± 27.46 | 0.528 |
| Shannon | 6.16 ± 0.18 | 6.15 ± 0.42 | 6.29 ± 0.38 | 5.97 ± 0.29 | 0.426 |
| Simpson | 0.96 ± 0.01 | 0.95 ± 0.03 | 0.96 ± 0.02 | 0.95 ± 0.01 | 0.572 |
| chao1 | 643.82 ± 44.96 | 621.40 ± 22.59 | 626.14 ± 35.73 | 616.97 ± 27.55 | 0.547 |
| ACE | 647.60 ± 44.69 | 623.57 ± 20.05 | 624.87 ± 33.66 | 615.07 ± 24.75 | 0.364 |
| PD_whole_tree | 39.11 ± 2.25 | 40.94 ± 3.65 | 41.12 ± 4.65 | 38.61 ± 3.05 | 0.516 |
| OTU catalogue | 1032 | Annotated on Class level: | 95.16% |
| Annotated on (database) | 1025 (99.32%) | Annotated on Order level | 91.47% |
| Annotated on Unclassified | 7 (0.68%) | Annotated on Family level | 81.69% |
| Annotated on Kingdom level | 99.32% | Annotated on Genus level | 40.50% |
| Annotated on Phylum level | 97.48% | Annotated on Species level | 14.24% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Yang, F.; Jiao, T.; Zhao, S. Effects of Dietary Oregano Essential Oil on Cecal Microorganisms and Muscle Fatty Acids of Luhua Chickens. Animals 2022, 12, 3215. https://doi.org/10.3390/ani12223215
Wu T, Yang F, Jiao T, Zhao S. Effects of Dietary Oregano Essential Oil on Cecal Microorganisms and Muscle Fatty Acids of Luhua Chickens. Animals. 2022; 12(22):3215. https://doi.org/10.3390/ani12223215
Chicago/Turabian StyleWu, Tao, Farong Yang, Ting Jiao, and Shengguo Zhao. 2022. "Effects of Dietary Oregano Essential Oil on Cecal Microorganisms and Muscle Fatty Acids of Luhua Chickens" Animals 12, no. 22: 3215. https://doi.org/10.3390/ani12223215
APA StyleWu, T., Yang, F., Jiao, T., & Zhao, S. (2022). Effects of Dietary Oregano Essential Oil on Cecal Microorganisms and Muscle Fatty Acids of Luhua Chickens. Animals, 12(22), 3215. https://doi.org/10.3390/ani12223215






