Relationship between the Dynamics of Gross Composition, Free Fatty Acids and Biogenic Amines, and Microbial Shifts during the Ripening of Raw Ewe Milk-Derived Idiazabal Cheese
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Sampling
2.2. Analysis of Gross Composition
2.3. Analysis of Free Fatty Acids
2.4. Analysis of Biogenic Amines
2.5. Statistical Analysis
2.6. Correlation with Bacterial Communities
3. Results
3.1. Changes in Gross Composition
3.2. Changes in Free Fatty Acids
3.3. Changes in Biogenic Amines
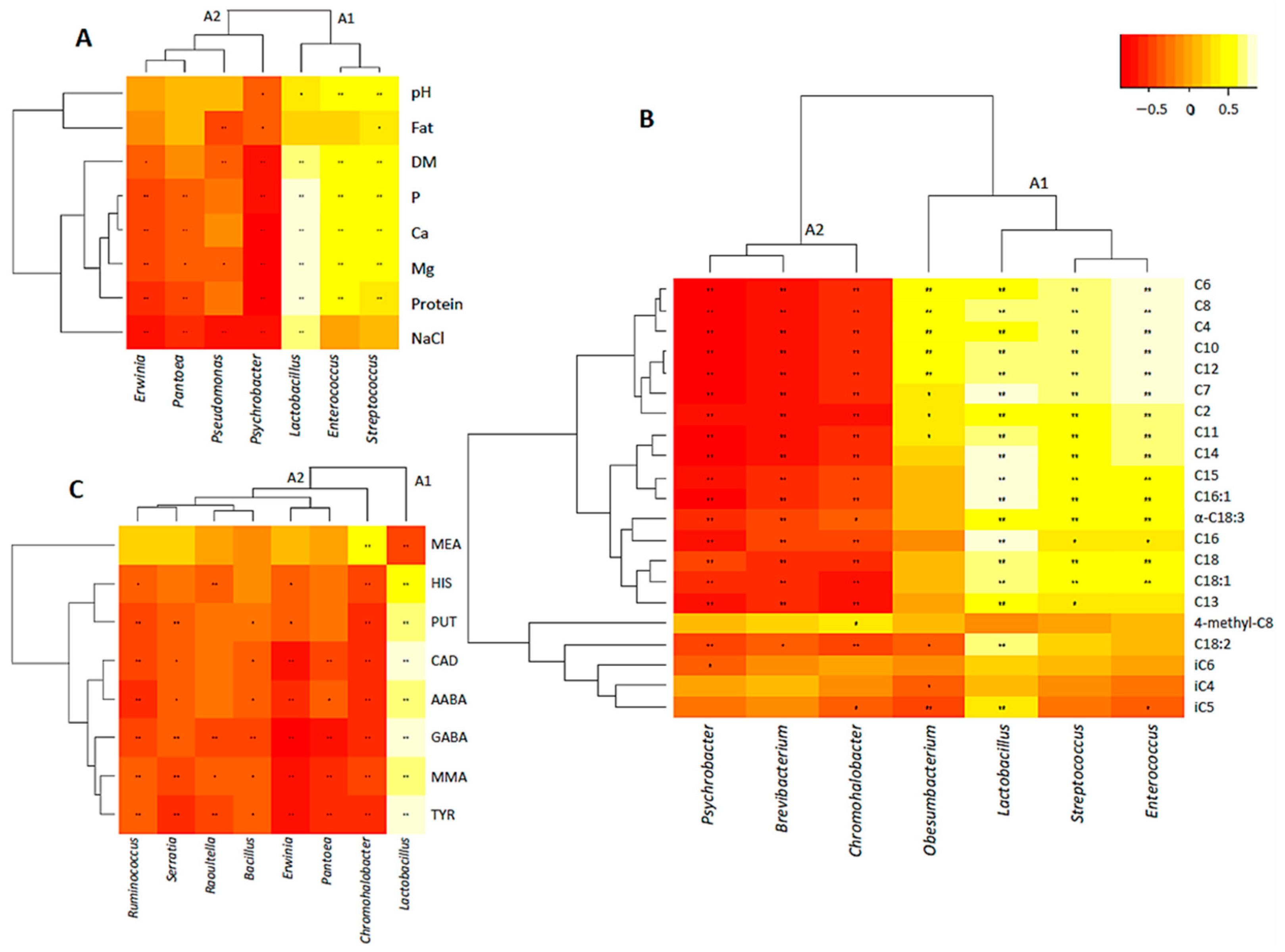
3.4. Correlation between Bacterial Dynamics and Physico-Chemical Parameters Evolution
3.4.1. Correlation between Main Bacterial Genera and Gross Composition
3.4.2. Correlation between Main Bacterial Genera and FFAs
3.4.3. Correlation between Main Bacterial Genera and BAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Official Journal of the European Communities. Reglamento (CE) N° 1107/96 de La Comisión de 12 de Junio de 1996 Relativo Al Registro de Las Indicaciones Geográficas y de Las Denominaciones de Origen Con Arreglo Al Procedimiento Establecido En El Artículo 17 Del Reglamento (CEE) No 2081/92 Del Consejo. Off. J. Eur. Union 1996, 148, 1–10. [Google Scholar]
- Ordoñez, A.I.; Ibañez, F.C.; Torre, P.; Barcina, Y.; Pérez-Elortondo, F.J. Application of multivariate analysis to sensory characterization of ewes’ milk cheese. J. Sens. Stud. 1998, 13, 45–55. [Google Scholar] [CrossRef]
- Grappin, R.; Beuvier, E. Possible Implications of Milk Pasteurization on the Manufacture and Sensory Quality of Ripened Cheese. Int. Dairy J. 1997, 7, 751–761. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Barron, L.J.R.; Redondo, Y.; Aramburu, M.; Gil, P.; Pérez-Elortondo, F.J.; Albisu, M.; Nájera, A.I.; de Renobales, M.; Fernández-García, E. Volatile Composition and Sensory Properties of Industrially Produced Idiazabal Cheese. Int. Dairy J. 2007, 17, 1401–1414. [Google Scholar] [CrossRef]
- Madrau, M.A.; Mangia, N.P.; Murgia, M.A.; Sanna, M.G.; Garau, G.; Leccis, L.; Caredda, M.; Deiana, P. Employment of Autochthonous Microflora in Pecorino Sardo Cheese Manufacturing and Evolution of Physicochemical Parameters during Ripening. Int. Dairy J. 2006, 16, 876–885. [Google Scholar] [CrossRef]
- Ballesteros, C.; Poveda, J.M.; González-Viñas, M.A.; Cabezas, L. Microbiological, Biochemical and Sensory Characteristics of Artisanal and Industrial Manchego Cheeses. Food Control 2006, 17, 249–255. [Google Scholar] [CrossRef]
- Ortigosa, M.; Arizcun, C.; Oneca, M.; Irigoyen, A.; Torre, P. Effects of Pasteurisation on Microorganisms and Sensory Attributes in Roncal Type Cheese from Ewe’s Milk. Milchwissenschaft 2004, 59, 392–396. [Google Scholar]
- Rehman, S.U.; Banks, J.M.; Brechany, E.Y.; Muir, D.D.; McSweeney, P.L.H.; Fox, P.F. Influence of Ripening Temperature on the Volatiles Profile and Flavour of Cheddar Cheese Made from Raw or Pasteurised Milk. Int. Dairy J. 2000, 10, 55–65. [Google Scholar] [CrossRef]
- Balkir, P.; Kemahlioglu, K.; Yucel, U. Foodomics: A New Approach in Food Quality and Safety. Trends Food Sci. Technol. 2021, 108, 49–57. [Google Scholar] [CrossRef]
- Rizal, N.S.M.; Neoh, H.M.; Ramli, R.; Periyasamy, P.R.A.L.K.; Hanafiah, A.; Samat, M.N.A.; Tan, T.L.; Wong, K.K.; Nathan, S.; Chieng, S.; et al. Advantages and Limitations of 16S RRNA Next-Generation Sequencing for Pathogen Identification in the Diagnostic Microbiology Laboratory: Perspectives from a Middle-Income Country. Diagnostics 2020, 10, 816. [Google Scholar] [CrossRef] [PubMed]
- Santamarina-García, G.; Hernández, I.; Amores, G.; Virto, M. Characterization of Microbial Shifts during the Production and Ripening of Raw Ewe Milk-Derived Idiazabal Cheese by High-Throughput Sequencing. Biology 2022, 11, 769. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; McSweeney, P.L.H.; Rea, M.C.; Kilcawley, K.N. Detection of Volatile Compounds of Cheese and Their Contribution to the Flavor Profile of Surface-Ripened Cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afshari, R.; Pillidge, C.J.; Read, E.; Rochfort, S.; Dias, D.A.; Osborn, A.M.; Gill, H. New Insights into Cheddar Cheese Microbiota-Metabolome Relationships Revealed by Integrative Analysis of Multi-Omics Data. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses during Ripening: A Review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and Free Fatty Acid Catabolism in Cheese: A Review of Current Knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Thierry, A.; Collins, Y.F.; Mukdsi, M.C.A.; McSweeney, P.L.H.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and Metabolism of Fatty Acids in Cheese. In Cheese: Chemistry, Physics, and Microbiology; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 423–444. [Google Scholar]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: New York, NT, USA, 2017; pp. 1–803. [Google Scholar]
- Virto, M.; Chávarri, F.; Bustamante, M.A.; Barron, L.J.R.; Aramburu, M.; Vicente, M.S.; Pérez-Elortondo, F.J.; Albisu, M.; de Renobales, M. Lamb Rennet Paste in Ovine Cheese Manufacture. Lipolysis and Flavour. Int. Dairy J. 2003, 13, 391–399. [Google Scholar] [CrossRef]
- Ardö, Y.; McSweeney, P.L.H.; Magboul, A.A.A.; Upadhyay, V.K.; Fox, P.F. Biochemistry of Cheese Ripening: Proteolysis. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Elsevier Inc.: London, UK, 2017; Volume 1, pp. 445–482. [Google Scholar]
- Fernández, M.; Hudson, J.A.; Korpela, R.; De Los Reyes-Gavilán, C.G. Impact on Human Health of Microorganisms Present in Fermented Dairy Products: An Overview. Biomed. Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şanli, T.; Şenel, E. Chapter 27: Formation of Biogenic Amines in Cheese. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Elsevier Inc.: London, UK, 2015; pp. 223–230. [Google Scholar]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic Amines in Fermented Foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida Møller, C.O.; Ücok, E.F.; Rattray, F.P. Histamine Forming Behaviour of Bacterial Isolates from Aged Cheese. Food Res. Int. 2020, 128, 108719. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; Özogul, F. Chapter 1: Biogenic Amines Formation, Toxicity, Regulations in Food. In Food Chemistry, Function and Analysis; Saad, B., Tofalo, R., Eds.; Royal Society of Chemistry: London, UK, 2020; pp. 1–17. [Google Scholar]
- Linares, D.M.; Martĺn, M.C.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic Amines in Dairy Products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Barone, C.; Barbera, M.; Barone, M.; Parisi, S.; Zaccheo, A. Biogenic Amines in Cheeses: Types and Typical Amounts. In Chemical Evolution of Nitrogen-Based Compounds in Mozzarella Cheeses; Barone, C., Barbera, M., Barone, M., Parisi, S., Zaccheo, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–18. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 1–93. [Google Scholar]
- Mah, J.H.; Park, Y.K.; Jin, Y.H.; Lee, J.H.; Hwang, H.J. Bacterial Production and Control of Biogenic Amines in Asian Fermented Soybean. Foods 2019, 8, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Liu, F.; Shi, X.; Wang, B.; Li, K.; Li, B.; Zhuge, B. Dynamic Correlations between Microbiota Succession and Flavor Development Involved in the Ripening of Kazak Artisanal Cheese. Food Res. Int. 2018, 105, 733–742. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, Z.; Lin, K.; Zhang, D.; Chen, Y.; Xiao, J.; Wang, B.; Shi, X. Dynamic Changes in Bacterial Microbiota Succession and Flavour Development during Milk Fermentation of Kazak Artisanal Cheese. Int. Dairy J. 2021, 113, 104878. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, K.; Luo, A.; Chen, J.; Shen, Y.; Wang, X.; He, Q.; Gao, H. Dynamic Changes of Volatile Compounds and Bacterial Diversity during Fourth to Seventh Rounds of Chinese Soy Sauce Aroma Liquor. Food Sci. Nutr. 2021, 9, 3500–3511. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. O2-PLS, a two-block (X-Y) latent variable regression (LVR) method with an integral OSC filter. J. Chemometr. 2003, 17, 53–64. [Google Scholar] [CrossRef]
- El Bouhaddani, S.; Houwing-Duistermaat, J.; Salo, P.; Perola, M.; Jongbloed, G.; Uh, H.-W. Evaluation of O2PLS in Omics Data Integration. BMC Bioinforma. 2016, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, L.; Toft, M.; Johansson, E.; Wold, S.; Trygg, J. Separating Y-Predictive and Y-Orthogonal Variation in Multi-Block Spectral Data. J. Chemom. 2006, 20, 352–361. [Google Scholar] [CrossRef]
- Trygg, J. O2-PLS for Qualitative and Quantitative Analysis in Multivariate Calibration. J. Chemom. 2002, 16, 283–293. [Google Scholar] [CrossRef]
- Zhao, D.; Hu, J.; Chen, W. Analysis of the Relationship between Microorganisms and Flavour Development in Dry-Cured Grass Carp by High-Throughput Sequencing, Volatile Flavour Analysis and Metabolomics. Food Chem. 2022, 368, 130889. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.M.; Yu, D.; Yang, F.; Jiang, Q. Correlations between Microbiota Succession and Flavor Formation during Fermentation of Chinese Low-Salt Fermented Common Carp (Cyprinus Carpio L.) Inoculated with Mixed Starter Cultures. Food Microbiol. 2020, 90, 103487. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, B.; Zhao, W.; Pang, X.; Lao, F.; Liao, X.; Wu, J. Correlation between Autochthonous Microbial Communities and Key Odorants during the Fermentation of Red Pepper (Capsicum Annuum L.). Food Microbiol. 2020, 91, 103510. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Yu, J.; Sun, J.; Lu, K.; Xie, W. Bacterial Composition Changes and Volatile Compounds during the Fermentation of Shrimp Paste: Dynamic Changes of Microbial Communities and Flavor Composition. Food Biosci. 2021, 43, 101169. [Google Scholar] [CrossRef]
- Kubinyi, H. 3D QSAR in Drug Design: Volume 1: Theory Methods and Applications; Springer: Berlin, Germany, 2000; pp. 1–759. [Google Scholar]
- Galindo-Prieto, B.; Eriksson, L.; Trygg, J. Variable Influence on Projection (VIP) for Orthogonal Projections to Latent Structures (OPLS). J. Chemom. 2014, 28, 623–632. [Google Scholar] [CrossRef]
- Unno, R.; Suzuki, T.; Matsutani, M.; Ishikawa, M. Evaluation of the Relationships Between Microbiota and Metabolites in Soft-Type Ripened Cheese Using an Integrated Omics Approach. Front. Microbiol. 2021, 12, 681185. [Google Scholar] [CrossRef]
- Bertuzzi, A.S.; Walsh, A.M.; Sheehan, J.J.; Cotter, P.D.; Crispie, F.; McSweeney, P.L.H.; Kilcawley, K.N.; Rea, M.C. Omics-Based Insights into Flavor Development and Microbial Succession within Surface-Ripened Cheese. mSystems 2018, 3, e00211–e00217. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, F.; Ferrocino, I.; Milanović, V.; Belleggia, L.; Corvaglia, M.R.; Garofalo, C.; Foligni, R.; Mannozzi, C.; Mozzon, M.; Cocolin, L.; et al. Microbial Communities and Volatile Profile of Queijo de Azeitão PDO Cheese, a Traditional Mediterranean Thistle-Curdled Cheese from Portugal. Food Res. Int. 2021, 147, 110537. [Google Scholar] [CrossRef]
- De Pasquale, I.; Calasso, M.; Mancini, L.; Ercolini, D.; La Storia, A.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Causal Relationship between Microbial Ecology Dynamics and Proteolysis during Manufacture and Ripening of Protected Designation of Origin (PDO) Cheese Canestrato Pugliese. Appl. Environ. Microbiol. 2014, 80, 4085–4094. [Google Scholar] [CrossRef] [Green Version]
- De Pasquale, I.; Di Cagno, R.; Buchin, S.; De Angelis, M.; Gobbetti, M. Spatial Distribution of the Metabolically Active Microbiota within Italian PDO Ewes’ Milk Cheeses. PLoS ONE 2016, 11, e0153213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungjaroenchai, W.; White, C.H.; Holmes, W.E.; Drake, M.A. Influence of Adjunct Cultures on Volatile Free Fatty Acids in Reduced-Fat Edam Cheeses. J. Dairy Sci. 2004, 87, 3224–3234. [Google Scholar] [CrossRef] [Green Version]
- Pérez Elortondo, F.J.; Albisu, M.; Barcina, Y. Brining Time Effect on Physicochemical and Microbiological Parameters in Idiazabal Cheese. Int. J. Food Microbiol. 1999, 49, 139–149. [Google Scholar] [CrossRef]
- Cleide, C.O.; Castro-Mejía, J.L.; Krych, L.; Rattray, F.P. Histamine-Forming Ability of Lentilactobacillus parabuchneri in Reduced Salt Cheddar Cheese. Food Microbiol. 2021, 98, 103789. [Google Scholar]
- Boletín Oficial del Estado. Orden del 30 de noviembre por la que se aprueba el Reglamento de la Denominación de Origen «Idiazabal» y su Consejo Regulador. BOE 1993, 289, 34591–34596. [Google Scholar]
- Bustamante, M.A.; Chávarri, F.; Santisteban, A.; Ceballos, G.; Hernández, I.; José Miguélez, M.; Aranburu, I.; Barrón, L.J.R.; Virto, M.; de Renobales, M. Coagulating and Lipolytic Activities of Artisanal Lamb Rennet Pastes. J. Dairy Res. 2000, 67, 393–402. [Google Scholar] [CrossRef]
- Aldalur, A. Technical Factors Affecting Cheese Properties, Yield and Whey Generated from Raw Sheep Milk during Cheesemaking in Small Rural Dairies. Ph.D. Thesis, University of the Basque Country, Vitoria-Gasteiz, Spain, 2019. [Google Scholar]
- ISO 5943/IDF 88: 2006; Cheese and Processed Cheese Products—Determination of Chloride Content—Potentiometric Titration Method. International Standardisation Organisation: Geneva, Switzerland, 2006.
- Chavarri, F.; Virto, M.; Martin, C.; Nájera, A.I.; Santisteban, A.; Barrón, L.J.R.; de Renobales, M. Determination of Free Fatty Acids in Cheese: Comparison of Two Analytical Methods. J. Dairy Res. 1997, 64, 445–452. [Google Scholar] [CrossRef]
- Busto, O.; Miracle, M.; Guasch, J.; Borrull, F. Determination of Biogenic Amines in Wines by High-Performance Liquid Chromatography with on-Column Fluorescence Derivatization. J. Chromatogr. A 1997, 757, 311–318. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 17 May 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package “Vegan”. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 15 January 2021).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Package “Gplots” Title Various R Programming Tools for Plotting Data [R Package Gplots Version 3.1.1]. Available online: https://cran.r-project.org/package=gplots (accessed on 15 January 2021).
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, 1–11. [Google Scholar] [CrossRef]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.M.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; et al. The Metagenomics RAST Server-A Public Resource for the Automatic Phylogenetic and Functional Analysis of Metagenomes. BMC Bioinform. 2008, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wang, Z.; Sun, J.; Ni, L. The Dynamics of Volatile Compounds and Their Correlation with the Microbial Succession during the Traditional Solid-State Fermentation of Gutian Hong Qu Glutinous Rice Wine. Food Microbiol. 2020, 86, 103347. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.; Rodríguez, J.; Vázquez, L.; Flórez, A.B. Microbial Interactions within the Cheese Ecosystem and Their Application to Improve Quality and Safety. Foods 2021, 10, 602. [Google Scholar] [CrossRef]
- Lv, J.; Yang, Z.; Xu, W.; Li, S.; Liang, H.; Ji, C.; Yu, C.; Zhu, B.; Lin, X. Relationships between Bacterial Community and Metabolites of Sour Meat at Different Temperature during the Fermentation. Int. J. Food Microbiol. 2019, 307, 108286. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, X.; Ma, Y.; Zhu, L.; Xiao, J.; Deng, L.; Shi, X.; Wang, B. Diversity and Potential Function of Bacterial Communities during Milk Fermentation of Kazak Artisanal Cheese. Process Biochem. 2021, 111, 191–200. [Google Scholar] [CrossRef]
- Nájera, A.I.; Barcina, Y.; de Renobales, M.; Barron, L.J.R. Influence of Salt Content on the Triglyceride Composition of Idiazabal Cheese during Ripening. Lait 1999, 79, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Etayo, I.; Pérez Elortondo, F.J.; Gil, P.F.; Albisu, M.; Virto, M.; Conde, S.; Barron, L.J.R.; Nájera, A.I.; Gómez-Hidalgo, M.E.; Delgado, C.; et al. Hygienic Quality, Lipolysis and Sensory Properties of Spanish Protected Designation of Origin Ewe’s Milk Cheeses Manufactured with Lamb Rennet Paste. Lait 2006, 86, 415–434. [Google Scholar] [CrossRef]
- Aldalur, A.; Bustamante, M.A.; Barron, L.J.R. Effects of Technological Settings on Yield, Curd, Whey, and Cheese Composition during the Cheese-Making Process from Raw Sheep Milk in Small Rural Dairies: Emphasis on Cutting and Cooking Conditions. J. Dairy Sci. 2019, 102, 7813–7825. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E.; Samelis, J. Microbiological and Biochemical Characteristics of Kashkaval Cheese Produced Using Pasteurised or Raw Milk. Int. Dairy J. 2019, 89, 60–67. [Google Scholar] [CrossRef]
- De Pasquale, I.; Di Cagno, R.; Buchin, S.; De Angelis, M.; Gobbetti, M. Use of Autochthonous Mesophilic Lactic Acid Bacteria as Starter Cultures for Making Pecorino Crotonese Cheese: Effect on Compositional, Microbiological and Biochemical Attributes. Food Res. Int. 2019, 116, 1344–1356. [Google Scholar] [CrossRef]
- Valdivielso, I.; Bustamante, M.A.; Buccioni, A.; Franci, O.; Ruiz de Gordoa, J.C.; de Renobales, M.; Barron, L.J.R. Commercial Sheep Flocks–Fatty Acid and Fat-Soluble Antioxidant Composition of Milk and Cheese Related to Changes in Feeding Management throughout Lactation. J. Dairy Res. 2015, 82, 334–343. [Google Scholar] [CrossRef]
- Valdivielso, I.; Bustamante, M.A.; Aldezabal, A.; Amores, G.; Virto, M.; Ruiz De Gordoa, J.C.; de Renobales, M.; Barron, L.J.R. Case Study of a Commercial Sheep Flock under Extensive Mountain Grazing: Pasture Derived Lipid Compounds in Milk and Cheese. Food Chem. 2016, 197, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Aldalur, A.; Bustamante, M.A.; Salmerón, J.; Barron, L.J.R. Relationships between cheese-processing conditions and curd and cheese properties to improve the yield of Idiazabal cheese made in small artisan dairies: A multivariate approach. J. Dairy Sci. 2021, 104, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Hayaloglu, A.A.; Karatekin, B.; Gurkan, H. Thermal Stability of Chymosin or Microbial Coagulant in the Manufacture of Malatya, a Halloumi Type Cheese: Proteolysis, Microstructure and Functional Properties. Int. Dairy J. 2014, 38, 136–144. [Google Scholar] [CrossRef]
- Soltani, M.; Sahingil, D.; Gokce, Y.; Hayaloglu, A.A. Effect of Blends of Camel Chymosin and Microbial Rennet (Rhizomucor Miehei) on Chemical Composition, Proteolysis and Residual Coagulant Activity in Iranian Ultrafiltered White Cheese. J. Food Sci. Technol. 2019, 56, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, E.; Saavedra, P.; Millán, R.; Castelo, M.; Fernández-Salguero, J. Effect of Ripening and Type of Rennet on the Mineral Content of Los Pedroches Cheese. J. Food Qual. 1998, 21, 187–200. [Google Scholar] [CrossRef]
- Possas, A.; Bonilla-Luque, O.M.; Valero, A. From Cheese-Making to Consumption: Exploring the Microbial Safety of Cheeses through Predictive Microbiology Models. Foods 2021, 10, 355. [Google Scholar] [CrossRef]
- Narvhus, J.A.; Axelsson, L. Lactic acid bacteria. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Elsevier Inc.: London, UK, 2003; pp. 3465–3472. [Google Scholar]
- An, F.; Li, M.; Zhao, Y.; Zhang, Y.; Mu, D.; Hu, X.; You, S.; Wu, J.; Wu, R. Metatranscriptome-Based Investigation of Flavor-Producing Core Microbiota in Different Fermentation Stages of Dajiang, a Traditional Fermented Soybean Paste of Northeast China. Food Chem. 2021, 343, 128509. [Google Scholar] [CrossRef]
- Wang, H.; Su, W.; Mu, Y.; Zhao, C. Correlation Between Microbial Diversity and Volatile Flavor Compounds of Suan Zuo Rou, a Fermented Meat Product from Guizhou, China. Front. Microbiol. 2021, 12, 3216. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Wang, Y.; Li, L.; Yang, X.; Chen, S.; Zhao, Y.; Zhou, W. Microbial Community Changes Induced by Pediococcus pentosaceus Improve the Physicochemical Properties and Safety in Fermented Tilapia Sausage. Food Res. Int. 2021, 147, 110476. [Google Scholar] [CrossRef] [PubMed]
- Chávarri, F.; Bustamante, M.A.; Santisteban, A.; Virto, M.; Barrón, L.J.R.; de Renobales, M. Changes in Free Fatty Acids During Ripening of Idiazabal Cheese Manufactured at Different Times of the Year. J. Dairy Sci. 1999, 82, 885–890. [Google Scholar] [CrossRef]
- Hernández, I.; Barrón, L.J.R.; Virto, M.; Pérez-Elortondo, F.J.; Flanagan, C.; Rozas, U.; Nájera, A.I.; Albisu, M.; Vicente, M.S.; de Renobales, M. Lipolysis, Proteolysis and Sensory Properties of Ewe’s Raw Milk Cheese (Idiazabal) Made with Lipase Addition. Food Chem. 2009, 116, 158–166. [Google Scholar] [CrossRef]
- Barron, L.J.R.; Hernández, I.; Bilbao, A.; Flanagan, C.E.; Nájera, A.I.; Virto, M.; Pérez-Elortondo, F.J.; Albisu, M.; de Renobales, M. Changes in Lipid Fractions and Sensory Properties of Idiazabal Cheese Induced by Lipase Addition. J. Dairy Res. 2004, 71, 372–379. [Google Scholar] [CrossRef]
- Hernández, I.; de Renobales, M.; Virto, M.; Pérez-Elortondo, F.J.; Barron, L.J.R.; Flanagan, C.; Albisu, M. Assessment of Industrial Lipases for Flavour Development in Commercial Idiazabal (Ewe’s Raw Milk) Cheese. Enzyme Microb. Technol. 2005, 36, 870–879. [Google Scholar] [CrossRef]
- Amores, G.; Pérez-Elortondo, F.J.; Albisu, M.; Barron, L.J.R. Short Communication: To What Extent Do Environmental or Technological Conditions Affect the Sensory Differentiation of Raw Ewe Milk Cheeses Produced in Valley or Mountain Farms? J. Dairy Sci. 2021, 104, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, P.; Ehsani, M.R.; Mizani, M.; Givianrad, M.H. Characterization of a Traditional Ripened Cheese, Kurdish Kope: Lipolysis, Lactate Metabolism, the Release Profile of Volatile Compounds, and Correlations with Sensory Characteristics. J. Food Sci. 2021, 86, 3303–3321. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Maxia, D.; Scano, P.; Addis, M.; Dedola, A.; Pes, M.; Murgia, A.; Casula, M.; Profumo, A.; Pirisi, A. A Gas Chromatography-Mass Spectrometry Untargeted Metabolomics Approach to Discriminate Fiore Sardo Cheese Produced from Raw or Thermized Ovine Milk. J. Dairy Sci. 2019, 102, 5005–5018. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.; Combarros-Fuertes, P.; Renes, E.; Abarquero, D.; Fresno, J.M.; Tornadijo, M.E. Influence of the Breed of Sheep on the Characteristics of Zamorano Cheese. Dairy 2021, 2, 242–255. [Google Scholar] [CrossRef]
- Larráyoz, P.; Martínez, M.T.; Barrón, L.J.R.; Torre, P.; Barcina, Y. The Evolution of Free Fatty Acids during the Ripening of Idiazábal Cheese: Influence of Rennet Type. Eur. Food Res. Technol. 1999, 210, 9–12. [Google Scholar] [CrossRef]
- Chávarri, F.; Santisteban, A.; Virto, M.; de Renobales, M. Alkaline Phosphatase, Acid Phosphatase, Lactoperoxidase, and Lipoprotein Lipase Activities in Industrial Ewe’s Milk and Cheese. J. Agric. Food Chem. 1998, 46, 2926–2932. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Flemetakis, E.; Kouri, E.; Sotirakoglou, K.; Zervas, G. The Effect of Long Term Under- and over-Feeding on the Expression of Genes Related to Lipid Metabolism in Mammary Tissue of Sheep. J. Dairy Res. 2015, 82, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Liu, S.Q.; Crow, V.L.; Delabre, M.L.; Lubbers, M.; Bennett, M.; Norris, G. Esterases of Lactic Acid Bacteria and Cheese Flavour: Milk Fat Hydrolysis, Alcoholysis and Esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Dellali, A.; Karam, H.Z.; Karam, N.-E. Lipase and Esterase Activities of Lactic Acid Bacteria Isolated from Different Biotopes. African J. Biotechnol. 2020, 19, 156–164. [Google Scholar]
- Katz, M.; Medina, R.; Gonzalez, S.; Oliver, G. Esterolytic and Lipolytic Activities of Lactic Acid Bacteria Isolated from Ewe’s Milk and Cheese. J. Food Prot. 2002, 65, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Torres, M.; Mancheño, J.M.; de las Rivas, B.; Muñoz, R. Characterization of a Halotolerant Lipase from the Lactic Acid Bacteria Lactobacillus plantarum Useful in Food Fermentations. LWT-Food Sci. Technol. 2015, 60, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Esteban-Torres, M.; Mancheño, J.M.; de las Rivas, B.; Muñoz, R. Production and Characterization of a Tributyrin Esterase from Lactobacillus plantarum Suitable for Cheese Lipolysis. J. Dairy Sci. 2014, 97, 6737–6744. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Goveas, L.C.; Suralikerimath, N.; Jampani, C.; Halami, P.M.; Narayan, B. Extraction and Purification of Lipase from Enterococcus faecium MTCC5695 by PEG/Phosphate Aqueous-Two Phase System (ATPS) and Its Biochemical Characterization. Biocatal. Agric. Biotechnol. 2016, 6, 19–27. [Google Scholar] [CrossRef]
- Han, D.M.; Chun, B.H.; Kim, H.M.; Jeon, C.O. Characterization and Correlation of Microbial Communities and Metabolite and Volatile Compounds in Doenjang Fermentation. Food Res. Int. 2021, 148, 110645. [Google Scholar] [CrossRef] [PubMed]
- Özer, B.; Yaman, H. Milk and Milk Products: Microbiology of Liquid Milk. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier Inc.: London, UK, 2014; pp. 721–727. [Google Scholar]
- Ozturkoglu-Budak, S.; Wiebenga, A.; Bron, P.A.; de Vries, R.P. Protease and Lipase Activities of Fungal and Bacterial Strains Derived from an Artisanal Raw Ewe’s Milk Cheese. Int. J. Food Microbiol. 2016, 237, 17–27. [Google Scholar] [CrossRef]
- Schmitz-Esser, S.; Dzieciol, M.; Nischler, E.; Schornsteiner, E.; Bereuter, O.; Mann, E.; Wagner, M. Abundance and Potential Contribution of Gram-Negative Cheese Rind Bacteria from Austrian Artisanal Hard Cheeses. Int. J. Food Microbiol. 2018, 266, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2009, 85, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, C. Influence of Trace Amounts of Fatty Acids on the Growth of Microorganisms. Bacteriol. Rev. 1954, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forquin, M.P.; Weimer, B.C. Brevibacterium. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier Inc.: London, UK, 2014; pp. 324–330. [Google Scholar]
- Ordóñez, A.I.; Ibáñez, F.C.; Torre, P.; Barcina, Y. Formation of Biogenic Amines in Idiazabal Ewe’s-Milk Cheese: Effect of Ripening, Pasteurization, and Starter. J. Food Prot. 1997, 60, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Perpetuini, G.; Battistelli, N.; Pepe, A.; Ianni, A.; Martino, G.; Suzzi, G. Accumulation γ-Aminobutyric Acid and Biogenic Amines in a Traditional Raw Milk Ewe’s Cheese. Foods 2019, 8, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torracca, B.; Pedonese, F.; López, M.B.; Turchi, B.; Fratini, F.; Nuvoloni, R. Effect of Milk Pasteurisation and of Ripening in a Cave on Biogenic Amine Content and Sensory Properties of a Pecorino Cheese. Int. Dairy J. 2016, 61, 189–195. [Google Scholar] [CrossRef]
- Schirone, M.; Tofalo, R.; Fasoli, G.; Perpetuini, G.; Corsetti, A.; Manetta, A.C.; Ciarrocchi, A.; Suzzi, G. High Content of Biogenic Amines in Pecorino Cheeses. Food Microbiol. 2013, 34, 137–144. [Google Scholar] [CrossRef]
- Linares, D.M.; del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Alvarez, M.A. Factors Influencing Biogenic Amines Accumulation in Dairy Products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nájera, A.I.; Nieto, S.; Barron, L.J.R.; Albisu, M. A Review of the Preservation of Hard and Semi-Hard Cheeses: Quality and Safety. Int. J. Environ. Res. Public Health 2021, 18, 9789. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Huo, W.; Liu, J.; Zhang, R.; Zhu, W. In Vitro Effects of Sodium Bicarbonate Buffer on Rumen Fermentation, Levels of Lipopolysaccharide and Biogenic Amine, and Composition of Rumen Microbiota. J. Sci. Food Agric. 2017, 97, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Steinmann, M.E. Structure, Function, and Modulation of GABAA Receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, S.; Heydari, A.; Alinaghipour, A.; Salami, M. Effect of Probiotic Supplementation on Seizure Activity and Cognitive Performance in PTZ-Induced Chemical Kindling. Epilepsy Behav. 2019, 95, 43–50. [Google Scholar] [CrossRef]
- Abdelazez, A.; Abdelmotaal, H.; Evivie, S.E.; Melak, S.; Jia, F.F.; Khoso, M.H.; Zhu, Z.T.; Zhang, L.J.; Sami, R.; Meng, X.C. Screening Potential Probiotic Characteristics of Lactobacillus brevis Strains in Vitro and Intervention Effect on Type i Diabetes in Vivo. Biomed Res. Int. 2018, 2018, 7356173. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as Food Supplements: The Effects of GABA on Brain and Behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhu, C.L.; Huang, Z.J.; Wang, G.; Huang, Q.; Liu, C.H.; Xie, F.; Wang, W. γ-Aminobutyric Acid Inhibits the Growth of Cholangiocarcinoma via CAMP/PKA Signal Pathway. Int. J. Clin. Exp. Med. 2016, 9, 9992–9998. [Google Scholar]
- Bustamante, M.A.; Virto, M.; Aramburu, M.; Barron, L.J.R.; Pérez-Elortondo, F.J.; Albisu, M.; de Renobales, M. Lamb Rennet Paste in Ovine Cheese (Idiazabal) Manufacture. Proteolysis and Relationship between Analytical and Sensory Parameters. Int. Dairy J. 2003, 13, 547–557. [Google Scholar] [CrossRef]
- Renes, E.; Fernández, D.; Abarquero, D.; Ladero, V.; Álvarez, M.A.; Tornadijo, M.E.; Fresno, J.M. Effect of Forage Type, Season, and Ripening Time on Selected Quality Properties of Sheep Milk Cheese. J. Dairy Sci. 2021, 104, 2539–2552. [Google Scholar] [CrossRef] [PubMed]
- Silla Santos, M.H. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Vasconcelos, H.; de Almeida, J.M.M.M.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; Coelho, L.C.C. Detection of Biogenic Amines in Several Foods with Different Sample Treatments: An Overview. Trends Food Sci. Technol. 2021, 113, 86–96. [Google Scholar] [CrossRef]
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic Amines and Their Production by Microorganisms in Food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Tittarelli, F.; Perpetuini, G.; Di Gianvito, P.; Tofalo, R. Biogenic Amines Producing and Degrading Bacteria: A Snapshot from Raw Ewes’ Cheese. LWT-Food Sci. Technol. 2019, 101, 1–9. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa-Pesqueira, D.; Roig-Sagués, A.X.; Manuela Hernández-Herrero, M. Screening Method to Evaluate Amino Acid-Decarboxylase Activity of Bacteria Present in Spanish Artisanal Ripened Cheeses. Foods 2018, 7, 182. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.; del Rio, B.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Isolation and Typification of Histamine-Producing Lactobacillus vaginalis Strains from Cheese. Int. J. Food Microbiol. 2015, 215, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Sánchez, S.; Ramos, I.M.; Seseña, S.; Poveda, J.M.; Palop, M.L. Potential of Lactobacillus Strains for Health-Promotion and Flavouring of Fermented Dairy Foods. LWT-Food Sci. Technol. 2021, 143, 111102. [Google Scholar] [CrossRef]
- Joosten, H.M.L.J.; Northolt, M.D. Conditions Allowing the Formation of Biogenic Amines in Cheese, 2: Decarboxylative Properties of Some Non-Starter Bacteria. Netherlands Milk Dairy J. 1987, 41, 259–280. [Google Scholar]
- Aliakbarlu, J.; Alizadeh, M.; Razavi-Rohani, S.M.; Agh, N. Biogenic Amines in Iranian White Brine Cheese: Modelling and Optimisation of Processing Factors. Int. J. Dairy Technol. 2011, 64, 417–424. [Google Scholar] [CrossRef]
- Body, P.; Greif, G.; Greifová, G.; Sliacká, M.; Greifová, M. Effects of Cultivation Media and NaCl Concentration on the Growth Kinetics and Biogenic Amine Production of Lactobacillus reuteri. Czech J. Food Sci. 2021, 39, 9–16. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Liu, D. Biochemical Changes and Microbial Community Dynamics during Spontaneous Fermentation of Zhacai, a Traditional Pickled Mustard Tuber from China. Int. J. Food Microbiol. 2021, 347, 109199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Li, C.; Li, L.; Yang, X.; Wu, Y.; Chen, S.; Zhao, Y. Novel Insight into Physicochemical and Flavor Formation in Naturally Fermented Tilapia Sausage Based on Microbial Metabolic Network. Food Res. Int. 2021, 141, 110122. [Google Scholar] [CrossRef]
- Santiyanont, P.; Chantarasakha, K.; Tepkasikul, P.; Srimarut, Y.; Mhuantong, W.; Tangphatsornruang, S.; Zo, Y.G.; Chokesajjawatee, N. Dynamics of Biogenic Amines and Bacterial Communities in a Thai Fermented Pork Product Nham. Food Res. Int. 2019, 119, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Chun, B.H.; Jeon, C.O. Chromohalobacter Is a Causing Agent for the Production of Organic Acids and Putrescine during Fermentation of Ganjang, a Korean Traditional Soy Sauce. J. Food Sci. 2015, 80, M2853–M2859. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, S. The Importance of Amine-Degrading Enzymes on the Biogenic Amine Degradation in Fermented Foods: A Review. Process Biochem. 2020, 99, 331–339. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kung, H.F.; Huang, C.Y.; Huang, T.C.; Tsai, Y.H. Reduction of Histamine and Biogenic Amines during Salted Fish Fermentation by Bacillus Polymyxa as a Starter Culture. J. Food Drug Anal. 2016, 24, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, C.Y.; Bakar, F.A.; Russly, A.R.; Jamilah, B.; Mahyudin, N.A. The Effects of Food Processing on Biogenic Amines Formation. Int. Food Res. J. 2011, 18, 867–876. [Google Scholar]
- Butor, I.; Pištěková, H.; Purevdorj, K.; Jančová, P.; Buňka, F.; Buňková, L. Biogenic Amines Degradation by Microorganisms Isolated from Cheese. Potravin. Slovak J. Food Sci. 2017, 11, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Fresno, A.; Martínez, N.; Sánchez-Llana, E.; Díaz, M.; Fernández, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. Lactobacillus casei Strains Isolated from Cheese Reduce Biogenic Amine Accumulation in an Experimental Model. Int. J. Food Microbiol. 2012, 157, 297–304. [Google Scholar] [CrossRef]

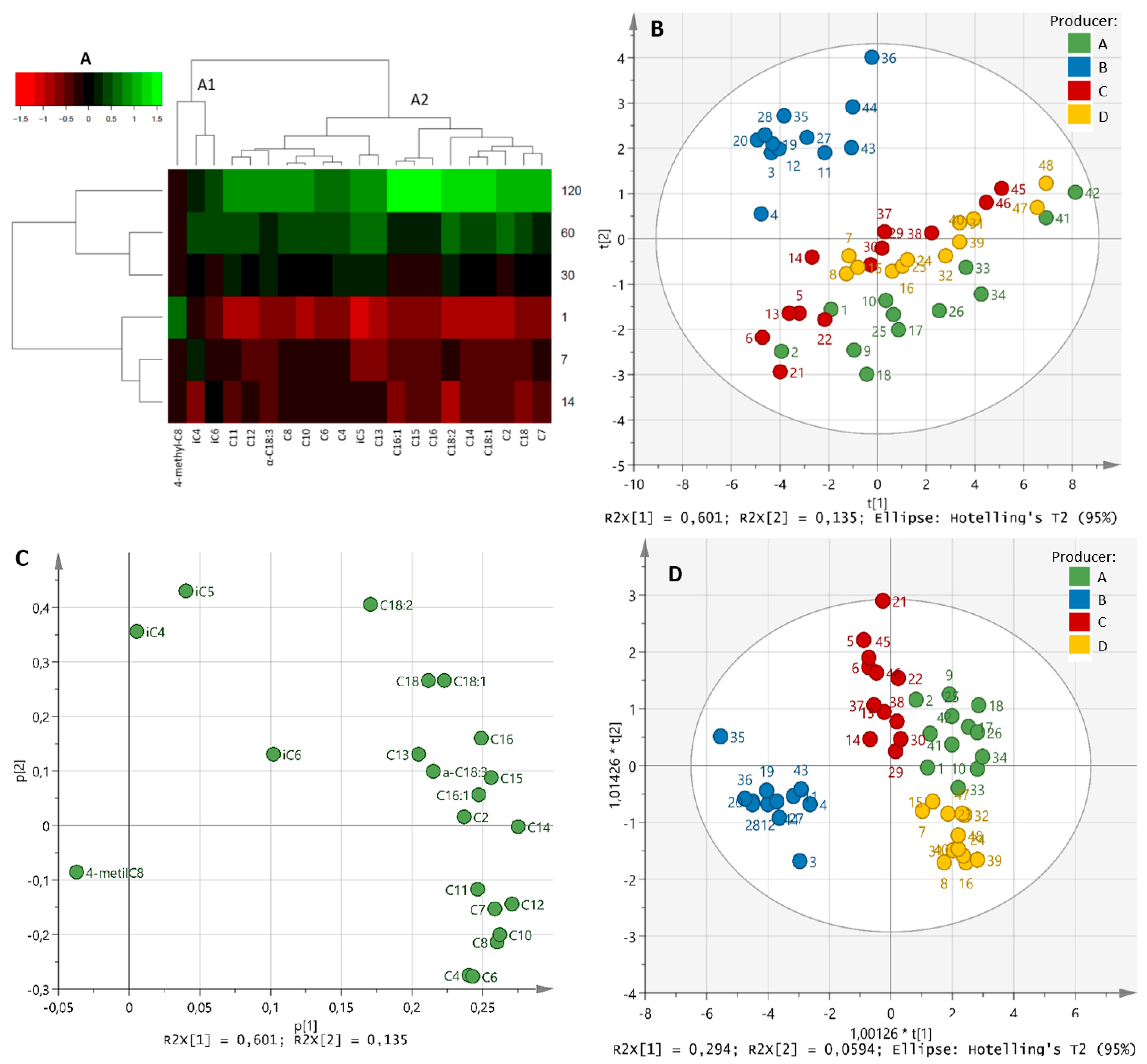

| Producer | Ripening Time (Days) | Total FFAs | Unsaturated FFAs | Saturated FFAs | Short-Chain FFAs | Medium-Chain FFAs | Long-Chain FFAs |
|---|---|---|---|---|---|---|---|
| A | 1 | 16.7 ± 4.36 | 0.331 ± 0.166 | 16.4 ± 4.19 | 15.4 ± 3.88 | 0.413 ± 0.0723 | 0.851 ± 0.412 |
| 7 | 32.6 ± 2.26 | 0.481 ± 0.214 | 32.1 ± 2.05 | 30.6 ± 1.91 | 0.814 ± 0.00560 | 1.15 ± 0.342 | |
| 14 | 37.2 ± 4.92 | 0.486 ± 0.0122 | 36.7 ± 4.93 | 35.0 ± 4.64 | 1.04 ± 0.190 | 1.19 ± 0.0939 | |
| 30 | 41.2 ± 7.01 | 0.712 ± 0.0524 | 40.5 ± 6.96 | 38.1 ± 6.36 | 1.42 ± 0.429 | 1.66 ± 0.219 | |
| 60 | 56.4 ± 1.00 | 1.31 ± 0.497 | 55.1 ± 0.507 | 51.4 ± 0.463 | 2.10 ± 0.0919 | 2.81 ± 0.634 | |
| 120 | 77.8 ± 8.39 | 2.92 ± 0.434 | 74.9 ± 7.96 | 68.3 ± 6.96 | 3.50 ± 0.248 | 5.94 ± 1.18 | |
| B | 1 | 6.04 ± 0.764 | 0.533 ± 0.0261 | 5.51 ± 0.738 | 4.47 ± 0.610 | 0.312 ± 0.0302 | 1.25 ± 0.124 |
| 7 | 10.5 ± 2.51 | 0.584 ± 0.0288 | 9.93 ± 2.54 | 8.55 ± 2.01 | 0.465 ± 0.231 | 1.50 ± 0.267 | |
| 14 | 9.47 ± 0.508 | 0.474 ± 0.0623 | 8.99 ± 0.446 | 7.96 ± 0.563 | 0.321 ± 0.0689 | 1.19 ± 0.0141 | |
| 30 | 10.0 ± 0.431 | 0.655 ± 0.082 | 9.34 ± 0.513 | 8.21 ± 0.720 | 0.372 ± 0.0805 | 1.41 ± 0.209 | |
| 60 | 20.3 ± 10.6 | 0.831 ± 0.312 | 19.5 ± 10.3 | 17.9 ± 9.64 | 0.519 ± 0.207 | 1.90 ± 0.770 | |
| 120 | 18.1 ± 1.65 | 1.05 ± 0.0411 | 17.0 ± 1.61 | 15.3 ± 1.59 | 0.569 ± 0.0238 | 2.22 ± 0.0816 | |
| C | 1 | 7.75 ± 1.30 | 0.227 ± 0.0409 | 7.52 ± 1.34 | 6.34 ± 1.65 | 0.476 ± 0.121 | 0.932 ± 0.23 |
| 7 | 7.63 ± 1.96 | 0.273 ± 0.00353 | 7.36 ± 1.96 | 6.00 ± 1.70 | 0.568 ± 0.118 | 1.06 ± 0.145 | |
| 14 | 7.43 ± 1.61 | 0.240 ± 0.067 | 7.19 ± 1.55 | 5.92 ± 1.22 | 0.598 ± 0.148 | 0.913 ± 0.244 | |
| 30 | 16.3 ± 2.77 | 0.447 ± 0.0478 | 15.8 ± 2.72 | 14.0 ± 2.63 | 0.877 ± 0.00720 | 1.41 ± 0.142 | |
| 60 | 30.8 ± 4.16 | 0.512 ± 0.112 | 30.2 ± 4.05 | 27.8 ± 3.37 | 1.21 ± 0.366 | 1.70 ± 0.427 | |
| 120 | 52.1 ± 6.96 | 1.02 ± 0.0542 | 51.1 ± 7.01 | 47.2 ± 6.76 | 2.12 ± 0.123 | 2.83 ± 0.0777 | |
| D | 1 | 17.5 ± 1.68 | 0.523 ± 0.132 | 17.0 ± 1.82 | 15.3 ± 1.72 | 0.719 ± 0.0749 | 1.51 ± 0.106 |
| 7 | 21.2 ± 0.441 | 0.700 ± 0.107 | 20.5 ± 0.334 | 18.6 ± 0.173 | 0.816 ± 0.0865 | 1.72 ± 0.182 | |
| 14 | 22.6 ± 2.46 | 0.817 ± 0.188 | 21.8 ± 2.27 | 19.5 ± 2.23 | 1.09 ± 0.00966 | 2.03 ± 0.241 | |
| 30 | 36.1 ± 1.77 | 1.15 ± 0.427 | 35.0 ± 1.34 | 31.8 ± 1.26 | 1.60 ± 0.0392 | 2.74 ± 0.547 | |
| 60 | 38.4 ± 1.90 | 1.42 ± 0.179 | 37.0 ± 1.72 | 33.7 ± 1.53 | 1.73 ± 0.0656 | 3.02 ± 0.307 | |
| 120 | 51.6 ± 1.92 | 2.45 ± 0.0444 | 49.2 ± 1.87 | 43.4 ± 1.63 | 3.12 ± 0.145 | 5.04 ± 0.142 | |
| p-value 1 | RT | *** | *** | *** | ** | ** | *** |
| P | *** | ** | *** | *** | *** | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamarina-García, G.; Amores, G.; López de Armentia, E.; Hernández, I.; Virto, M. Relationship between the Dynamics of Gross Composition, Free Fatty Acids and Biogenic Amines, and Microbial Shifts during the Ripening of Raw Ewe Milk-Derived Idiazabal Cheese. Animals 2022, 12, 3224. https://doi.org/10.3390/ani12223224
Santamarina-García G, Amores G, López de Armentia E, Hernández I, Virto M. Relationship between the Dynamics of Gross Composition, Free Fatty Acids and Biogenic Amines, and Microbial Shifts during the Ripening of Raw Ewe Milk-Derived Idiazabal Cheese. Animals. 2022; 12(22):3224. https://doi.org/10.3390/ani12223224
Chicago/Turabian StyleSantamarina-García, Gorka, Gustavo Amores, Emma López de Armentia, Igor Hernández, and Mailo Virto. 2022. "Relationship between the Dynamics of Gross Composition, Free Fatty Acids and Biogenic Amines, and Microbial Shifts during the Ripening of Raw Ewe Milk-Derived Idiazabal Cheese" Animals 12, no. 22: 3224. https://doi.org/10.3390/ani12223224






