Natural Mycoplasma Infection Reduces Expression of Pro-Inflammatory Cytokines in Response to Ovine Footrot Pathogens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Primary Cell Isolation and Culture
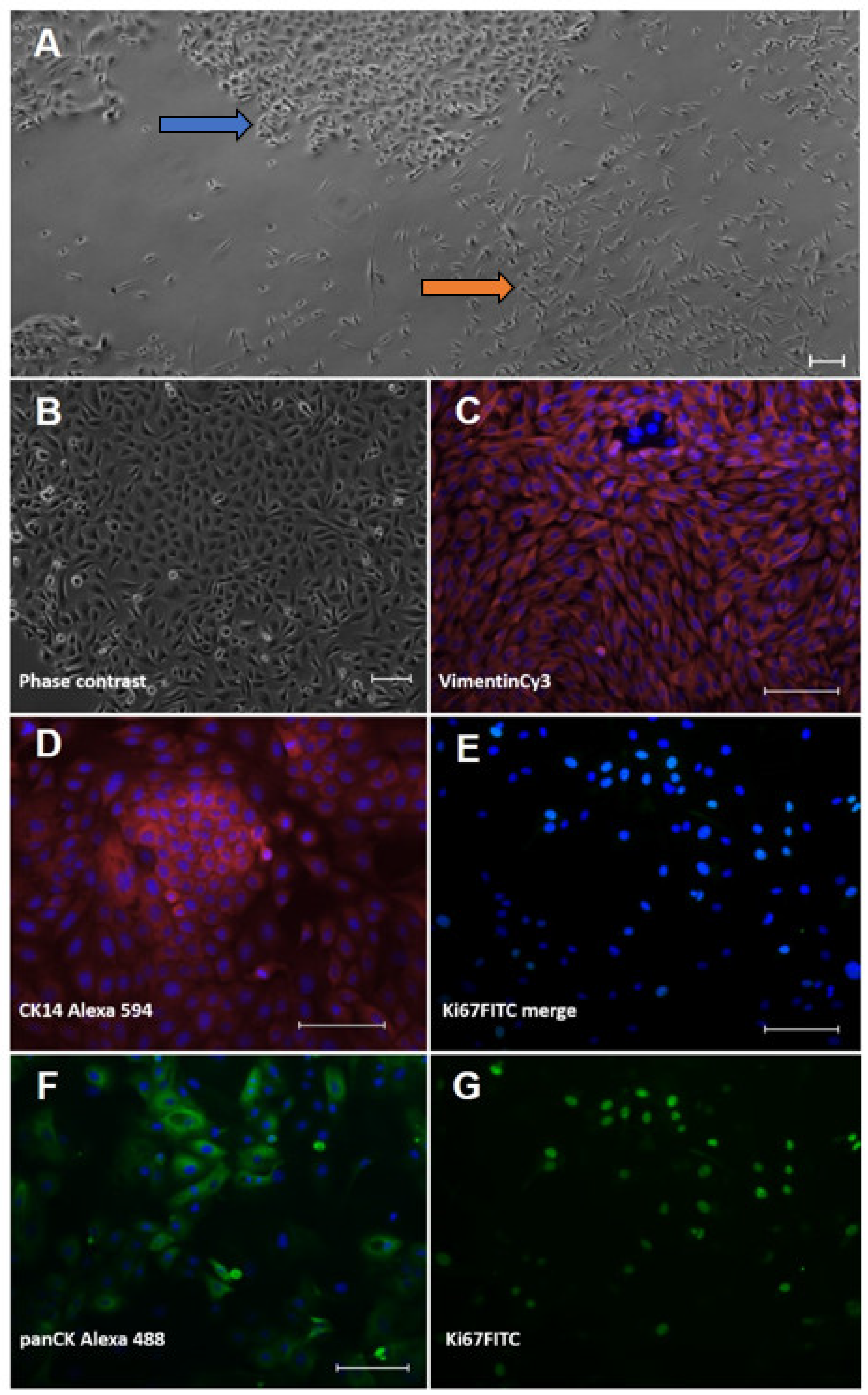
2.2. Immunofluorescent Analysis of Primary Isolated Cells
2.3. Cell Stimulation
2.4. RNA Isolation, cDNA Synthesis and RT-qPCR
2.5. ELISA
2.6. Statistical Analysis
3. Results
3.1. Characterisation of Ovine Interdigital Skin Keratinocytes and Fibroblasts
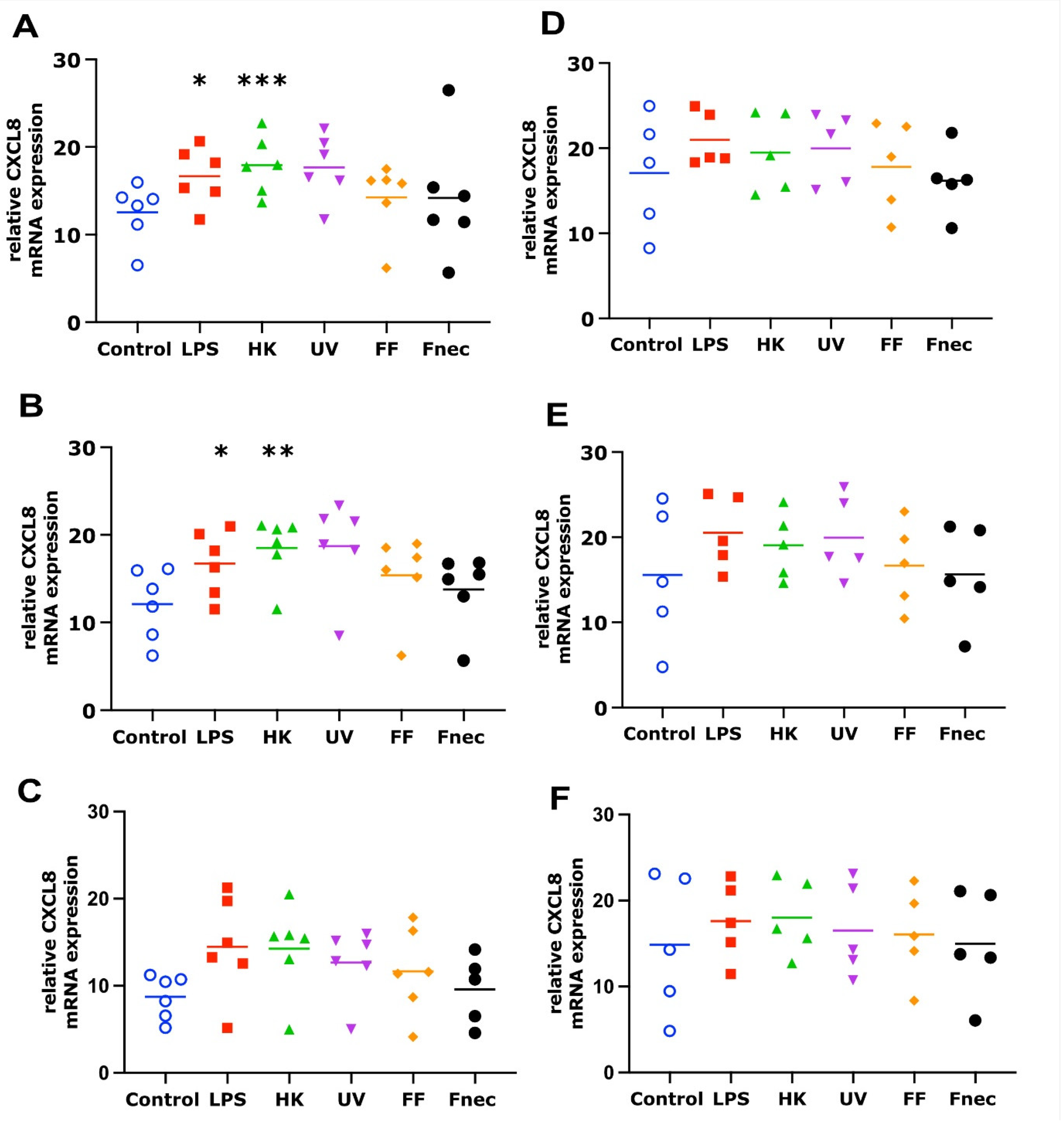
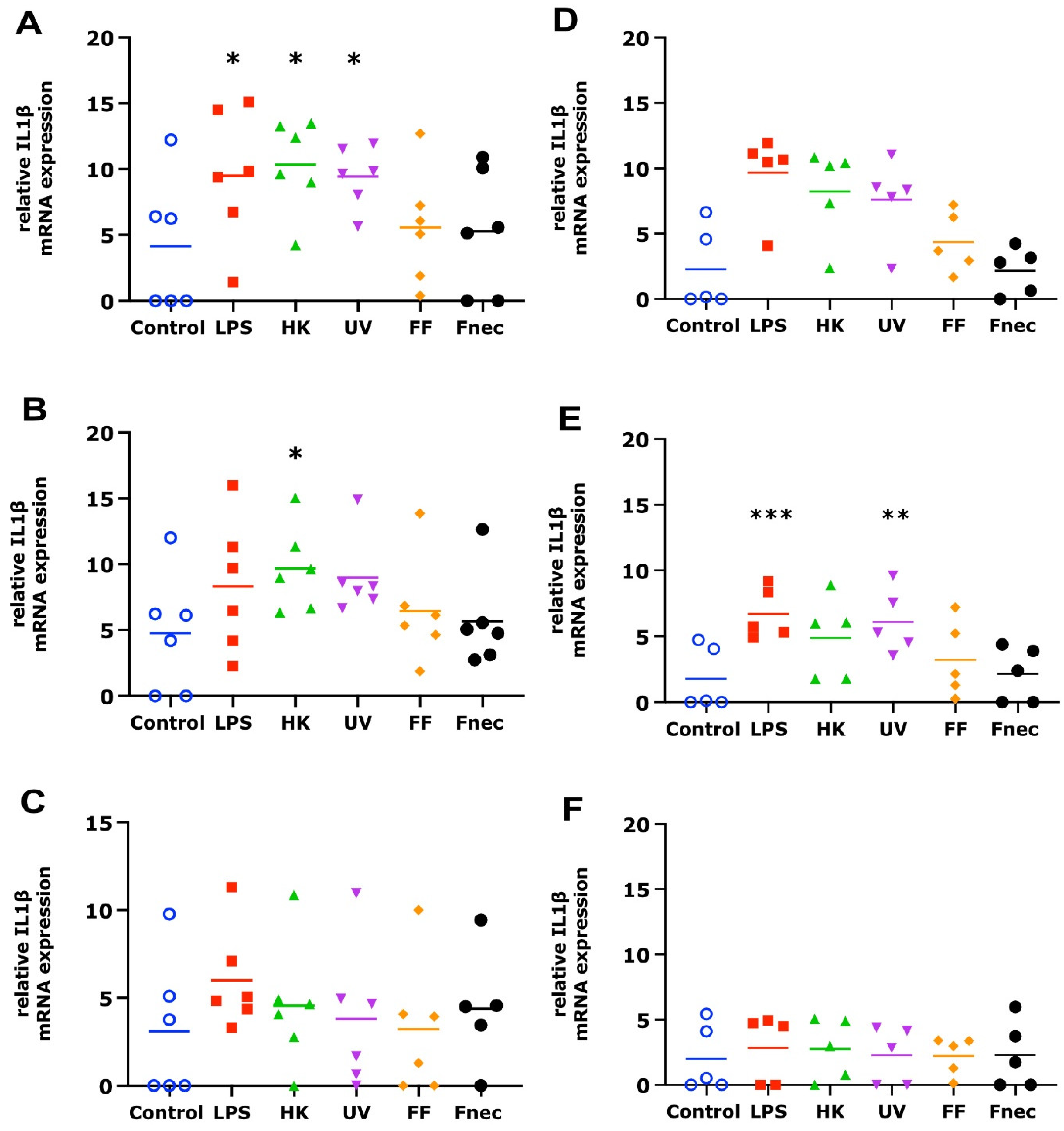
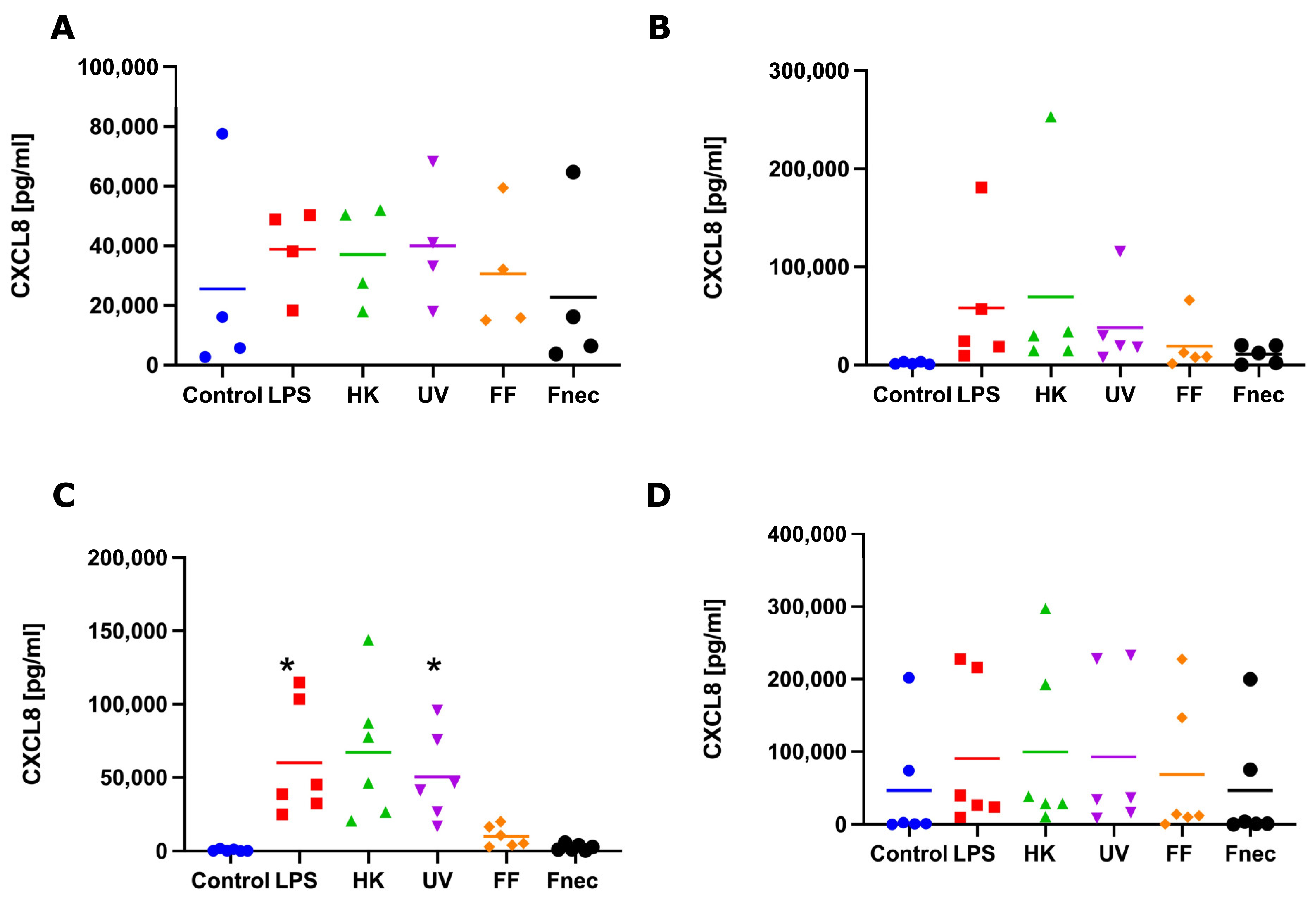
3.2. Expression of Pro-Inflammatory Mediators in Primary Ovine Skin Keratinocytes and Fibroblasts in Response to D. nodosus Stimulation
4. Discussion
4.1. Primary Ovine Interdigital Skin Cell Cultures as a Model for Infection
4.2. Response of Ovine Interdigital Skin Fibroblasts and Keratinocytes to Microbial Preparations
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Winter:, J.R.; Kaler, J.; Ferguson, E.; KilBride, A.L.; Green, L.E. Changes in prevalence of, and risk factors for, lameness in random samples of English sheep flocks: 2004–2013. Prev. Vet. Med. 2015, 122, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Prosser, N.S.; Purdy, K.J.; Green, L.E. Increase in the flock prevalence of lameness in ewes is associated with a reduction in farmers using evidence-based management of prompt treatment: A longitudinal observational study of 154 English sheep flocks 2013–2015. Prev. Vet. Med. 2019, 173, 104801. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Remnant, J.G.; Green, M.; Gascoigne, E.; Gibbon, N.; Hyde, R.; Porteous, J.R.; Schubert, K.; Lovatt, F.; Corbishley, A. Quantitative analysis of antibiotic usage in British sheep flocks. Vet. Rec. 2017, 181, 511. [Google Scholar] [CrossRef]
- Kennan, R.M.; Gilhuus, M.; Frosth, S.; Seemann, T.; Dhungyel, O.P.; Whittington, R.J.; Boyce, J.D.; Powell, D.R.; Aspán, A.; Jørgensen, H.J.; et al. Genomic Evidence for a Globally Distributed, Bimodal Population in the Ovine Footrot Pathogen Dichelobacter nodosus. mBio 2014, 5, e01821-14. [Google Scholar] [CrossRef] [Green Version]
- Agbaje, M.; Rutland, C.; Maboni, G.; Blanchard, A.; Bexon, M.; Stewart, C.; Jones, M.A.; Totemeyer, S. Novel inflammatory cell infiltration scoring system to investigate healthy and footrot affected ovine interdigital skin. PeerJ 2018, 6, e5097. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, A.M.; Jolley, K.A.; Maiden, M.C.J.; Coffey, T.J.; Maboni, G.; Staley, C.E.; Bollard, N.J.; Warry, A.; Emes, R.D.; Davies, P.L.; et al. The Applied Development of a Tiered Multilocus Sequence Typing (MLST) Scheme for Dichelobacter nodosus. Front. Microbiol. 2018, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, A.M.; Staley, C.E.; Shaw, L.; Wattegedera, S.R.; Baumbach, C.-M.; Michler, J.K.; Rutland, C.; Back, C.; Newbold, N.; Entrican, G.; et al. A Trifecta of New Insights into Ovine Footrot for Infection Drivers, Immune Response, and Host-Pathogen Interactions. Infect. Immun. 2021, 89, IAI0027021. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, W.I.B. Foot-Rot in Sheep: A Transmissible Disease due to Infection with Fusiformis nodosus(n. sp.). Studies on Its Cause, Epidemiology, and Control; H.E. Daw for Council for Scientific and Industrial Research: Melbourne, Australia, 1941. [Google Scholar] [CrossRef]
- Clifton, R.; Giebel, K.; Liu, N.L.B.H.; Purdy, K.J.; Green, L.E. Sites of persistence of Fusobacterium necrophorum and Dichelobacter nodosus: A paradigm shift in understanding the epidemiology of footrot in sheep. Sci. Rep. 2019, 9, 14429. [Google Scholar] [CrossRef] [Green Version]
- Egerton, J.R.; Roberts, D.S.; Parsonson, I.M. The aetiology and pathogenesis of ovine foot-rot. A histological study of the bacterial invasion. J. Comp. Pathol. 1969, 79, 207-IN7. [Google Scholar] [CrossRef]
- Roberts, D.S.; Egerton, J.R. The aetiology and pathogenesis of ovine foot-rot. The pathogenic association of Fusiformis nodosus and F. necrophorus. J. Comp. Pathol. 1969, 79, 217–227. [Google Scholar] [CrossRef]
- Kennan, R.M.; Wong, W.; Dhungyel, O.P.; Han, X.; Wong, D.; Parker, D.; Rosado, C.J.; Law, R.H.P.; McGowan, S.; Reeve, S.B.; et al. The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates. PLoS Pathog. 2010, 6, e1001210. [Google Scholar] [CrossRef]
- Kennan, R.M.; Han, X.; Porter, C.J.; Rood, J.I. The pathogenesis of ovine footrot. Vet. Microbiol. 2011, 153, 59–66. [Google Scholar] [CrossRef]
- Calvo-Bado, L.A.; Oakley, B.B.; Dowd, S.E.; Green, L.E.; Medley, G.F.; Ul-Hassan, A.; Bateman, V.; Gaze, W.; Witcomb, L.; Grogono-Thomas, R.; et al. Ovine pedomics: The first study of the ovine foot 16S rRNA-based microbiome. ISME J. 2011, 5, 1426–1437. [Google Scholar] [CrossRef]
- Maboni, G.; Blanchard, A.; Frosth, S.; Stewart, C.; Emes, R.; Tötemeyer, S. A distinct bacterial dysbiosis associated skin inflammation in ovine footrot. Sci. Rep. 2017, 7, 45220. [Google Scholar] [CrossRef] [Green Version]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, C.D.; Pilling, D.; Lord, J.M.; Akbar, A.N.; Scheel-Toellner, D.; Salmon, M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001, 22, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.; Heawood, C.; Sessford, K.; Baker, M.; Baiker, K.; Blacklaws, B.; Kaler, J.; Green, L.; Tötemeyer, S. Differential expression of Toll-like receptors and inflammatory cytokines in ovine interdigital dermatitis and footrot. Vet. Immunol. Immunopathol. 2014, 161, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maboni, G.; Davenport, R.; Sessford, K.; Baiker, K.; Jensen, T.K.; Blanchard, A.; Wattegedera, S.; Entrican, G.; Tötemeyer, S. A Novel 3D Skin Explant Model to Study Anaerobic Bacterial Infection. Front. Cell. Infect. Microbiol. 2017, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Witcomb, L.A.; Green, L.E.; Kaler, J.; Ul-Hassan, A.; Calvo-Bado, L.A.; Medley, G.F.; Grogono-Thomas, R.; Wellington, E.M. A longitudinal study of the role of Dichelobacter nodosus and Fusobacterium necrophorum load in initiation and severity of footrot in sheep. Prev. Vet. Med. 2014, 115, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sow, F.B.; Gallup, J.M.; Olivier, A.; Krishnan, S.; Patera, A.C.; Suzich, J.; Ackermann, M.R. Respiratory syncytial virus is associ-ated with an inflammatory response in lungs and architectural remodeling of lung-draining lymph nodes of newborn lambs. Am. J. Physiol-Lung. C. 2011, 300, L12–L24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, J.; Kwong, W.Y.; Li, D.; Salter, A.M.; Lea, R.G.; Sinclair, K.D. Effects of omega-3 and -6 polyunsaturated fatty acids on ovine follicular cell steroidogenesis, embryo development and molecular markers of fatty acid metabolism. Reproduction 2011, 141, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darlay, R.J.; McCarthy, A.J.; Illot, N.E.; Smith, J.E.; Shaw, M.-A. Novel polymorphisms in ovine immune response genes and their association with abortion. Anim. Genet. 2011, 42, 535–543. [Google Scholar] [CrossRef]
- Haig, D.; Deane, D.; Percival, A.; Myatt, N.; Thomson, J.; Inglis, L.; Rothel, J.; Seow, H.; Wood, P.; Miller, H.R.P.; et al. The cytokine response of afferent lymph following orf virus reinfection of sheep. Vet. Dermatol. 1996, 7, 11–20. [Google Scholar] [CrossRef]
- Wattegedera, S.R.; Corripio-Miyar, Y.; Pang, Y.; Frew, D.; McNeilly, T.N.; Palarea-Albaladejo, J.; McInnes, C.J.; Hope, J.C.; Glass, E.J.; Entrican, G. Enhancing the toolbox to study IL-17A in cattle and sheep. Vet. Res. 2017, 48, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanolari, P.; Dürr, S.; Jores, J.; Steiner, A.; Kuhnert, P. Ovine footrot: A review of current knowledge. Vet. J. 2021, 271, 105647. [Google Scholar] [CrossRef] [PubMed]
- Gstraunthaler, G.; Lindl, T. Zell- und Gewebekultur; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Watkins, C.A.; Mackellar, A.; Frew, D.; Mackie, C.; George, A.; Hopkins, J.; Burgess, S.T.G.; Mcneilly, T.N.; Huntley, J.F. Gene expression profiling of ovine keratinocytes stimulated with Psoroptes ovis mite antigen—A preliminary study. Parasite Immunol. 2009, 31, 304–311. [Google Scholar] [CrossRef]
- Pisansarakit, P.; du Cros, D.; Moore, G.P.M. Cultivation of keratinocytes derived from epidermal explants of sheep skin and the roles of growth factors in the regulation of proliferation. Arch Derm. Res. 1990, 281, 530–535. [Google Scholar] [CrossRef]
- Saito, M.; Payne, M.S.; Miura, Y.; Ireland, D.J.; Stock, S.; Kallapur, S.G.; Kannan, P.S.; Newnham, J.P.; Kramer, B.W.; Jobe, A.H.; et al. Polymyxin B Agonist Capture Therapy for Intrauterine Inflammation: Proof-of-Principle in a Fetal Ovine Model. Reprod. Sci. 2014, 21, 623–631. [Google Scholar] [CrossRef]
- Schneider, L.E.; Protschka, M.; Müller, U.; Muhsen, M.; Magin, T.; Anderegg, U.; Saalbach, A.; Büttner, M.; Alber, G.; Siegemund, S. Orf virus infection of human keratinocytes and dermal fibroblasts: Limited virus detection and interference with intercellular adhesion molecule-1 up-regulation. Exp. Dermatol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Scagliarini, A.; Pozzo, F.D.; Gallina, L.; Guercio, A.; De Clercq, E.; Snoeck, R.; Andrei, G. Ovine Skin Organotypic Cultures Applied to the Ex vivo Study of Orf Virus Infection. Vet. Res. Commun. 2005, 29, 245–247. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Pastor, D.M.; Poritz, L.S.; Olson, T.L.; Kline, C.L.; Harris, L.R.; Koltun, W.A.; Chinchilli, V.M.; Irby, R.B. Primary cell lines: False representation or model system? a comparison of four human colorectal tumors and their coordinately established cell lines. Int. J. Clin. Exp. Med. 2010, 3, 69–83. [Google Scholar]
- Hegde, S.; Gabriel, C.; Kragl, M.; Chopra-Dewasthaly, R. Sheep primary cells as in vitro models to investigate Mycoplasma agalactiae host cell interactions. Pathog. Dis. 2015, 73, ftv048. [Google Scholar] [CrossRef] [Green Version]
- Evans, N.J.; Brown, J.M.; Scholey, R.; Murray, R.D.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Differential inflammatory responses of bovine foot skin fibroblasts and keratinocytes to digital dermatitis treponemes. Vet. Immunol. Immunopathol. 2014, 161, 12–20. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Zhao, H.; Qiao, J.; Liu, S.; Deng, Z.; Lei, X.; Ning, L.; Cao, Y.; Zhao, Y.; et al. Ovine Hair Follicle Stem Cells Derived from Single Vibrissae Reconstitute Haired Skin. IJMS 2015, 16, 17779–17797. [Google Scholar] [CrossRef] [Green Version]
- Gauglitz, G.G.; Zedler, S.; Spiegel, F.V.; Fuhr, J.; Donnersmarck, G.H.V.; Faist, E. Functional Characterization of Cultured Keratinocytes after Acute Cutaneous Burn Injury. PLoS ONE 2012, 7, e29942. [Google Scholar] [CrossRef] [Green Version]
- Michler, J.K.; Hillmann, A.; Savkovic, V.; Mülling, C.K.W. Horse hair follicles: A novel dermal stem cell source for equine regenerative medicine: Dermal Stem Cells from Horse Hair Follicles. Cytometry 2018, 93, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Scholey, R.; Evans, N.; Blowey, R.; Massey, J.; Murray, R.; Smith, R.; Ollier, W.; Carter, S. Identifying host pathogenic pathways in bovine digital dermatitis by RNA-Seq analysis. Vet. J. 2013, 197, 699–706. [Google Scholar] [CrossRef]
- Nolan, T.J.; Gadsby, N.J.; Hellyer, T.P.; Templeton, K.E.; McMullan, R.; McKenna, J.P.; Rennie, J.; Robb, C.T.; Walsh, T.S.; Rossi, A.G.; et al. Low-pathogenicity Mycoplasma spp. alter human monocyte and macrophage function and are highly prevalent among patients with ventilator-acquired pneumonia. Thorax 2016, 71, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, E.; Grandhi, J.; Wewers, M.D.; Gavrilin, M.A. Mycoplasma Suppression of THP-1 Cell TLR Responses Is Corrected with Antibiotics. PLoS ONE 2010, 5, e9900. [Google Scholar] [CrossRef]
- Drexler, H.G.; Uphoff, C.C. Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology 2002, 39, 75–90. [Google Scholar] [CrossRef] [PubMed]

| Target | Primer | Size | GenBank ID | Source |
|---|---|---|---|---|
| Ov_Actin_F | TGTGCGTGACATCAAGGAGAA | 67 bp | AF129289 | [21] |
| Ov_Actin_R | CGCAGTGGCCATCTCCTG | |||
| Ov_PPIA_F | TGAGCACTGGAGAGAAAGGATTTG | 84 bp | AY251270 | [22] |
| Ov_PPIA_R | AGTCACCACCCTGGCACATAA | |||
| Ov_IL1 β _F | TTCTGCATGAGCTTCGTACAA | 115 bp | X54796 | [23] |
| Ov_IL1 β _R | GGGTCGGTGTATCACCTTTTT | |||
| Ov_CXCL8_F | GAGAAGTCCTCTGGGACAGC | 102 bp | NM_001009401.1 | [15] |
| Ov_CXCL8_R | CAGCCAGCTTGGAAGTCATA |
| Target | Capture Antibody | Protein Standard | Secondary Antibody | Tertiary Antibody | Standard Curve Range |
|---|---|---|---|---|---|
| IL-1β | Bio-Rad Mouse anti Ovine IL-1β clone 1D4 (MCA1658, 1:200) | Ovine IL-1β RP0656V-005 | rabbit anti sheep IL-1β polyclonal (AHP423, 1:500) | Dako (PO448) Goat anti rabbit-HRP conjugate (1:500) | 375–30,000 pg/mL |
| CXCL8 | Bio-Rad Mouse anti Ovine IL-8 clone 8M6 (MCA1660, 1:200) | Kingfisher Biotech Ovine & Caprine IL-8 (CXCL8) RP0488V-005 | Bio-Rad rabit anti-sheep IL-8 polyclonal (AHP425, 1:500) | Dako (PO448) goat anti rabbit-HRP (1:500) | 25.6–1640 pg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanchard, A.M.; Baumbach, C.-M.; Michler, J.K.; Pickwell, N.D.; Staley, C.E.; Franklin, J.M.; Wattegedera, S.R.; Entrican, G.; Tötemeyer, S. Natural Mycoplasma Infection Reduces Expression of Pro-Inflammatory Cytokines in Response to Ovine Footrot Pathogens. Animals 2022, 12, 3235. https://doi.org/10.3390/ani12233235
Blanchard AM, Baumbach C-M, Michler JK, Pickwell ND, Staley CE, Franklin JM, Wattegedera SR, Entrican G, Tötemeyer S. Natural Mycoplasma Infection Reduces Expression of Pro-Inflammatory Cytokines in Response to Ovine Footrot Pathogens. Animals. 2022; 12(23):3235. https://doi.org/10.3390/ani12233235
Chicago/Turabian StyleBlanchard, Adam M., Christina-Marie Baumbach, Jule K. Michler, Natalie D. Pickwell, Ceri E. Staley, Jemma M. Franklin, Sean R. Wattegedera, Gary Entrican, and Sabine Tötemeyer. 2022. "Natural Mycoplasma Infection Reduces Expression of Pro-Inflammatory Cytokines in Response to Ovine Footrot Pathogens" Animals 12, no. 23: 3235. https://doi.org/10.3390/ani12233235






