A Smart Textile Band Achieves High-Quality Electrocardiograms in Unrestrained Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing Conditions
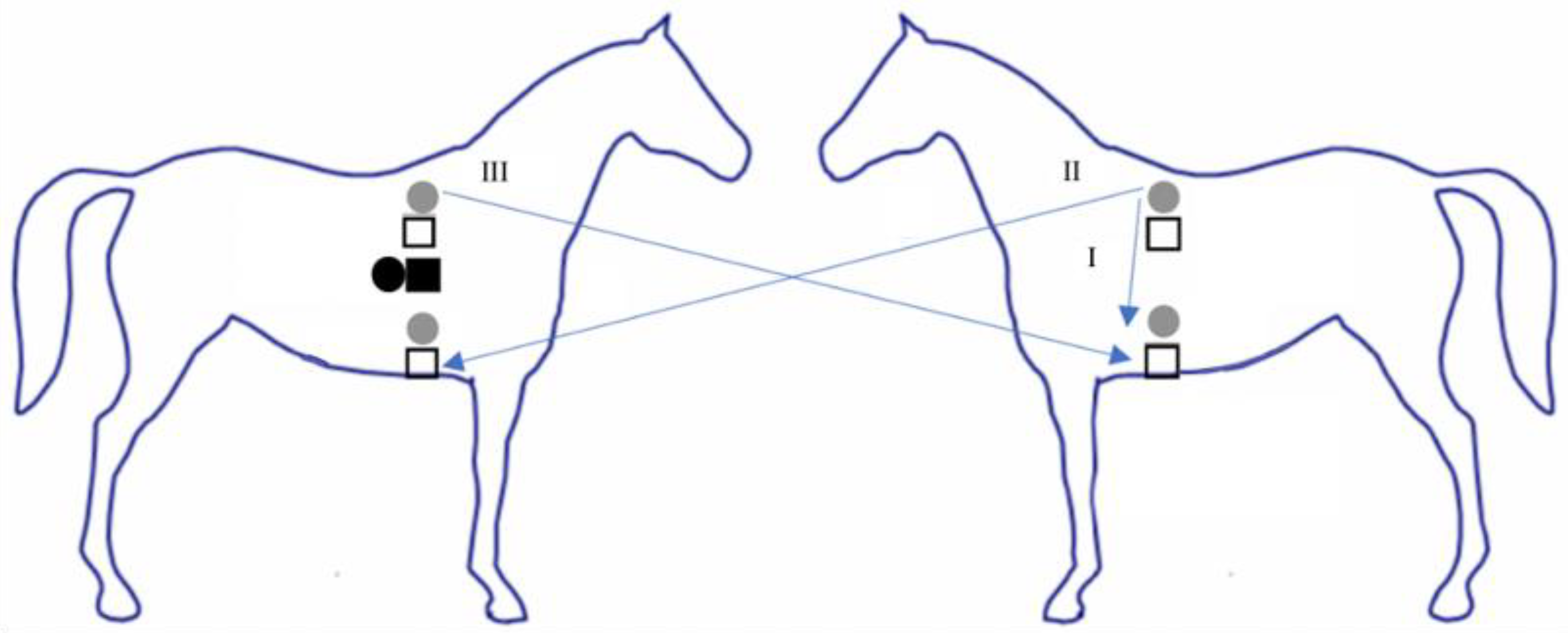
2.2. Experimental Protocol
2.3. Data Analysis
3. Results
3.1. Kurtosis and kSQI
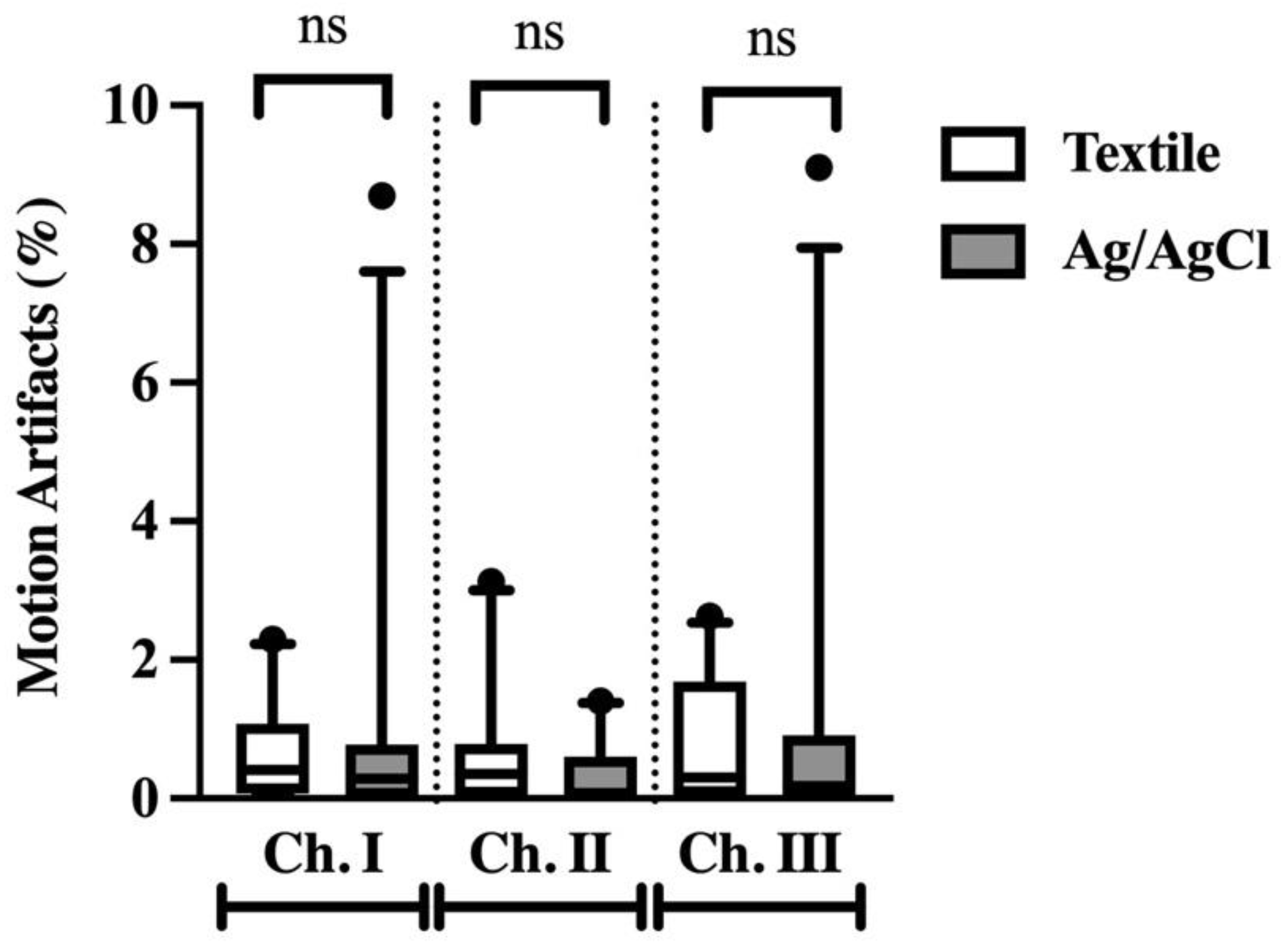
3.2. Percentage of Motion Artifacts
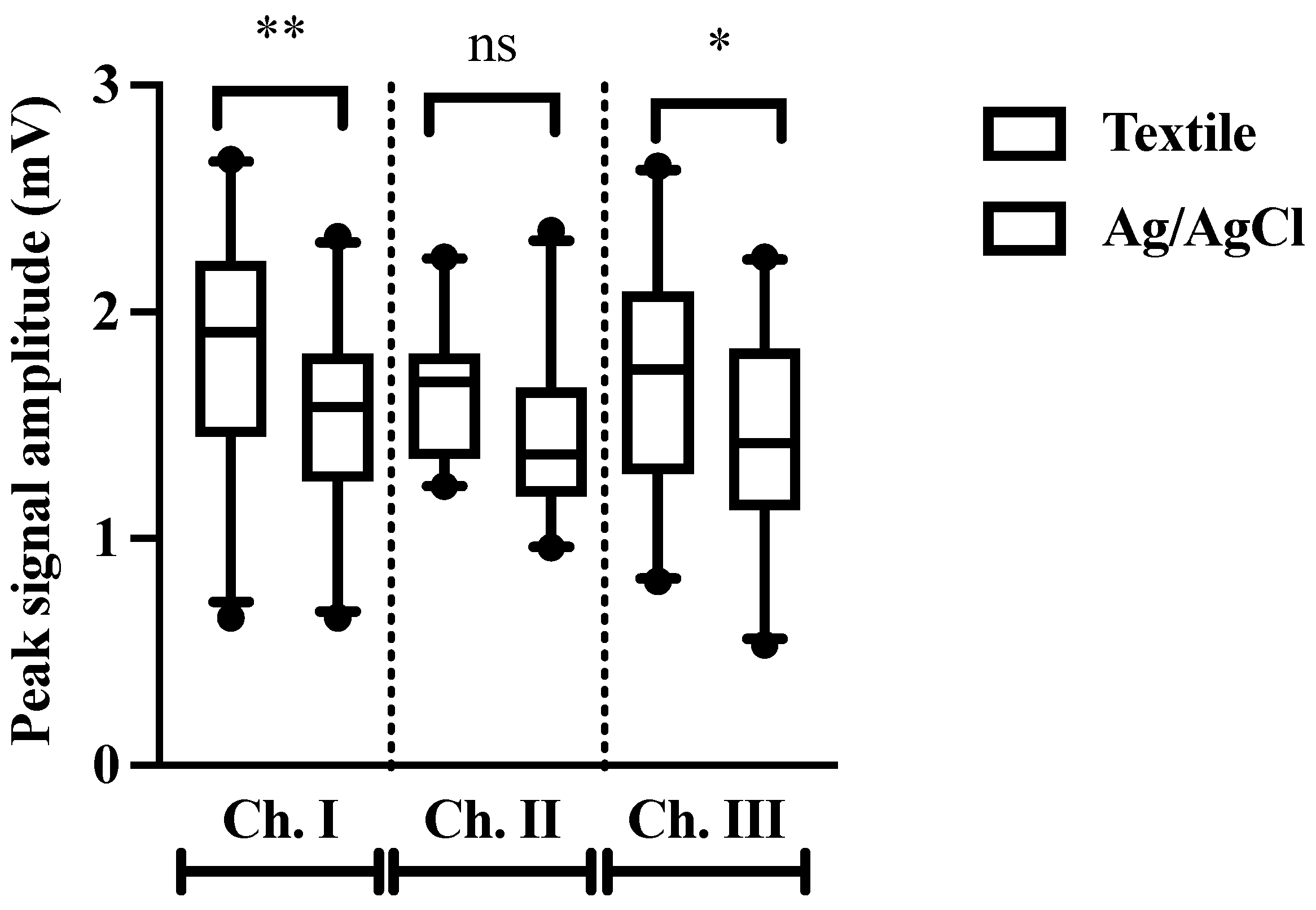
3.3. Peak Amplitude
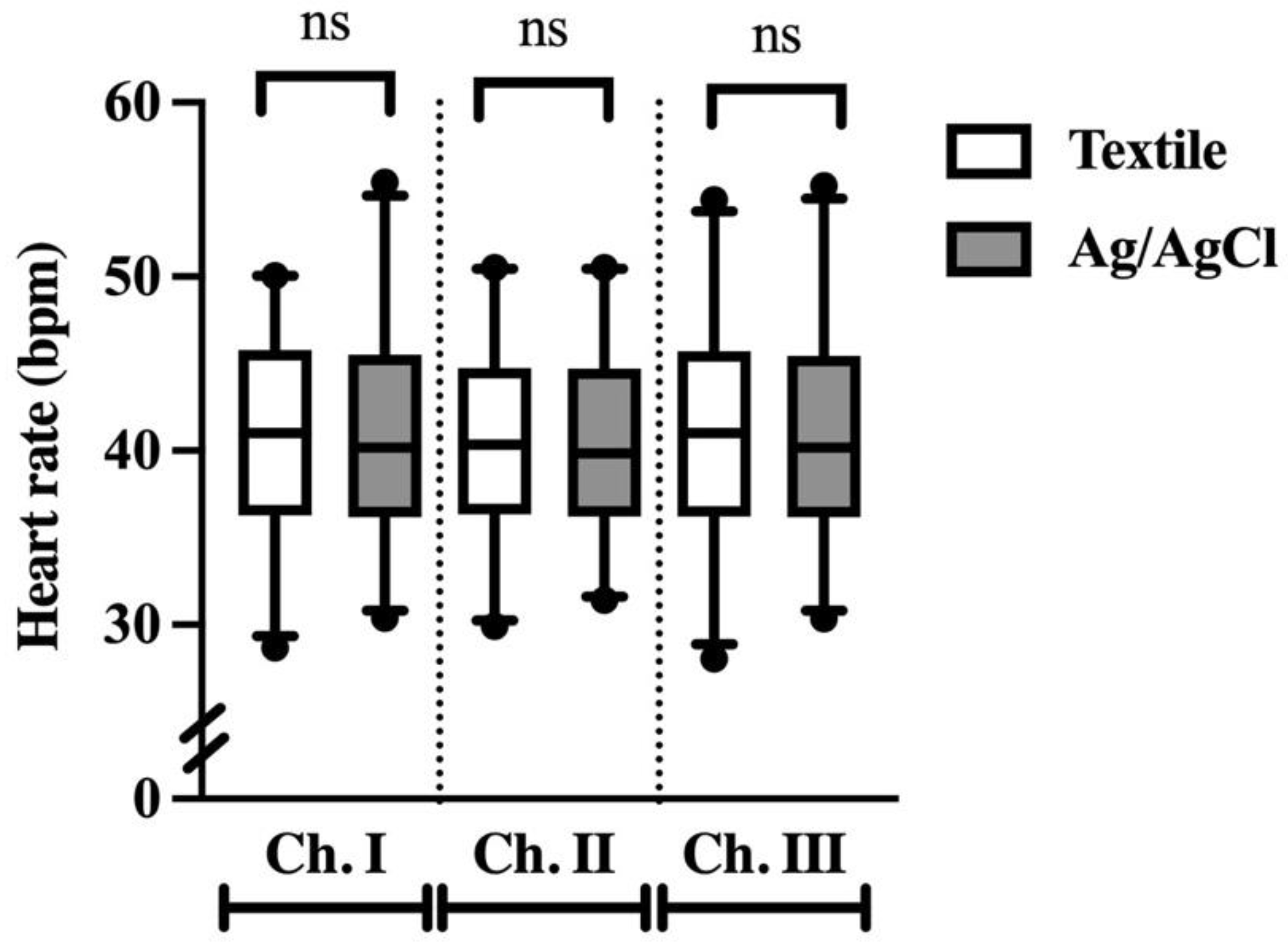
3.4. Heart Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamlin, R.L.; Klepinger, W.L.; Gilpin, K.W.; Smith, C.R. Autonomic Control of Heart Rate in the Horse. Am. J. Physiol. 1972, 222, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Young, L.E.; van Loon, G. Diseases of the Heart and Vessels, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; ISBN 978-0-70-204771-8. [Google Scholar]
- Ryan, N.; Marr, C.M.; McGladdery, A.J. Survey of Cardiac Arrhythmias during Submaximal and Maximal Exercise in Thoroughbred Racehorses. Equine Vet. J. 2005, 37, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Physick-Sheard, P.W.; McGurrin, M.K.J. Ventricular arrhythmias during race recovery in Standardbred racehorses and associations with autonomic activity. J. Vet. Intern. Med. 2010, 24, 1158–1166. [Google Scholar] [CrossRef]
- Buhl, R.; Petersen, E.E.; Lindholm, M.; Bak, L.; Nostell, K. Cardiac Arrhythmias in Standardbreds during and after Racing-Possible Association between Heart Size, Valvular Regurgitations, and Arrhythmias. J. Equine Vet. Sci. 2013, 33, 590–596. [Google Scholar] [CrossRef]
- Barbesgaard, L.; Buhl, R.; Meldgaard, C. Prevalence of Exercise-Associated Arrhythmias in Normal Performing Dressage Horses. Equine Vet. J. 2010, 42, 202–207. [Google Scholar] [CrossRef]
- Lyle, C.H.; Uzal, F.A.; Mcgorum, B.C.; Aida, H.; Blissitt, K.J.; Case, J.T.; Charles, J.T.; Gardner, I.; Horadagoda, N.; Kusano, K.; et al. Sudden Death in Racing Thoroughbred Horses: An International Multicentre Study of Post Mortem Findings. Equine Vet. J. 2011, 43, 324–331. [Google Scholar] [CrossRef]
- Lyle, C.H.; Blissitt, K.J.; Kennedy, R.N.; McGorum, B.C.; Newton, J.R.; Parkin, T.D.H.; Stirk, A.; Boden, L.A. Risk Factors for Race-Associated Sudden Death in Thoroughbred Racehorses in the UK (2000–2007). Equine Vet. J. 2012, 44, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Corrado, D.; Basso, C.; Schiavon, M.; Pelliccia, A.; Thiene, G. Pre-Participation Screening of Young Competitive Athletes for Prevention of Sudden Cardiac Death. J. Am. Coll. Cardiol. 2008, 52, 1981–1989. [Google Scholar] [CrossRef]
- Brown, C.M.; Kaneene, J.B.; Taylor, R.F. Sudden and Unexpected Death in Horses and Ponies: An Analysis of 200 Cases. Equine Vet. J. 1988, 20, 99–103. [Google Scholar] [CrossRef]
- Lyle, C.H.; Turley, G.; Blissitt, K.J.; Pirie, R.S.; Mayhew, I.G.; Mcgorum, B.C.; Keen, J.A. Retrospective evaluation of episodic collapse in the horse in a referred population: 25 cases (1995–2009). J. Vet. Intern. Med. 2010, 24, 1498–1502. [Google Scholar] [CrossRef]
- Lyle, C.H.; Keen, J.A. Episodic Collapse in the Horse. Equine Vet. Educ. 2010, 22, 576–586. [Google Scholar] [CrossRef]
- Mitchell, K.J. Equine Electrocardiography. Vet. Clin. N. Am.-Equine Pract. 2019, 35, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Lymberis, A.; Olsson, S. Intelligent biomedical clothing for personal health and disease management: State of the art and future vision. Telemed. J. e-Health 2003, 9, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Pantelopoulos, A.; Bourbakis, N.G. A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2010, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cherenack, K.; Van Pieterson, L. Smart Textiles: Challenges and Opportunities. J. Appl. Phys. 2012, 112, 091301. [Google Scholar] [CrossRef] [Green Version]
- McGreevy, P.D.; Sundin, M.; Karlsteen, M.; Berglin, L.; Ternström, J.; Hawson, L.; Richardsson, H.; McLean, A.N. Problems at the Human-Horse Interface and Prospects for Smart Textile Solutions. J. Vet. Behav. Clin. Appl. Res. 2014, 9, 34–42. [Google Scholar] [CrossRef]
- Le, K.; Narayana, H.; Servati, A.; Bahi, A.; Soltanian, S.; Servati, P.; Ko, F. Electronic Textiles for Electrocardiogram Monitoring: A Review on the Structure–Property and Performance Evaluation from Fiber to Fabric. Text. Res. J. 2022. [Google Scholar] [CrossRef]
- Reef, V.B.; Bonagura, J.; Buhl, R.; Mcgurrin, M.K.J.; Schwarzwald, C.C.; van Loon, G.; Young, L.E. Recommendations for Management of Equine Athletes with Cardiovascular Abnormalities. J. Vet. Intern. Med. 2014, 28, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Vitale, V.; Balocchi, R.; Varanini, M.; Sgorbini, M.; Macerata, A.; Sighieri, C.; Baragli, P. The Effects of Restriction of Movement on the Reliability of Heart Rate Variability Measurements in the Horse (Equus caballus). J. Vet. Behav. Clin. Appl. Res. 2013, 8, 400–403. [Google Scholar] [CrossRef]
- Guidi, A.; Lanata, A.; Valenza, G.; Scilingo, E.P.; Baragli, P. Validation of Smart Textile Electrodes for Electrocardiogram Monitoring in Free-Moving Horses. J. Vet. Behav. 2017, 17, 19–23. [Google Scholar] [CrossRef]
- Felici, M.; Nardelli, M.; Lanatà, A.; Sgorbini, M.; Pasquale Scilingo, E.; Baragli, P. Smart Textiles Biotechnology for Electrocardiogram Monitoring in Horses during Exercise on Treadmill: Validation Tests. Equine Vet. J. 2021, 53, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, S.; Chen, K.C. The Effects of Washing Environment on the Resistance of Silver Plated Nylon and Stainless Steel Conductive Threads; Cal Poly State University: San Luis Obispo, CA, USA, 2015. [Google Scholar]
- Schwarz, A.; Kazani, I.; Cuny, L.; Hertleer, C.; Ghekiere, F.; de Clercq, G.; Van Langenhove, L. Comparative Study on the Mechanical Properties of Elastic, Electro-Conductive Hybrid Yarns and Their Input Materials. Text. Res. J. 2011, 81, 1713–1723. [Google Scholar] [CrossRef]
- Penhaker, M.; Polomik, J.; Kubicek, J.; Kasik, V. Biopotential Conducting Polymer Electrodes Design and Realization for ECG Measurement. In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies, Porto, Portugal, 21–23 February 2017; pp. 134–141. [Google Scholar] [CrossRef]
- Neuman, M.R. Biopotential Electrodes. In Medical Instrumentation Application and Design; Webster, J.G., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 189–240. [Google Scholar]
- Li, Q.; Mark, R.G.; Clifford, G.D. Robust Heart Rate Estimation from Multiple Asynchronous Noisy Sources Using Signal Quality Indices and a Kalman Filter. Physiol. Meas. 2008, 29, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Clifford, G.D.; Behar, J.; Li, Q.; Rezek, I. Signal Quality Indices and Data Fusion for Determining Clinical Acceptability of Electrocardiograms. Physiol. Meas. 2012, 33, 1419–1433. [Google Scholar] [CrossRef] [Green Version]
- Behar, J.; Oster, J.; Li, Q.; Clifford, G.D. ECG Signal Quality during Arrhythmia and Its Application to False Alarm Reduction. IEEE Trans. Biomed. Eng. 2013, 60, 1660–1666. [Google Scholar] [CrossRef]
- Scilingo, E.P.; Gemignani, A.; Paradiso, R.; Taccini, N.; Ghelarducci, B.; De Rossi, D. Performance Evaluation of Sensing Fabrics for Monitoring Physiological and Biomechanical Variables. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 345–352. [Google Scholar] [CrossRef]
- Vezzosi, T.; Sgorbini, M.; Bonelli, F.; Buralli, C.; Pillotti, M.; Meucci, V.; Tognetti, R. Evaluation of a Smartphone Electrocardiograph in Healthy Horses: Comparison With Standard Base-Apex Electrocardiography. J. Equine Vet. Sci. 2018, 67, 61–65. [Google Scholar] [CrossRef]
- Vezzosi, T.; Tognetti, R.; Buralli, C.; Marchesotti, F.; Patata, V.; Zini, E.; Domenech, O. Home Monitoring of Heart Rate and Heart Rhythm with a Smartphone-Based ECG in Dogs. Vet. Rec. 2019, 184, 96. [Google Scholar] [CrossRef]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.F.; Abächerli, R.; Meyer, V.R.; Rossi, R.M. Embroidered Electrode with Silver/Titanium Coating for Long-Term ECG Monitoring. Sensors 2015, 15, 1750–1759. [Google Scholar] [CrossRef]
- Abu-Saude, M.; Morshed, B.I. Characterization of a Novel Polypyrrole (PPy) Conductive Polymer Coated Patterned Vertical CNT (PvCNT) Dry ECG Electrode. Chemosensors 2018, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Clifford, G.; Tarassenko, L. Application of Independent Component Analysis in Removing Artefacts from the Electrocardiogram. Neural Comput. Appl. 2006, 15, 105–116. [Google Scholar] [CrossRef]
- Clifford, G.D.; Azuaje, F.; McSharry, P.E. Advanced Methods and Tools for ECG Data Analysis; Artech House: Boston, MA, USA, 2006; pp. 1–400. [Google Scholar]
- Webster, J.G. Reducing Motion Artifacts and Interference in Biopotential Recording. IEEE Trans. Biomed. Eng. 1984, BME-31, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, D.S.; Bitschnau, C.; Waldern, N.; Weishaupt, M.A.; Schwarzwald, C.C. Observer Agreement for Detection of Cardiac Arrhythmias on Telemetric ECG Recordings Obtained at Rest, during and after Exercise in 10 Warmblood Horses. Equine Vet. J. 2010, 42, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Buhl, R.; Meldgaard, C.; Barbesgaard, L. Cardiac Arrhythmias in Clinically Healthy Showjumping Horses. Equine Vet. J. 2010, 42, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Cottin, F.; Barrey, E.; Lopes, P.; Billat, V. Effect of Repeated Exercise and Recovery on Heart Rate Variability in Elite Trotting Horses during High Intensity Interval Training. Equine Vet. J. 2006, 38, 204–209. [Google Scholar] [CrossRef]
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of Textile Electrodes and Conductors Using Standardized Measurement Setups. Physiol. Meas. 2010, 31, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Pani, D.; Bonfiglio, A. Characterization of Screen-Printed Textile Electrodes Based on Conductive Polymer for ECG Acquisition. Comput. Cardiol. 2017, 44, 1–4. [Google Scholar] [CrossRef]
- Kim, S.; Leonhardt, S.; Zimmermann, N.; Kranen, P.; Kensche, D.; Müller, E.; Quix, C. Influence of Contact Pressure and Moisture on the Signal Quality of a Newly Developed Textile ECG Sensor Shirt. In Proceedings of the 2008 5th International Summer School and Symposium on Medical Devices and Biosensors, Hong Kong, China, 1–3 June 2008; pp. 256–259. [Google Scholar] [CrossRef]
- Taji, B.; Chan, A.D.C.; Shirmohammadi, S. Effect of Pressure on Skin-Electrode Impedance in Wearable Biomedical Measurement Devices. IEEE Trans. Instrum. Meas. 2018, 67, 1900–1912. [Google Scholar] [CrossRef]
- Takeshita, T.; Yoshida, M.; Takei, Y.; Ouchi, A.; Hinoki, A.; Uchida, H.; Kobayashi, T. Relationship between Contact Pressure and Motion Artifacts in ECG Measurement with Electrostatic Flocked Electrodes Fabricated on Textile. Sci. Rep. 2019, 9, 5897. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Hwang, S.H.; Yoon, H.N.; Lee, W.K.; Park, K.S. Heart Rate Variability Monitoring during Sleep Based on Capacitively Coupled Textile Electrodes on a Bed. Sensors 2015, 15, 11295–11311. [Google Scholar] [CrossRef] [Green Version]
- An, X.; Tangsirinaruenart, O.; Stylios, G.K. Investigating the Performance of Dry Textile Electrodes for Wearable End-Uses. J. Text. Inst. 2019, 110, 151–158. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCrae, P.; Spong, H.; Rutherford, A.-A.; Osborne, V.; Mahnam, A.; Pearson, W. A Smart Textile Band Achieves High-Quality Electrocardiograms in Unrestrained Horses. Animals 2022, 12, 3254. https://doi.org/10.3390/ani12233254
McCrae P, Spong H, Rutherford A-A, Osborne V, Mahnam A, Pearson W. A Smart Textile Band Achieves High-Quality Electrocardiograms in Unrestrained Horses. Animals. 2022; 12(23):3254. https://doi.org/10.3390/ani12233254
Chicago/Turabian StyleMcCrae, Persephone, Hannah Spong, Ashley-Ann Rutherford, Vern Osborne, Amin Mahnam, and Wendy Pearson. 2022. "A Smart Textile Band Achieves High-Quality Electrocardiograms in Unrestrained Horses" Animals 12, no. 23: 3254. https://doi.org/10.3390/ani12233254
APA StyleMcCrae, P., Spong, H., Rutherford, A.-A., Osborne, V., Mahnam, A., & Pearson, W. (2022). A Smart Textile Band Achieves High-Quality Electrocardiograms in Unrestrained Horses. Animals, 12(23), 3254. https://doi.org/10.3390/ani12233254




