Evaluation of an Innovative Zn Source on Feed Efficiency, Growth Performance, Skin and Bone Quality of Broilers Suffering Heat Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds
2.2. Diets
2.3. Samples Analysis
2.4. Experimental Design
2.5. Induced Heat Stress
2.6. Skin Resistance Test
2.7. Footpad Dermatitis Score
2.8. Bone Physical Characteristics
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajati, H.; Zaghari, M.; Oliveira, H. Arthrospira (Spirulina) Platensis Can Be Considered as a Probiotic Alternative to Reduce Heat Stress in Laying Japanese Quails. Braz. J. Poult. Sci. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M.; Oskoueian, E.; Le, H.H.; Shakeri, M. Strategies to combat heat stress in broiler chickens: Unveiling the roles of selenium, vitamin E and vitamin C. Vet. Sci. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Bao, Y.; Choct, M.; Iji, P.; Bruerton, K. Optimal dietary inclusion of organically complexed zinc for broiler chickens. Br. Poult. Sci. 2009, 50, 95–102. [Google Scholar] [CrossRef]
- Zaghari, M.; Avazkhanllo, M.; Ganjkhanlou, M. Reevaluation of male broiler zinc requirement by dose response trial using practical diet with added exogenous phytase. J. Agr. Sci. Tech. 2015, 17, 333–343. [Google Scholar]
- National Research Council. Nutrient Requirements for Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Aviagen. Broiler Nutrition Specification Ross 308; Aviagen: Huntsville, AL, USA, 2019. [Google Scholar]
- Ramiah, S.K.; Awad, E.A.; Mookiah, S.; Idrus, Z. Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions. Poult. Sci. 2019, 98, 3828–3838. [Google Scholar] [CrossRef]
- Poulsen, H.D. Zinc Oxide for Weanling Piglets. Acta Agric. Scand. Sect. A Anim. Sci. 1995, 45, 159–167. [Google Scholar] [CrossRef]
- Carlson, D.; Sehested, J.; Feng, Z.; Poulsen, H. Serosal zinc attenuate serotonin and vasoactive intestinal peptide induced secretion in piglet small intestinal epithelium in vitro. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Morgan, N.; Roberts, J.; Wu, S.-B.; Swick, R.; Toghyani, M. Zinc hydroxychloride supplementation improves tibia bone development and intestinal health of broiler chickens. Poult. Sci. 2021, 100, 101254. [Google Scholar] [CrossRef]
- Edwards, H.M.; Baker, D.H. Bioavailability of zinc in several sources of zinc oxide, zinc sulfate, and zinc metal. J. Anim. Sci. 1999, 77, 2730–2735. [Google Scholar] [CrossRef]
- Jondreville, C.; Lescoat, P.; Magnin, M.; Feuerstein, D.; Gruenberg, B.; Nys, Y. Sparing effect of microbial phytase on zinc supplementation in maize–soya-bean meal diets for chickens. Animal 2007, 1, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Nys, Y.; Jondreville, C. Zinc availability and digestive zinc solubility in piglets and broilers fed diets varying in their phytate contents, phytase activity and supplemented zinc source. Animal 2010, 4, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Sahin, K.; Sahin, N.; Kucuk, O.; Hayirli, A.; Prasad, A. Role of dietary zinc in heat-stressed poultry: A review. Poult. Sci. 2009, 88, 2176–2183. [Google Scholar] [CrossRef]
- Chandra, R.K.; Dayton, D.H. Trace element regulation of immunity and infection. Nutr. Res. 1982, 2, 721–733. [Google Scholar] [CrossRef]
- Dardenne, M.; Savino, W.; Borrih, S.; Bach, J.F. A zinc dependent epitope of the molecule of thymulin, a thymichormone. Proc. Natl. Acad. Sci. USA 1985, 82, 7035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, A.R. Zinc, copper and iron nutrition and immunity. J. Nutr. 1992, 122, 604–609. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Su, W.; Ying, Z.; He, J.; Zhang, L.; Zhong, X.; Wang, T. Zinc oxide nanoparticles as a substitute for zinc oxide or colistin sulfate: Effects on growth, serum enzymes, zinc deposition, intestinal morphology and epithelial barrier in weaned piglets. PLoS ONE 2017, 12, e0181136. [Google Scholar] [CrossRef] [Green Version]
- Medeiros-Ventura, W.; Rabello, C.; Barros, M.; Junior, R.S.; Oliveira, H.; Faria, A.; Silva, A.; Soares, P.; Pereira, C.; Santos, M.; et al. Zinc, manganese, and copper amino acid complexes improve performance and bone characteristics of layer-type chicks under thermoneutral and cold stress conditions. Poult. Sci. 2020, 99, 5718–5727. [Google Scholar] [CrossRef]
- Iranian Council of Animal Care. Guide to the Care and Use of Experimental Animals; Isfahan University of Technology: Isfahan, Iran, 1995. [Google Scholar]
- Noori, O.; Zaghari, M.; Mehrvarz, H. Scrutinizing Mixer Efficiency and Poultry Feed Homogeneity. In Proceedings of the European Symposium on the Quality of Eggs and Egg Products, Çeşme, Turkey, 23–26 June 2019. [Google Scholar]
- Rossi, P.; Rutz, F.; Anciuti, M.A.; Rech, J.L.; Zauk, N.H.F. Influence of Graded Levels of Organic Zinc on Growth Performance and Carcass Traits of Broilers. J. Appl. Poult. Res. 2007, 16, 219–225. [Google Scholar] [CrossRef]
- Michel, V.; Prampart, E.; Mirabito, L.; Allain, V.; Arnould, C.; Huonnic, D.; Le Bouquin, S.; Albaric, O. Histologically-validated footpad dermatitis scoring system for use in chicken processing plants. Br. Poult. Sci. 2012, 53, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Baranova, D.; Pesek, L.; Saly, J. Mechanical properties of bones. Folia Vet. 2008, 52, 168–173. [Google Scholar]
- Baird, H.T.; Eggett, D.L.; Fullmer, S.O. Varying ratios of omega-6: Omega-3 fatty acids on the pre and postmortem bone mineral density, bone ash, and bone breaking strength of laying chickens. Poult. Sci. 2008, 87, 223–328. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide: Statistics: Release 9.1 Ed.; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- De Grande, A.; Leleu, S.; Delezie, E.; Rapp, C.; De Smet, S.; Goossens, E.; Haesebrouck, F.; Van Immerseel, F.; Ducatelle, R. Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult. Sci. 2020, 99, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.A.; Abol-Ela, S.S.; Askar, A.A.; Mohamed, L.A.; El-Sayed, S.A.; Ahmed, S.Y.; Moustafa, A.A.; Alagawany, M. Supplementation of different zinc sources to low-CP diets and its effect on performance, carcass traits, liver and kidney functions, immunological, and antioxidant parameters of quail chicks. Poult. Sci. 2021, 100, 101463. [Google Scholar] [CrossRef]
- Lonnerdal, B. Dietary factor influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, A.K.; Pfaender, S.; Hagmeyer, S.; Tarana, L.; Mattes, A.-K.; Briel, F.; Küry, S.; Boeckers, T.M.; Grabrucker, A.M. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. BioMetals 2017, 30, 643–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, Y.A.; El-Hamid, A.E.A.; Zeweil, H.S.; Qota, E.M.; Bovera, F.; Monastra, M.; Sahledom, M.D. Effect of dietary amounts of organic and inorganic Zinc on productive and physiological traits of white peckin ducks. Animal 2013, 7, 695–700. [Google Scholar] [CrossRef]
- Attia, Y.A.; Addeo, N.F.; Al-Hamid, A.A.-H.E.A.; Bovera, F. Effects of Phytase Supplementation to Diets with or without Zinc Addition on Growth Performance and Zinc Utilization of White Pekin Ducks. Animals 2019, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Ensminger, M.E.; Oldfield, J.E.; Heinemann, W.W. Feeds and Nutrition: Formerly Feeds and Nutrition, Complete; Ensminger Pub. Co.: Clovis, CA, USA, 1990. [Google Scholar]
- Bartlett, J.R.; Smith, M. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 2003, 82, 1580–1588. [Google Scholar] [CrossRef]
- Goel, A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, C.; Yan, M.; Xie, H.; Li, S.; Yu, Q.; He, S.; He, J. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016, 7, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.; Anthony, N.; Lee, S.R. Progeny Performance When Dams and Chicks Are Fed Supplemental Zinc. Poult. Sci. 1992, 71, 1201–1206. [Google Scholar] [CrossRef]
- Oteiza, P.L.; Olin, K.L.; Fraga, C.G.; Keen, C.L. Oxidant defense systems in testes from Zn deficient rats. Proc. Soc. Exp. Biol. Med. 1996, 213, 85–91. [Google Scholar] [CrossRef]
- Liu, Z.H.; Lu, L.; Wang, R.L.; Lei, H.L.; Li, S.F.; Zhang, L.Y.; Luo, X.G. Effects of supplemental zinc source and level on antioxidant ability and fat metabolism-related enzymes of broilers. Poult. Sci. 2015, 94, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Smith, M.O.; Onderci, M.; Sahin, N.; Gursu, M.F.; Kucuk, O. Supplementation of zinc from organic or inorganic source improves performance and antioxidant status of heat-distressed quail. Poult. Sci. 2005, 84, 882–887. [Google Scholar] [CrossRef]
- Qudsieh, R.I.; Smith, D.P.; Brake, J. Effect of elevated dietary inorganic zinc on live performance, carcass yield, and quality of male and female broilers. Poult. Sci. 2018, 97, 4122–4130. [Google Scholar] [CrossRef]
- Sunder, G.S.; Panda, A.K.; Gopinath, N.C.S.; Rao, S.V.R.; Raju, M.V.L.N.; Reddy, M.R.; Kumar, C.V. Effects of Higher Levels of Zinc Supplementation on Performance, Mineral Availability, and Immune Competence in Broiler Chickens. J. Appl. Poult. Res. 2008, 17, 79–86. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, L.; Li, S.; Zhang, L.; Xi, L.; Zhang, K.; Luo, X. Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult. Sci. 2011, 90, 1782–1790. [Google Scholar] [CrossRef]
- Peric, L.; Nollet, L.; Milosevic, N.; Zikic, D. Effect of Bioplex and Sel-Plex substituting inorganic trace mineral sources on performance of broilers. Arch. Geflugelkd. 2007, 71, 122–129. [Google Scholar]
- Krane, S.M.; Inada, M. Matrix metalloproteinases and bone. Bone 2008, 43, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.D.; Zhao, J.; Harrell, R.J.; Atwell, C.A.; Dibner, J.J. Trace Mineral Nutrition in Poultry and Swine. Asian Australas. J. Anim. Sci. 2010, 23, 1527–1534. [Google Scholar] [CrossRef]
- Stryer, L. Protein conformation, dynamics, and function. In Biochemistry; W. H. Freeman and Co.: New York, NY, USA, 1988; pp. 143–314. [Google Scholar]
- Salim, H.; Lee, H.R.; Jo, C.; Lee, S.K.; Lee, B.D. Effect of Sources and Levels of Zinc on the Tissue Mineral Concentration and Carcass Quality of Broilers. Avian Biol. Res. 2010, 3, 23–29. [Google Scholar] [CrossRef]
- Sharideh, H.; Zhandi, M.; Zaghari, M.; Akhlaghi, A. Effect of dietary zinc oxide and phytase on the plasma metabolites and enzyme activities in aged broiler breeder hens. Iran. J. Vet. Med. 2015, 9, 263–270. [Google Scholar] [CrossRef]
- Chen, J.; Tellez, G.; Escobar, J.; Vazquez-Anon, M. Impact of Trace Minerals on Wound Healing of Footpad Dermatitis in Broilers. Sci. Rep. 2017, 7, 1894. [Google Scholar] [CrossRef] [Green Version]
- Langmade, S.J.; Ravindra, R.; Daniels, P.J.; Andrews, G.K. The Transcription Factor MTF-1 Mediates Metal Regulation of the Mouse ZnT1 Gene. J. Biol. Chem. 2000, 275, 34803–34809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liuzzi, J.P.; Blanchard, R.K.; Cousins, R.J. Differential Regulation of Zinc Transporter 1, 2, and 4 mRNA Expression by Dietary Zinc in Rats. J. Nutr. 2001, 131, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Lu, L.; Luo, X.; Liu, B. An Optimal Dietary Zinc Level of Broiler Chicks Fed a Corn-Soybean Meal Diet. Poult. Sci. 2007, 86, 2582–2589. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Cowin, S. Bone Mechanics Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Scrimgeour, A.G.; Stahl, C.H.; McClung, J.P.; Marchitelli, L.J.; Young, A.J. Moderate zinc deficiency negatively affects biomechanical properties of rat tibiae independently of body composition. J. Nutr. Biochem. 2007, 18, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. BoneKEy Rep. 2013, 2, 447. [Google Scholar] [CrossRef] [PubMed]
- Vakili, R.; Rashidi, A.A.; Sobhanirad, S. Effects of dietary fat, vitamin E and zinc supplementation on tibia breaking strength in female broilers under heat stress. Afr. J. Agric. Res. 2010, 5, 3151–3156. [Google Scholar]
- NRC. Mineral Tolerance of Animals, 2nd ed.; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
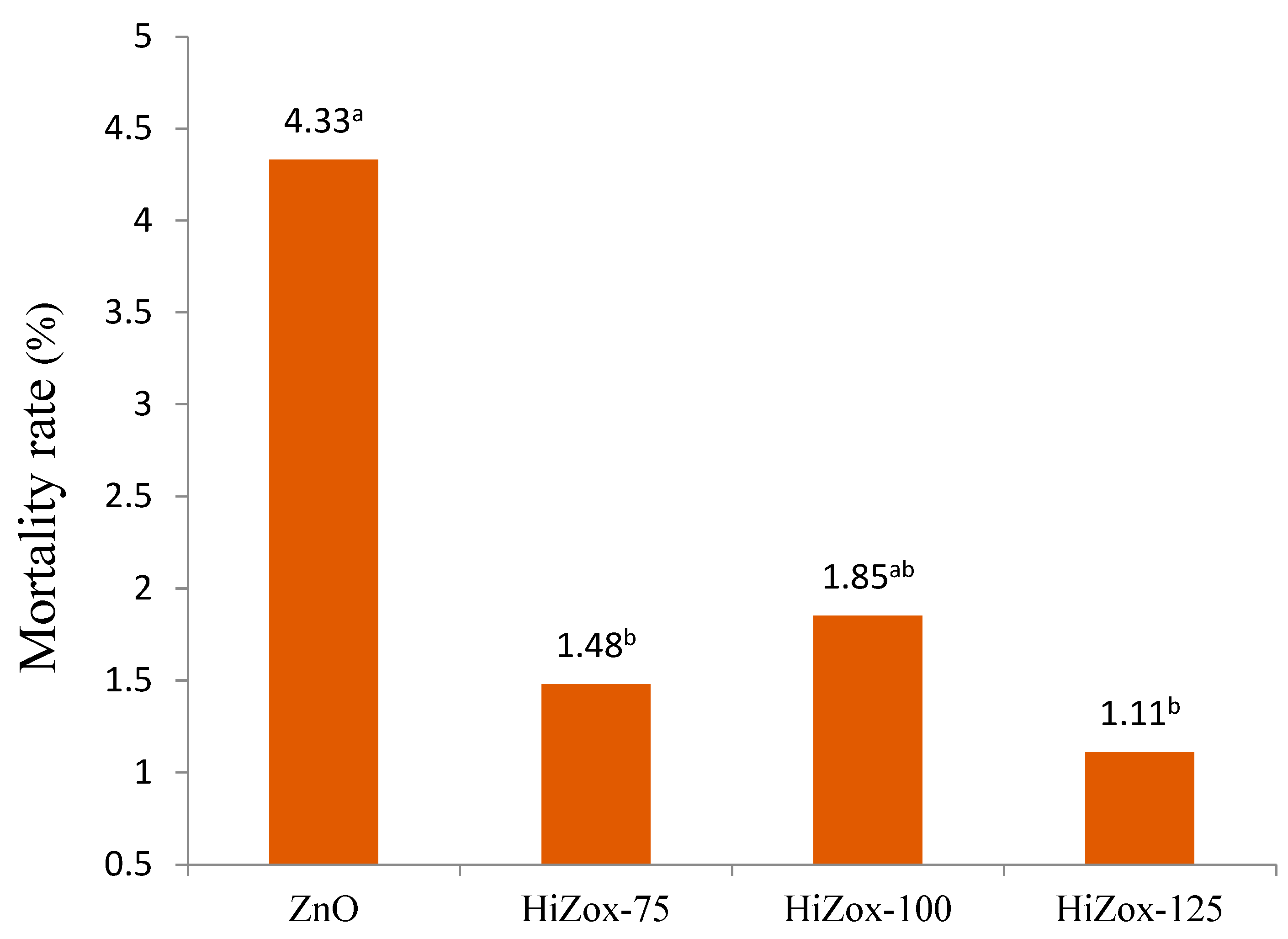


| Ingredients (g/kg) | Treatment | |||
|---|---|---|---|---|
| ZnO-100 | HiZox-75 | HiZox-100 | HiZox-125 | |
| Corn grain | 505.5 | 505.5 | 505.5 | 505.5 |
| Soybean meal | 408 | 408 | 408 | 408 |
| Corn oil | 39.9 | 39.9 | 39.9 | 39.9 |
| Di calcium phosphate | 20.9 | 20.9 | 20.9 | 20.9 |
| Oyster shell | 8.7 | 8.7 | 8.7 | 8.7 |
| Common salt | 3.7 | 3.7 | 3.7 | 3.7 |
| Sodium bicarbonate | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin supplement 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| Mineral supplement 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| D L Methionine | 3.5 | 3.5 | 3.5 | 3.5 |
| L Lysine HCl | 2 | 2 | 2 | 2 |
| L Threonine | 1.3 | 1.3 | 1.3 | 1.3 |
| Sum | 1000 | 1000 | 1000 | 1000 |
| ZnO 3 | 0.13158 | - | - | - |
| HiZox 3 | - | 0.098680 | 0.13158 | 0.16447 |
| Nutrients (calculated %) | ||||
| MEn (kcal/kg) | 3000 | 3000 | 3000 | 3000 |
| Crude protein | 23 | 23 | 23 | 23 |
| Calcium | 0.96 | 0.96 | 0.96 | 0.96 |
| Available phosphorus | 0.48 | 0.48 | 0.48 | 0.48 |
| Na | 0.2 | 0.2 | 0.2 | 0.2 |
| (Na + K)-Cl (meq/kg) | 250 | 250 | 250 | 250 |
| Dig. Lys 4 | 1.28 | 1.28 | 1.28 | 1.28 |
| Dig. Met | 0.65 | 0.65 | 0.65 | 0.65 |
| Dig. Met + Cys | 0.95 | 0.95 | 0.95 | 0.95 |
| Dig. Thr | 0.86 | 0.86 | 0.86 | 0.86 |
| Nutrients (analyzed %) | ||||
| Crude protein | 23.12 | 23.50 | 22.07 | 23.39 |
| Crude fat | 6.1 | 6.5 | 6.7 | 6.4 |
| Zinc (ppm) 5 | 126.3 | 113.45 | 137.40 | 183.06 |
| Ingredients (g/kg) | Treatment | |||
|---|---|---|---|---|
| ZnO-100 | HiZox-75 | HiZox-100 | HiZox-125 | |
| Corn grain | 536.8 | 536.8 | 536.8 | 536.8 |
| Soybean meal | 372 | 372 | 372 | 372 |
| Corn oil | 49.5 | 49.5 | 49.5 | 49.5 |
| Di calcium phosphate | 18.7 | 18.7 | 18.7 | 18.7 |
| Oyster shell | 8 | 8 | 8 | 8 |
| Common salt | 2.4 | 2.4 | 2.4 | 2.4 |
| Sodium bicarbonate | 2.3 | 2.3 | 2.3 | 2.3 |
| Vitamin supplement 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| Mineral supplement 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| D L Methionine | 3 | 3 | 3 | 3 |
| L Lysine HCl | 1.4 | 1.4 | 1.4 | 1.4 |
| L Threonine | 0.9 | 0.9 | 0.9 | 0.9 |
| Sum | 1000 | 1000 | 1000 | 1000 |
| ZnO 3 | 0.13158 | - | - | - |
| HiZox 3 | - | 0.098680 | 0.13158 | 0.16447 |
| Nutrients (calculated %) | ||||
| MEn (kcal/kg) | 3100 | 3100 | 3100 | 3100 |
| Crude protein | 21.5 | 21.5 | 21.5 | 21.5 |
| Calcium | 0.87 | 0.87 | 0.87 | 0.87 |
| Available phosphorus | 0.43 | 0.43 | 0.43 | 0.43 |
| Na | 0.17 | 0.17 | 0.17 | 0.17 |
| (Na + K)-Cl (meq/kg) | 247 | 247 | 247 | 247 |
| Dig. Lys 4 | 1.15 | 1.15 | 1.15 | 1.15 |
| Dig. Met | 0.59 | 0.59 | 0.59 | 0.59 |
| Dig. Met + Cys | 0.87 | 0.87 | 0.87 | 0.87 |
| Dig. Thr | 0.77 | 0.77 | 0.77 | 0.77 |
| Nutrients (analyzed %) | ||||
| Crude protein | 21.09 | 21.04 | 21.4 | 21.90 |
| Crude fat | 7.6 | 7.2 | 7.1 | 7.4 |
| Zinc (ppm) 5 | 120.95 | 98.95 | 128.15 | 158.35 |
| Ingredients (g/kg) | Treatment | |||
|---|---|---|---|---|
| ZnO-100 | HiZox-75 | HiZox-100 | HiZox-125 | |
| Corn grain | 585.4 | 585.4 | 585.4 | 585.4 |
| Soybean meal | 320 | 320 | 320 | 320 |
| Corn oil | 56.3 | 56.3 | 56.3 | 56.3 |
| Di calcium phosphate | 16.8 | 16.8 | 16.8 | 16.8 |
| Oyster shell | 7.4 | 7.4 | 7.4 | 7.4 |
| Common salt | 2.3 | 2.3 | 2.3 | 2.3 |
| Sodium bicarbonate | 2 | 2 | 2 | 2 |
| Vitamin supplement 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| Mineral supplement 2 | 2.5 | 2.5 | 2.5 | 2.5 |
| D L Methionine | 2.7 | 2.7 | 2.7 | 2.7 |
| L Lysine HCl | 1.4 | 1.4 | 1.4 | 1.4 |
| L Threonine | 0.7 | 0.7 | 0.7 | 0.7 |
| Sum | 1000 | 1000 | 1000 | 1000 |
| ZnO 3 | 0.13158 | - | - | - |
| HiZox 3 | - | 0.098680 | 0.13158 | 0.16447 |
| Nutrients (calculated %) | ||||
| MEn (kcal/kg) | 3200 | 3200 | 3200 | 3200 |
| Crude protein | 19.5 | 19.5 | 19.5 | 19.5 |
| Calcium | 0.79 | 0.79 | 0.79 | 0.79 |
| Available phosphorus | 0.39 | 0.39 | 0.39 | 0.39 |
| Na | 0.16 | 0.16 | 0.16 | 0.16 |
| (Na + K)-Cl (meq/kg) | 221 | 221 | 221 | 221 |
| Dig. Lys 4 | 1.03 | 1.03 | 1.03 | 1.03 |
| Dig. Met | 0.54 | 0.54 | 0.54 | 0.54 |
| Dig. Met + Cys | 0.80 | 0.80 | 0.80 | 0.80 |
| Dig. Thr | 0.69 | 0.69 | 0.69 | 0.69 |
| Nutrients (analyzed %) | ||||
| Crude protein | 19.20 | 19.80 | 19.61 | 19.25 |
| Crude fat | 8.1 | 8.0 | 8.3 | 8.1 |
| Zinc (ppm) 5 | 119.85 | 97.15 | 129.01 | 151.55 |
| Characteristics | Regular ZnO | Hizox |
|---|---|---|
| Particle size (nm) | 100–1000 | <100 |
| Area to weight ratio (m2/g) | 2.4 | 42 |
| CV 1 (%) | 5.61 | 3.65 |
| Angle of repose (degree) | 35 | 28 |
| Mixability | poor | good |
| ZnO-100 | HiZox-75 | HiZox-100 | HiZox-125 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| 1–7 days | ||||||
| Live body weight (g) | 159.9 | 160.8 | 159.2 | 158.1 | 1.9 | 0.8 |
| Average daily feed intake (g) | 19.9 | 19.7 | 19.9 | 20.0 | 0.2 | 0.87 |
| Feed conversion ratio | 0.87 | 0.86 | 0.88 | 0.89 | 0.01 | 0.26 |
| 1–14 days | ||||||
| Live body weight (g) | 422.1 | 420.4 | 420.6 | 417.1 | 5.1 | 0.71 |
| Average daily feed intake (g) | 33.7 | 33.7 | 33.7 | 32.7 | 0.3 | 0.29 |
| Feed conversion ratio | 1.11 | 1.11 | 1.11 | 1.10 | 0.01 | 0.9 |
| 1–28 days | ||||||
| Live body weight (g) | 1355.4 | 1378.0 | 1342.7 | 1364.4 | 20.3 | 0.66 |
| Average daily feed intake (g) | 65.5 | 65.2 | 64.6 | 64.1 | 0.8 | 0.63 |
| Feed conversion ratio | 1.34 a | 1.32 ab | 1.34 a | 1.31 b | 0.007 | 0.01 |
| 1–42 days | ||||||
| Live body weight (g) | 2504.5 | 2509.3 | 2471.0 | 2461.6 | 24.1 | 0.41 |
| Average daily feed intake (g) | 91.7 | 91.5 | 90.5 | 90.2 | 1.08 | 0.72 |
| Feed conversion ratio | 1.52 b | 1.55 a | 1.55 a | 1.54 ab | 0.007 | 0.04 |
| ZnO-100 | HiZox-75 | HiZox-100 | HiZox-125 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| Carcass fractional weights | ||||||
| Carcass | 75.6 | 75.6 | 75.4 | 75.5 | 0.28 | 0.95 |
| Breast | 26.8 | 27.2 | 26.8 | 26.8 | 0.38 | 0.84 |
| Thighs | 21.0 | 21.0 | 20.6 | 20.7 | 0.17 | 0.20 |
| Abdominal fat pad | 1.16 | 1.21 | 1.27 | 1.33 | 0.08 | 0.45 |
| liver | 1.67 b | 1.80 ab | 1.90 a | 1.83 ab | 0.05 | 0.04 |
| Plasma Zn (µg/dL) | 103.1 | 103.8 | 100.7 | 106.0 | 2.4 | 0.55 |
| ALP (u/L) | 5282 | 5356 | 4619 | 5448 | 392 | 0.44 |
| Footpad dermatitis score | 1.19 | 1.40 | 1.55 | 1.48 | 1.5 | 0.30 |
| Gizzard pH | 2.59 | 2.56 | 2.65 | 2.61 | 0.08 | 0.90 |
| Small intestine pH | 5.92 | 5.95 | 5.87 | 6.01 | 0.13 | 0.89 |
| Tibia weight (g) | 6.99 | 7.17 | 6.96 | 6.84 | 0.16 | 0.56 |
| Tibia length (mm) | 98.2 | 99.3 | 98.6 | 97.5 | 0.09 | 0.53 |
| Tibia diameter (widest, mm) | 9.0 | 9.5 | 9.3 | 9.3 | 0.01 | 0.36 |
| Tibia diameter (narrowest, mm) | 7.4 | 7.6 | 7.7 | 7.5 | 0.01 | 0.54 |
| Treatment | Peak | Break | ||||
|---|---|---|---|---|---|---|
| Elongation (%) | Force (N) | Extension (mm) | Elongation (%) | Force (N) | Extension (mm) | |
| ZnO-100 | 1.0381 b | 247.83 | 1.5571 b | 1.0623 b | 247.66 | 1.5934 b |
| HiZox-75 | 1.4517 a | 278.72 | 2.1776 a | 1.4649 a | 273.37 | 2.1974 a |
| HiZox-100 | 1.0325 b | 243.31 | 1.5488 b | 1.0344 b | 242.67 | 1.5516 b |
| HiZox-125 | 1.0000 b | 229.47 | 1.5000 b | 1.0034 b | 229.12 | 1.5051 b |
| SEM | 0.11 | 14.7 | 0.16 | 0.11 | 14.9 | 0.16 |
| p-Value | 0.10 | 0.30 | 0.10 | 0.09 | 0.40 | 0.09 |
| Treatment | Gizzard | Jejunum | ||||
|---|---|---|---|---|---|---|
| Zn (mg/kg DM) | Soluble Zn (mg/kg DM) | Zn Solubility (%) | Zn (mg/kg DM) | Soluble Zn (mg/kg DM) | Zn Solubility (%) | |
| ZnO-100 | 64.1 a | 17.5 | 29.1 b | 291.6 b | 35.1 | 12.2 |
| HiZox-75 | 53.6 ab | 19.1 | 37.7 ab | 222.1 c | 25.3 | 12.5 |
| HiZox-100 | 41.3 b | 19.1 | 45.7 a | 387.3 a | 33.1 | 8.9 |
| HiZox-125 | 64.5 a | 17.3 | 33.0 b | 420.9 a | 37.4 | 9.4 |
| SEM | 6.7 | 1.5 | 4.3 | 24.3 | 3.2 | 1.2 |
| p-Value | 0.08 | 0.78 | 0.03 | 0.0001 | 0.09 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaghari, M.; Mehrvarz, H.; Hajati, H.; Moravej, H. Evaluation of an Innovative Zn Source on Feed Efficiency, Growth Performance, Skin and Bone Quality of Broilers Suffering Heat Stress. Animals 2022, 12, 3272. https://doi.org/10.3390/ani12233272
Zaghari M, Mehrvarz H, Hajati H, Moravej H. Evaluation of an Innovative Zn Source on Feed Efficiency, Growth Performance, Skin and Bone Quality of Broilers Suffering Heat Stress. Animals. 2022; 12(23):3272. https://doi.org/10.3390/ani12233272
Chicago/Turabian StyleZaghari, Mojtaba, Hossein Mehrvarz, Hosna Hajati, and Hossein Moravej. 2022. "Evaluation of an Innovative Zn Source on Feed Efficiency, Growth Performance, Skin and Bone Quality of Broilers Suffering Heat Stress" Animals 12, no. 23: 3272. https://doi.org/10.3390/ani12233272
APA StyleZaghari, M., Mehrvarz, H., Hajati, H., & Moravej, H. (2022). Evaluation of an Innovative Zn Source on Feed Efficiency, Growth Performance, Skin and Bone Quality of Broilers Suffering Heat Stress. Animals, 12(23), 3272. https://doi.org/10.3390/ani12233272






