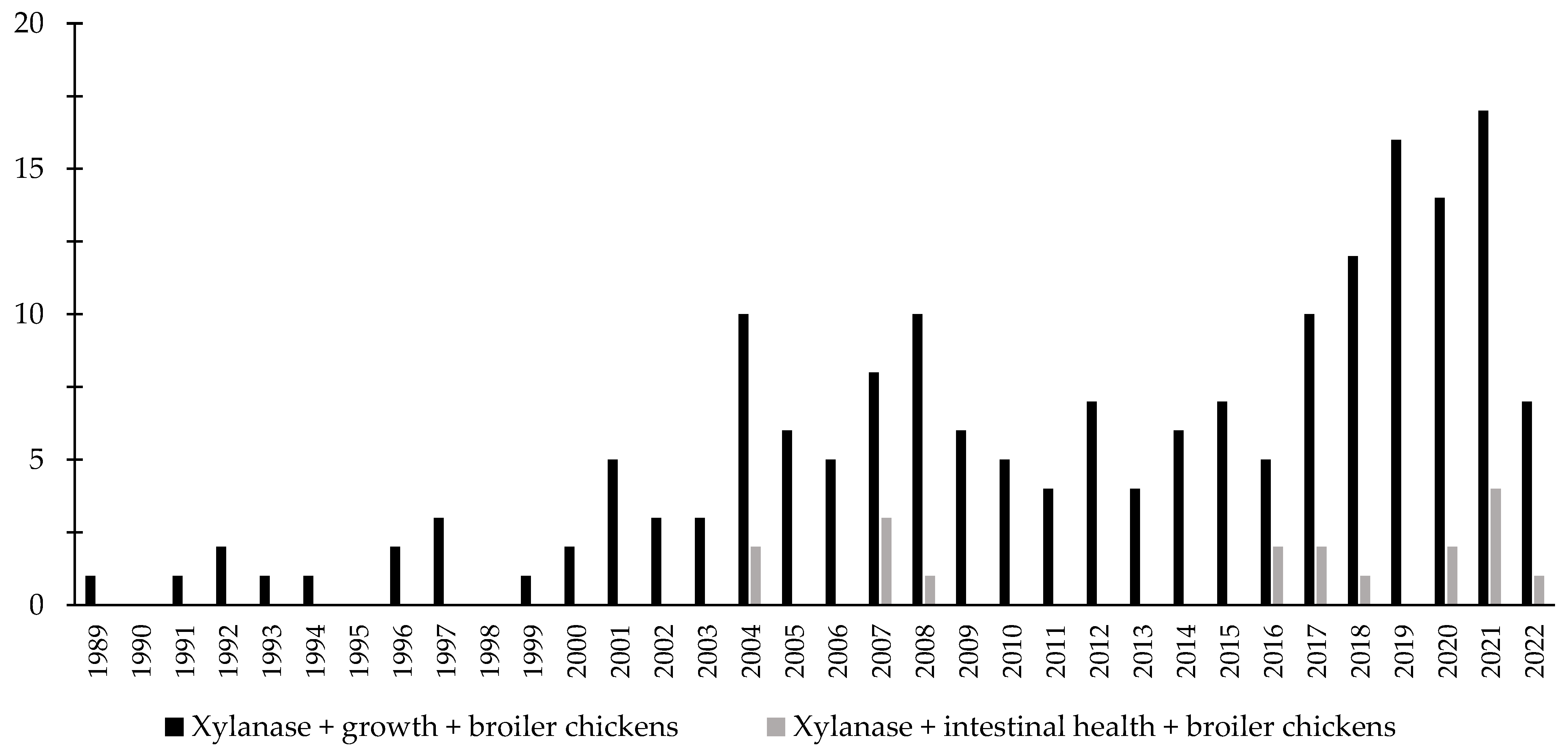Simple Summary
Feed enzymes have been widely used with the goal to improve nutrient digestibility and growth performance of pigs. However, recent studies have shown that feed enzymes, especially phytase and xylanase, may provide potential benefits associated with the intestinal health and microbiota of nursery pigs and broiler chickens. Phytase and xylanase are catalyzers for the hydrolysis of phytic acid and β-1,4- xylan bonds, respectively. With a reduction in the antinutritional properties of phytic acid and non-starch polysaccharides (NSP) by the supplementation of these enzymes, there may be a possibility to improve the intestinal health of nursery pigs and broiler chickens. Intestinal health can be a determinant for the overall health and subsequent performance of the animals. Some of the factors affecting the intestinal health of nursery pigs could be related to antinutritional properties from phytic acid and NSP. Thus, this review paper aimed to discuss the nutritional and functional roles associated phytase and xylanase supplementation enhancing the intestinal health and growth of nursery pigs and broiler chickens.
Abstract
This review paper discussed the nutritional and functional roles of phytase and xylanase enhancing the intestinal and growth of nursery pigs and broiler chickens. There are different feed enzymes that are currently supplemented to feeds for nursery pigs and broiler chickens. Phytase and xylanase have been extensively studied showing consistent results especially related to enhancement of nutrient digestibility and growth performance of nursery pigs and broiler chickens. Findings from recent studies raise the hypothesis that phytase and xylanase could play functional roles beyond increasing nutrient digestibility, but also enhancing the intestinal health and positively modulating the intestinal microbiota of nursery pigs and broiler chickens. In conclusion, the supplementation of phytase and xylanase for nursery pigs and broiler chickens reaffirmed the benefits related to enhancement of nutrient digestibility and growth performance, whilst also playing functional roles benefiting the intestinal microbiota and reducing the intestinal oxidative damages. As a result, it could contribute to a reduction in the feed costs by allowing the use of a wider range of feedstuffs without compromising the optimal performance of the animals, as well as the environmental concerns associated with a poor hydrolysis of antinutritional factors present in the diets for swine and poultry.
Keywords:
broiler chickens; functional roles; intestinal health; nursery pigs; 3-phytase; 6-phytase; xylanase 1. Introduction
Intestinal health is one of the most discussed contemporary issues in animal nutrition, due to its significance in the overall biological response of the animals. It is usually described as based on a combination of different parameters from different metabolic and physiological reactions that can impact the overall growth and health [1]. According to the literature, some of those parameters could be the quantification of anti and proinflammatory cytokines, immunoglobulins, and oxidative damage products in the digestive tract; assessment of intestinal morphology; and relative abundance and diversity quantification of the mucosa-associated microbiota in the jejunum [1,2,3,4,5,6,7].
In the first weeks after weaning and hatching, nursery pigs and broiler chickens start consuming diets with a greater amount of plant-based feedstuffs, which can contain different antinutritional factors and allergenic compounds that can lead to negative impacts on nutrient digestibility, growth performance, and intestinal health [8,9,10]. Corn and soybean meal are the most commonly used plant-based feedstuffs in diets for nursery pigs and broiler chickens, which can contain antinutritional and allergic compounds, such as phytic acid, non-starch polysaccharides (NSP), glycinin, and β-conglycinin [11,12]. The negative effects associated with phytic acid and NSP altering the digestion process may also lead to negative impacts in the intestinal health and intestinal microbiota of the animals, such as increases in the oxidative stress and increases in the abundance of pathogenic bacteria, which can be determinant for the intestinal health and subsequent growth of animals [5,13,14,15].
Enzymes are organic catalysts that accelerate reactions and act on specific substrates and reagents [16,17]. The enzyme activity can be affected by different factors, such as feed mixing temperature, levels of targeted substrate in the feedstuffs, different levels, types, and combinations of enzymes, and optimal gastric and intestinal pH and temperature [16,18]. The use of feed enzymes has been notarized as an alternative to increase nutrient digestibility and growth performance of swine and poultry through the active hydrolysis of antinutritional factors and allergenic compounds present in their diets [19,20]. Monogastric animals, especially pigs, at their first stages of growth, cannot effectively secrete endogenous enzymes, and consequently have their nutrient digestibility and utilization affected [6,21]. Recent studies have shown feed enzymes, especially phytase and xylanase, might play functional roles on intestinal health of nursery pigs and broiler chickens, whilst still providing the benefits on nutrient digestibility and growth performance [5,13,22,23].
Phytase is a feed enzyme that catalyzes the hydrolysis of phytic acid increasing the bioavailability of nutrients, especially phosphorus (P) and consequently leading to benefits on nutrient digestibility and growth performance [24,25]. Changes in the bioavailability of nutrients, such as calcium (Ca) and phosphorus, may express different effects on the intestinal and bone health, and composition and diversity of the intestinal microbiota of nursery pigs and broiler chickens [14,26,27,28]. The supplementation of xylanase will catalyze the depolymerization of the xylan structure into shorter chains and to the breaking down of the cell wall matrix [29,30]. Changes in the chemical structure of xylan and in the physicochemical properties of the digesta may lead to alterations on intestinal health parameters, especially the composition and diversity of the intestinal microbiota [15,31].
Based on the mechanisms of action and benefits of supplemental phytase and xylanase described above, it has been hypothesized that these enzymes could also play functional roles associated with the intestinal health of nursery pigs and broiler chickens. These functional roles could be related with a reduction in oxidative damage products and inflammatory cytokines, and a positive modulation of the intestinal microbiota [5,13,15,32]. This review focused on the characterization and discussion of the nutritional and functional roles of phytase and xylanase enhancing the intestinal health and growth of nursery pigs and broiler chickens.
2. Antinutritional Factors in Feeds for Nursery Pigs and Broiler Chickens
2.1. Phytic Acid
Phytate is a mixed salt of phytic acid (myo-inositol hexaphosphate; InsP6) present in plant-based feedstuffs and is constantly present in diets of monogastric animals [33,34]. Phytic acid is described as an antinutritional factor because it reduces the absorption and digestibility of trace minerals, such as Ca, zinc (Zn) and copper (Cu) forming insoluble and indigestible compounds [35,36,37,38]. Additionally, it can bind to amino acids, proteins, and enzymes like trypsin and α-amylase, inhibiting their activity and affecting protein and carbohydrate digestibility [39,40,41].
In general, around 70% of the P content in cereal grains and oil seeds used in diets for nursery pigs and broiler chickens is present in the form of phytic acid, which is not bioavailable for animal utilization [25,42]. Genetics, climate conditions, location, irrigation, soil nutrition, season, and fertilizer application may have an impact on phytic acid levels in the plant-based feedstuffs included in the diets [42]. The enzyme responsible for catalyzing the hydrolysis of phytic acid is phytase; however, monogastric animals lack on the production of endogenous phytase, which means that high levels of inorganic P sources were included in their diets to match their specific nutritional requirements [24,43]. As a result, there may be an increase in the costs associated with feed since inorganic P sources tend to be more expensive than other sources of P [44]. Additionally, environmental concerns are arising due to an increase in the excretion of undigested minerals in the manure, leading to soil and water contamination in the surrounding areas of pig and broiler chicken production systems [45,46].
During the digestion process for nursery pigs and broiler chickens, phytic acid is soluble under the acid pH of the gastric phase, which means that it is less susceptible to binding to other nutrients, reducing their bioavailability [24,47]. As the digestion process goes on, the digesta will move to the small intestine, where with different pH conditions, phytic acid will become insoluble increasing its ability to form indigestible phytate-complexes with other nutrients [34,48]. Thus, the main site for catalyzing the hydrolysis of phytic acid will be under acid pH in the stomach of pigs and proventriculus of broiler chickens, which will also provide better conditions for an optimal phytase activity [20,49,50].
2.2. Non-Starch Polysaccharides
The NSP have been described as antinutritional factors for nursery pigs and broiler chickens due to their inability to digest these compounds as a consequence of the absence of NSP degrading enzymes [5,12,51]. According to [12], around 30% of the composition of the main plant-based feedstuffs used in diets for nursery pigs and broiler chickens is NSP. The NSP content in plant-based feedstuffs can vary based on the plant genetics, environment, and storage conditions after harvesting [31]. Xylan, arabinoxylan, β-glucans, and cellulose are some of the main NSP present in those plant-based feedstuffs [52,53,54].
Non-starch polysaccharides are described as soluble and insoluble due to their structure, solubility, and impacts on the physicochemical properties of the digesta [55,56]. Cereal grains are described as containing higher levels of soluble NSP when compared to cereal by-products that contain higher levels of insoluble NSP [52,53]. Soluble NSP may alter the digesta viscosity, bulkiness, and passage rate due to its water holding capacity, which may lead to negative effects associated with nutrient digestibility and intestinal health [5,23,30,57]. Moreover, insoluble NSP may impact intestinal motility and transit time by acting as a barrier reducing the side activities of other endogenous enzymes and leading to detrimental effects on nutrient digestibility [29,56,58]. Plant-based feedstuffs used in diets for nursery pigs and broiler chickens can be classified as viscous and non-viscous based on the concentrations of soluble and insoluble NSP in their composition [52,54].
Xylan is described as the major class of hemicellulose and one of the main soluble NSP present in plant-based feedstuffs included in the diets of nursery pigs and broiler chickens [12,31,59]. It is also described as a family of structurally diverse NSP sharing a β-1,4-linked xylopyranose backbone as a common feature [60,61]. Classifications of xylan are usually based on the degree of substitution and type of side groups attached to the backbone [61,62]. Arabinoxylan (AX) is the most common form of xylan found in plant-based feedstuffs such as corn, sorghum, soybean meal, and wheat [63].
As previously mentioned, soluble NSP, especially xylan, can affect the digesta properties leading to negative impacts not only associated with nutrient digestibility but also on the intestinal health and mucosa-associated microbiota in the jejunum [1,64,65]. The negative effects of xylan on the digesta viscosity can be related to its chemical structure, molecular weight, swelling, and water-holding capacity [57,59]. When the digesta viscosity is increased, there are alterations to its passage rate and bulkiness [66,67]. An increase in the digesta bulk can cause a distention of the digestive tract walls leading to a greater secretion of satiety hormones and pancreatic secretions that can affect the daily intake of the animals [23,68].
In nursery pigs and broiler chickens, AX is poorly digested and can lead to a production of a viscous chyme in the small intestine resulting in an increase in the relative abundance of pathogenic bacteria, inflammatory and immune response, oxidative stress, and impairment of the intestinal barrier function [12,64,69]. An in vitro study simulating the digestion process of monogastric animals showed that the supplementation of xylanase, one of the NSP degrading enzymes, increased the availability of soluble minerals in corn, wheat, and soybean meal [70]. The authors attributed the positive effects on mineral availability to the inclusion of xylanase that reduced the antinutritional properties of xylan by breaking down the structural bonds of the xylan. The supplementation of xylanase may be a potential solution to the antinutritional properties of xylan and other NSP.
3. Phytase and Xylanase Enhancing the Intestinal Health of Nursery Pigs and Broiler Chickens
3.1. Phytase
3.1.1. Characteristics and Mechanisms of Action
Phytases (myo-inositol hexakiphosphate phosphohydrolase) are a class of phosphatases enzymes responsible for catalyzing the hydrolysis and release of P of phytic acid present in plant-based feedstuffs used in diets for monogastric animals [24,50]. Throughout a series of stepwise dephosphorylation reactions, phytase will increase the bioavailability of P and will allow a reduction on the inclusion of inorganic P sources in the diets [71,72]. Phosphorus is an essential nutrient especially for pigs and broiler chickens playing important roles as a component of co-enzymes in important metabolic pathways, bone mineralization, and intestinal health [43,73,74]. Therefore, it is important to optimize the P utilization by the animals due to economic and environmental concerns associated with the inclusion of inorganic P sources in the diets [75]. The activity of each phytase is expressed as phytase units (FTU), where one FTU was defined as the amount of phytase needed for the release of 1 μmol of inorganic P per minute from an excess of 15 µM sodium phytate at pH 5.5 and 37 °C [76].
Phytase is a heat labile enzyme, which means that its optimal activity is more susceptible to be affected at high temperatures usually applied to ensure the quality of some feed mixing processes [24,77]. Some of the plant-based feedstuffs used in the diets for nursery pigs and broiler chickens expresses intrinsic phytase activity; however, in the case of corn and soybean meal, expresses a negligible activity [42,78]. A great portion of phytase activity in cereal grains is found at the aleurone layers; however, it can be reduced when exposed to heat during feed mixing [78].
At the same time that the gastric phase of digestion provides better conditions for the hydrolysis of phytic acid, it also provides better conditions for optimal phytase activity [24,49]. Generally, phytases tends to work under a pH range of 3 to 5.5, which is accomplished in the stomach of pigs and birds by acidic secretions [20,33]. Since the pH in the small intestine is more favorable for proteolytic enzymes, there is a possibility that phytase could be broken down or inactivated by other active endogenous proteases [24,49]. There are many other factors besides heat that can also affect the optimal activity of phytase, such as diet composition, storing conditions, and gastric pH and temperature [24,79].
With the improvement of manufacturing technologies in the last few years, there was an increase in the production and development of new types of phytase [20,44,80]. They can be classified based on the form (liquid, powder, granule), microorganism sources (bacterial, fungi, yeast) optimal pH range (acidic or alkaline), and the position of the carbon where the dephosphorylation of phytate will initiate (3- and 6-phytase products) [25,81,82]. Recently, phytase has been supplemented beyond the manufacturer standard dose levels, also known as “super dosing”, and showed potential results that are discussed as “extra-phosphoric effects” [83,84,85,86]. The “extra-phosphoric” effects due high doses of phytase are usually described as a greater release and uptake of P and other nutrients, and the subsequent generation of lower inositol esters and myo-inositol [33,38,83,86].
3.1.2. 3- and 6-Phytase
Phytase can be classified based on the location of the carbon where the hydrolysis of the inositol ring will initiate [25,82,87]. The 3-phytase (EC 3.1.3.8) is described by initiating the dephosphorylation at the third carbon atom of the inositol ring, whereas the 6-phytase (EC 3.1.3.26) initiate at the sixth carbon atom, which may result in an enhanced hydrolysis of the inositol ring [88,89]. The pH changes towards a less acidic environment whereas the digesta goes to the small intestine can be a determinant factor for phytase activity because the pH in the small intestine is favorable for other endogenous proteolytic enzymes [24,49]. As a result, phytase may have natural decrease in its optimal activity and may be broken down by other endogenous proteases, becoming almost inactive in the small intestine [24,49].
Theoretically, 6-phytase may express optimal activity at a broader range of pH being more active and resistant than 3-phytase at the small intestine environment [19,24,50]. However, previous research has shown a variation among the results associated with growth performance, nutrient digestibility, and utilization between 3-phytase and 6-phytase, especially related to the growth stage when the animals were supplemented [84,90,91,92,93,94,95].
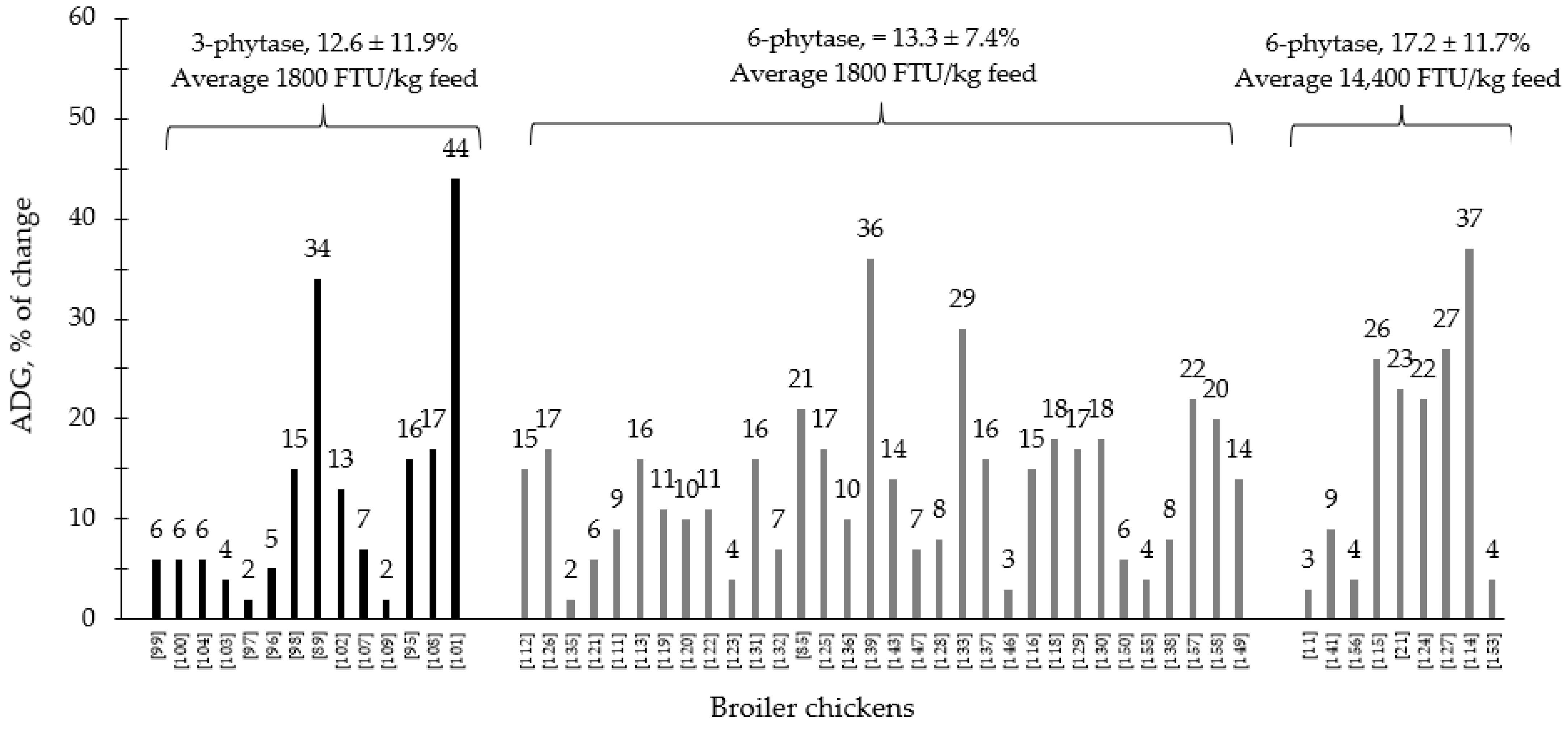
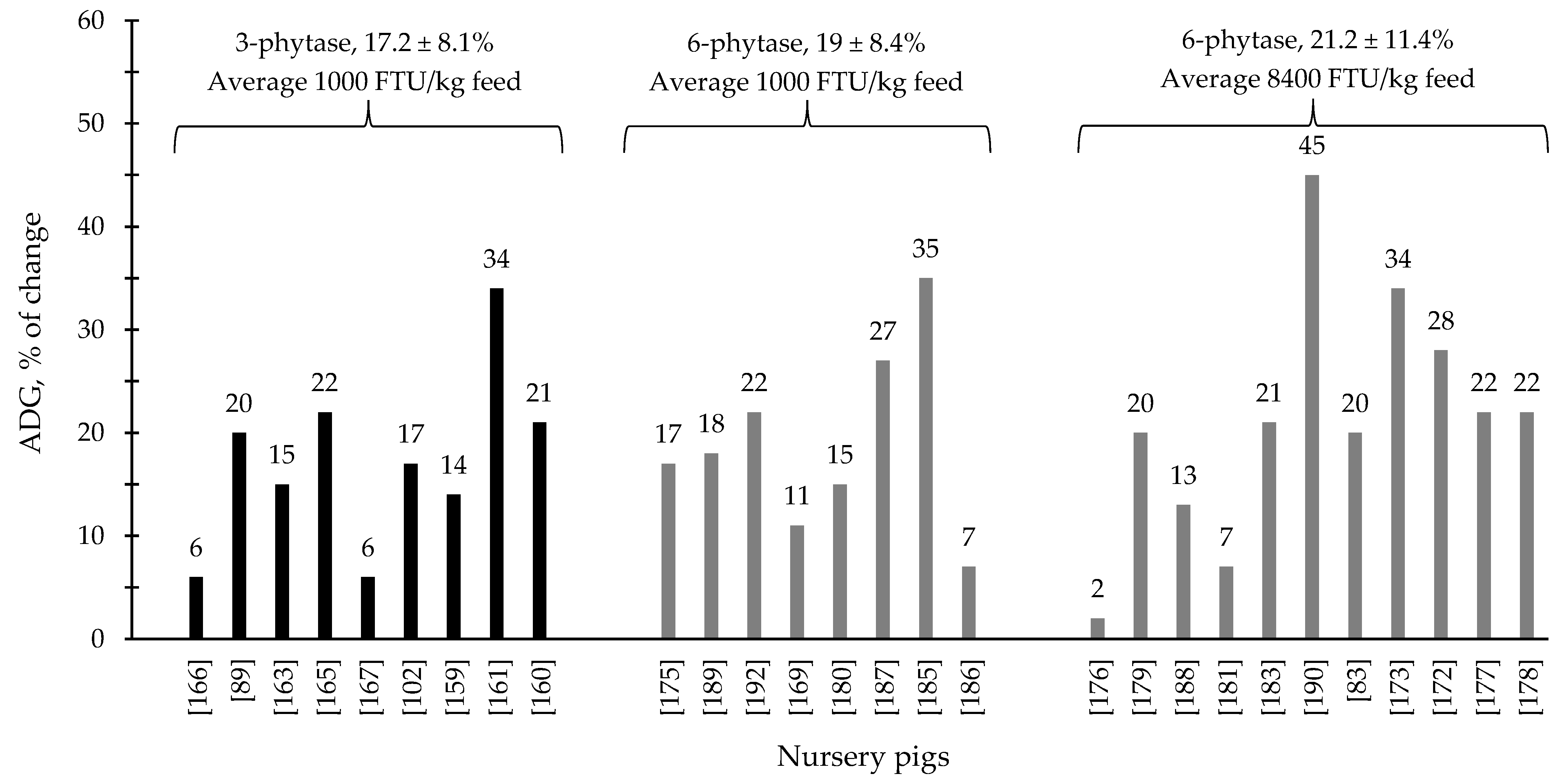
During the last few years, the supplementation of 3- and 6-phytase at different levels for swine and poultry at different stages of growth has shown consistent results, ranging from improvements on nutrient digestibility and growth performance to intestinal health parameters and modulation of the intestinal associated microbiota (Table 1 and Table 2). Generally, 6-phytase are more commonly used in swine and poultry production systems compared to 3-phytase, due to the benefits associated with the mode of action of this phytase. Based on the compiled data from Table 1 and Table 2, when 6-phytase was supplemented on average at 1800 and 14,400 FTU/kg feed showed respectively 13.3 and 17.2% of improvement in the ADG of broiler chickens compared to 12.6% from 3-phytase when supplemented 1800 FTU/kg feed (Figure 1). For nursery pigs, when 6-phytase was supplemented on average at 1000 and 8400 FTU/kg feed showed respectively 19 and 21.2% of improvement in the ADG compared to 17.2% from 3-phytase when supplemented 960 FTU/kg feed. (Figure 2). The changes in the ADG of broiler chickens and nursery pigs reported in Figure 1 and Figure 2 may indicate that 6-phytase could be supplemented at similar levels of 3- phytase and still provide greater changes in the ADG of the animals. Additionally, when 6-phytase was supplemented above the traditional levels it showed greater changes in the ADG of both broiler chickens and nursery pigs, when compared with the traditional supplemental levels (500–1000 FTU/kg feed).

Table 1.
A list of studies since 2000 describing effects of 3- and 6-phytase supplemented individually at different levels in diets for broiler chickens.

Table 2.
A list of studies since 2000 describing effects of 3- and 6-phytase supplemented individually at different levels in diets for nursery pigs.
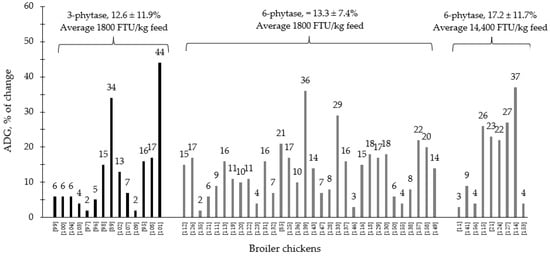
Figure 1.
Changes in the average daily gain (ADG) of broiler chickens by 3- and 6-phytase supplementation among the studies displayed in Table 2 that showed changes in the ADG. The studies were selected from peer-reviewed literature available after 2000. The percentage of change refers to a statistically significant (p < 0.05) and tendency (0.05 ≤ p < 0.10) effects of phytase individually on the ADG reported from each respective study. The described effects were in comparison with treatments containing no phytase. The average changes in the ADG of broiler chickens by 3- and 6-phytase supplementation were calculated excluding the studies that showed no effect.
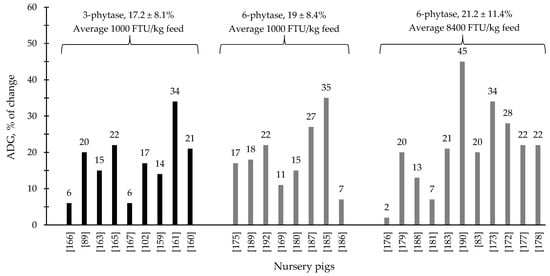
Figure 2.
Changes in the average daily gain (ADG) of nursery pigs by 3- and 6-phytase supplementation among the studies displayed in Table 2 that showed changes in the ADG. The studies were selected from peer-reviewed literature available after 2000. The percentage of change refers to a statistically significant (p < 0.05) and tendency (0.05 ≤ p < 0.10) effects of phytase individually on the ADG reported from each respective study. The described effects were in comparison with treatments containing no phytase. The average changes in the ADG of nursery pigs by 3- and 6-phytase supplementation were calculated excluding the studies that showed no effect.
3.1.3. Supplementing Phytase beyond Traditional Levels
The concept of supplementing phytase beyond traditional levels, also known as “super dosing” phytase, emerged due to economic and environmental concerns associated with high amounts of inorganic P sources in diets of swine and poultry, as well as the quality and availability of the main plant-based feedstuffs, such as corn and soybean meal [50,85,194]. Thus, it was hypothesized that an inclusion of phytase 3-fold or greater than the recommended manufacturer dose could enhance the phytic acid dephosphorylation leading to an increase in the bioavailability of P and other nutrients, and the generation of myo-inositol and lower inositol phosphate esters [38,186,194].
Inositol can be found in mammalian cells and tissues in the forms of myo-inositol or phosphatidylinositol, and it is considered an important cellular mediator for signal transduction and a regulator of growth metabolism [195,196,197]. Additionally, it is known to have insulin-like effects, such as increasing the insulin sensitivity by enhancing the concentration of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), and by increasing the insulin secretion by pancreatic β-cells [186,198,199]. Part of the benefits of the “extra phosphoric effects” are attributed by authors due to a greater generation of myo-inositol, and lower inositol phosphate esters more susceptible to hydrolysis and side activities of other endogenous enzymes [38,81,186,200]. The other part is attributed due to a greater generation and uptake of P, Ca, amino acids, and other nutrients that were trapped in the phytate-complexes and not bioavailable for the animals [38,185,186,194].
The “extra phosphoric effects” associated with supplementing phytase beyond traditional levels, are well documented and show a wide range of benefits, such as enhancement of nutrient digestibility, growth performance and intestinal health of pigs and broiler chickens [14,84,85,86,179,185,186,194,201,202].
3.1.4. Effects of Supplementing Phytase on the Intestinal Health of Nursery Pigs and Broiler Chickens
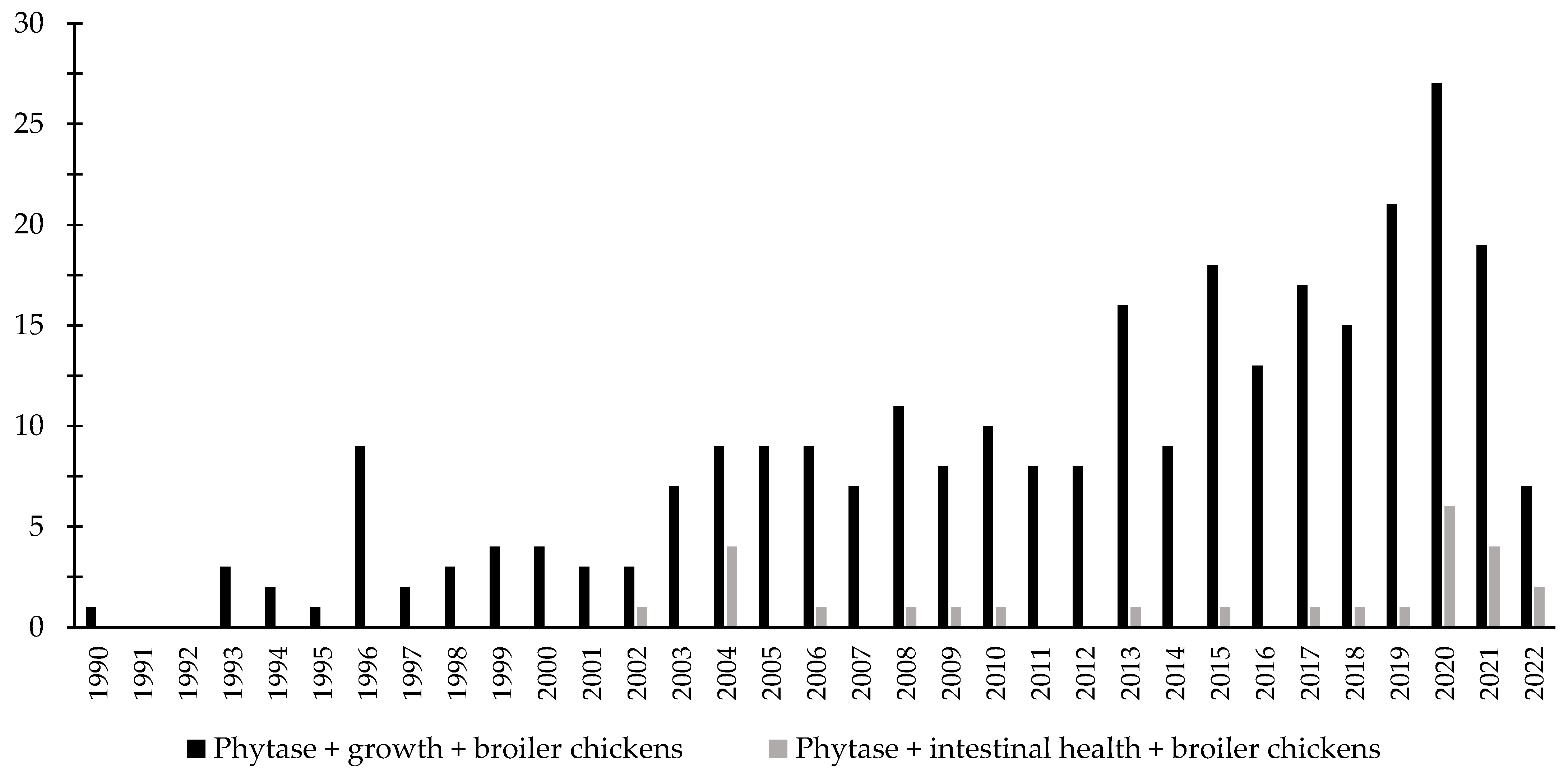
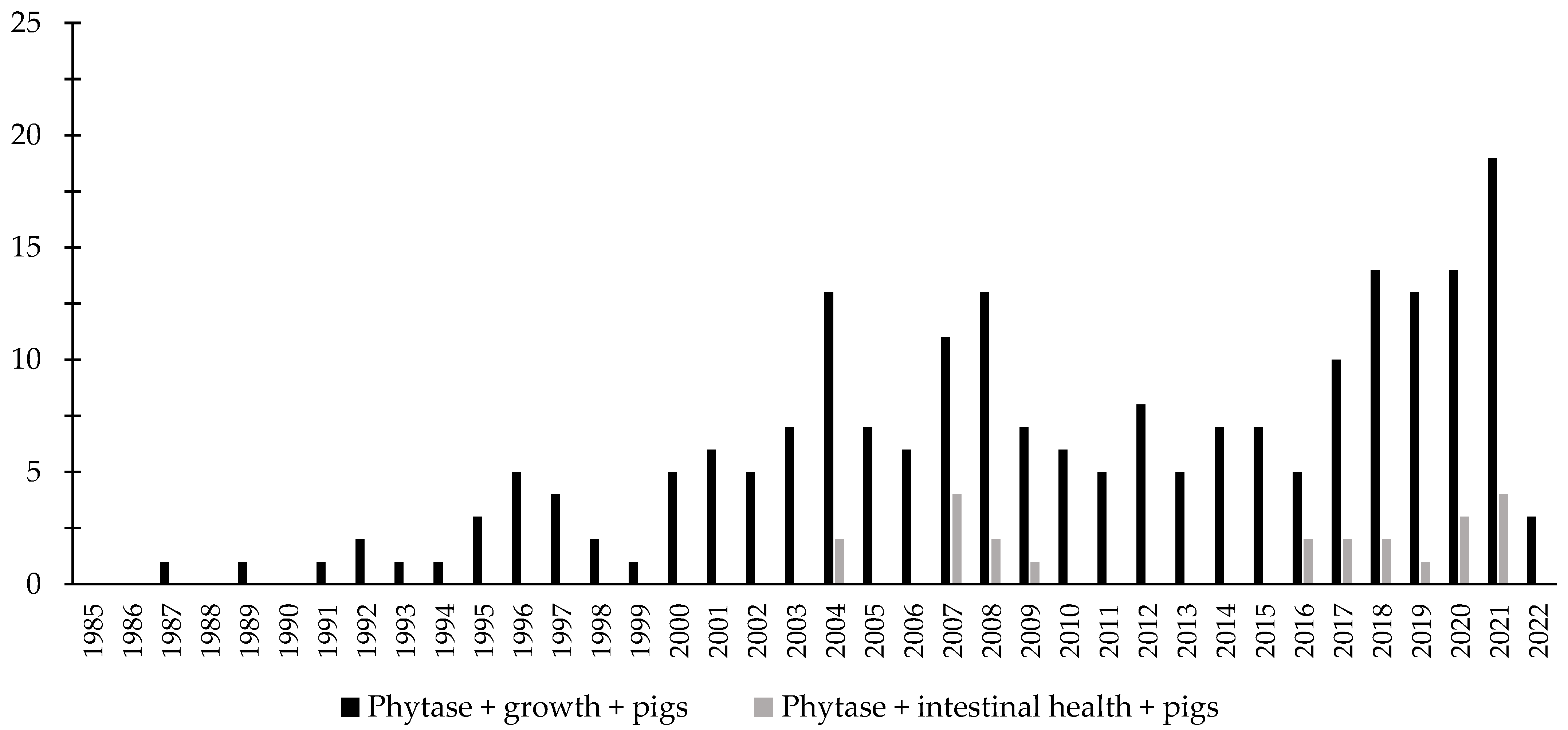
Over the last few decades, there has been an increase in the interest in the investigation of the effects of phytase associated with the intestinal health of broiler chickens and nursery pigs (Figure 3 and Figure 4). Different studies were conducted aiming to investigate the effects of phytase on the intestinal health and microbiota of nursery pigs and broiler chickens [13,14,32,191,203,204,205,206]. The authors reported a wide range of positive effects of phytase supplementation not only related to growth performance, nutrient digestibility, and bone health, but especially related to intestinal health.
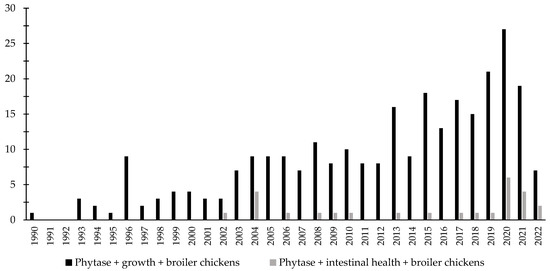
Figure 3.
A list of the number of peer-reviewed papers found in the PubMed using different keywords. Black bars: phytase, growth, broiler chickens as key words; grey bars: phytase, intestinal health, broiler chickens as keywords.
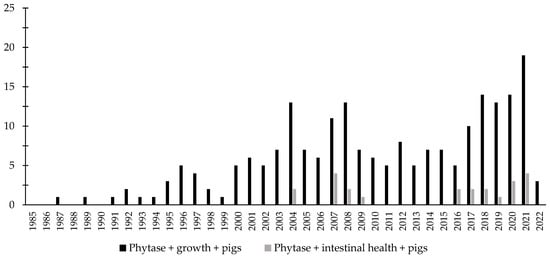
Figure 4.
A list of the number of peer-reviewed papers found in the PubMed using different keywords. Black bars: phytase, growth, pigs as key words; grey bars: phytase, intestinal health, pigs as keywords.
The authors of [14] reported a tendency for the relative abundance of Lactobacillus to increase and for Helicobacter and Pelomonas populations of the mucosa-associated microbiota to decrease in the jejunum of animals fed with phytase at 2000 FTU/kg feed. The authors attributed these effects to pH alterations possibly caused by a reduction in dietary Ca levels in the diets with phytase, and by the active hydrolysis of the phytate-complexes by phytase increasing the bioavailability of Ca and other minerals that can also affect the gastric and intestinal pH. Additionally, an increase was reported in the jejunal villus height, apparent ileal digestibility of nutrients, and bone health. Although the authors observed positive effects in the modulation of the mucosa-associated microbiota in the jejunum, no effects were observed in the inflammatory and oxidative stress parameters.
In another study conducted by [13], the authors observed increases in Lactobacillus and decreases in Streptococcus populations in the ileum of broiler chickens supplemented with phytase at 5000 FTU/kg feed. The authors explained these effects with alterations in the pH values in the crop, ileum, and caeca and short chain fatty acids production caused by the supplementation of phytase. Phytase supplementation increased the pH values in different gastric and intestinal sections, which may lead to a creation of a more acidic environment favoring beneficial bacteria and leading to a bacteriostatic effect against pathogenic bacteria. Additionally, an increase in the total short chain fatty acids, DL-lactate, and acetic acid in the ileum was reported with the addition of phytase, all of which are considered antioxidants and potential bacterial substrate. According to the authors, these findings coupled with the pH alterations in the crop, ileum, and caeca may be responsible for the positive effects observed in the microbiota of broiler chickens.
The authors of [207] reported increased concentrations of coenzyme Q10 in the liver of broiler chickens and turkeys when phytase was supplemented at 500 and 2500 FTU/kg feed. Coenzyme Q10 can enhance the antioxidant status of the animals by either targeting generated free radicals or through the regeneration of tocopherols and ascorbate, which are also antioxidant compounds [208,209]. Additionally, it can provide protective effects against metabolites originated from lipid peroxidation and protein oxidation [135,136], such as malondialdehyde (MDA) and protein carbonyl (PC). According to the authors, the increased bioavailability and uptake of nutrients, especially minerals, through the active hydrolysis of phytate by phytase may enhanced the antioxidant status of birds, by increasing the concentration of coenzyme Q10. On the other hand, the authors also believed that the greater bioavailability and uptake of metal ions from the minerals may have triggered the secretion of coenzyme Q10 as a response to increased oxidative damage.
In a study evaluating the effects of a corn-expressed phytase for nursery pigs [204], reported positive effects not only related to growth performance, but especially to intestinal health. Phytase supplementation increased villus height in the duodenum, jejunum, and on the villus height to crypt depth ratio in the duodenum. Additionally, it was also observed a tendency towards the reduction in the concentrations of tumor necrosis factor alpha (TNF-α) in the duodenum and MDA in the jejunum. These findings are in accordance with previously discussed studies reaffirming the positive effects of phytase through an active hydrolysis of phytate, and consequently a greater bioavailability and uptake of nutrients.
Another possible mechanism of phytase related to intestinal health, may be associated the “extra-phosphoric” effects aimed with the supplementation of high doses of this enzyme. As previously mentioned, these effects are characterized and discussed as a greater bioavailability and uptake of nutrients, and a greater generation of myo-inositol and lower inositol esters [83,186,194,200]. The authors of [202] observed an increase in plasma myo-inositol, and serum zinc and copper levels of nursery pigs when phytase was supplemented at 2500 FTU/kg feed. Although the authors did not measure other intestinal health related parameters, they observed positive effects on the growth performance of the pigs during their first ten days after weaning, which is one of the most critical periods for the pig. The authors suggested that the greater generation of myo-inositol and other essential minerals due to the higher doses of phytase, may positively affected the post-weaning performance of the animals.
In another study [83], also observed an increase in plasma myo-inositol and lower inositol esters concentrations, together with increased growth performance when phytase was supplemented above 500 FTU/kg feed for nursery pigs. An increase was also reported in the concentrations of glucose transporter type 4 (GLUT4) in the muscle plasma membranes and correlated with the increase in plasma myo-inositol concentrations. Glucose transporter type 4 is known to increase tissue glucose uptake during insulin signaling [210]. In this study, the authors hypothesized that phytase could be contributing to enhance the growth performance of nursery pigs through a different mechanism besides the increase in the bioavailability and uptake of nutrients, especially P.
Even though different studies reported positive effects on the intestinal health of nursery pigs and broiler chickens by supplementing phytase, there is still no consensus among authors regarding the mechanisms behind these benefits.
3.2. Xylanase
3.2.1. Characteristics and Mechanisms of Action
Xylanases are described as a carbohydrase and classified under the glycosyl hydrolase enzyme family, which means that they catalyze the hydrolysis of glycosidic bonds in complex sugars compounds [12,211,212]. In this context, xylanase will catalyze the hydrolysis of 1,4-β-D-xylopyranosyl linkages of xylan, mitigating the antinutritional effects associated with this type of NSP [212,213]. As a result, xylanase can contribute to the reduction in digesta viscosity and release otherwise entrapped nutrients by facilitating increased access of other endogenous enzymes to their substrates leading to benefits in nutrient digestibility and growth performance [5,23,211,214]. Additionally, it can also contribute to the generation of fermented NSP-released compounds such as, xylooligosaccharides (XOS) and arabinoxylooligosaccharides (AXOS), that can increase the subsequent energy generation and play a prebiotic role that can lead to alterations in the composition and diversity of the intestinal microbiota [12,65,215].
Endo-xylanases (endo β-1,4-xylanase, EC 3.2.1.8) are described as hydrolyzing β-D-xylosidic bonds of the interior xylan backbone [212] and exo-xylanases (exo-β-1,4-xylanase, EC 3.2.1.37) by hydrolyzing the reducing and/or non-reducing end from long-chain xylo-oligomers [216]. From a commercial and industrial point of view, endo-xylanases are more commonly used in swine and poultry production due to its manufacturing process and availability [216]. Xylanase can be produced from different sources such as, yeast, fungi, and bacteria, which can determine the standard supplemental level, optimal conditions, and enzyme activity [212]. The genetic and functional information, as well as the classification of different xylanases under the glycoside hydrolase family, can be found at CAZy database (http://www.cazy.org, accessed on 16 November 2022). The activity of xylanase can be expressed as xylanase unit (XU), where one unit will liberate 1 µmol of reducing sugar measured as xylose equivalents from xylan per minute under standard assay conditions.
Besides optimal temperature and pH conditions, it is important to ensure the presence of the targeted substrate in the diets in order to optimize dietary enzyme activity and enhance its associated benefits. Monogastric animals, especially swine and poultry, do not endogenously secrete the necessary enzymes to hydrolyze xylan [12], which according to [65] may require nine enzymes to completely hydrolyze and saccharify it. The main plant-based dietary feedstuffs (corn, soybean meal, wheat, sorghum) can contain up to 30% of NSP in their composition. Increased price and availability concerns coupled with relatively high levels of NSP among these feedstuffs offers opportunities for the supplementation of xylanase in swine and poultry diets to aid in the mediation of deleterious effects induced by NSP due to the inability of the animal to produce endogenous enzymes to properly hydrolyze xylan.
Compared to growing and finishing pigs, which also shows potential results supplementing xylanase, nursery pigs have an immature digestive system and capacity as a result of the wide range of stressors affecting them after weaning. As the pigs grow during nursery phase, the levels of NSP in their diets tend to increase since there will be a greater inclusion of plant-based feedstuffs. Recently, more studies are being conducted evaluating the supplementation xylanase for nursery pigs, and they have been showing potential results, especially related to enhancements in nutrient digestibility and intestinal health [5,23,29,30]. These findings may raise an opportunity for increasing the inclusion xylanase in diets for nursery pigs.
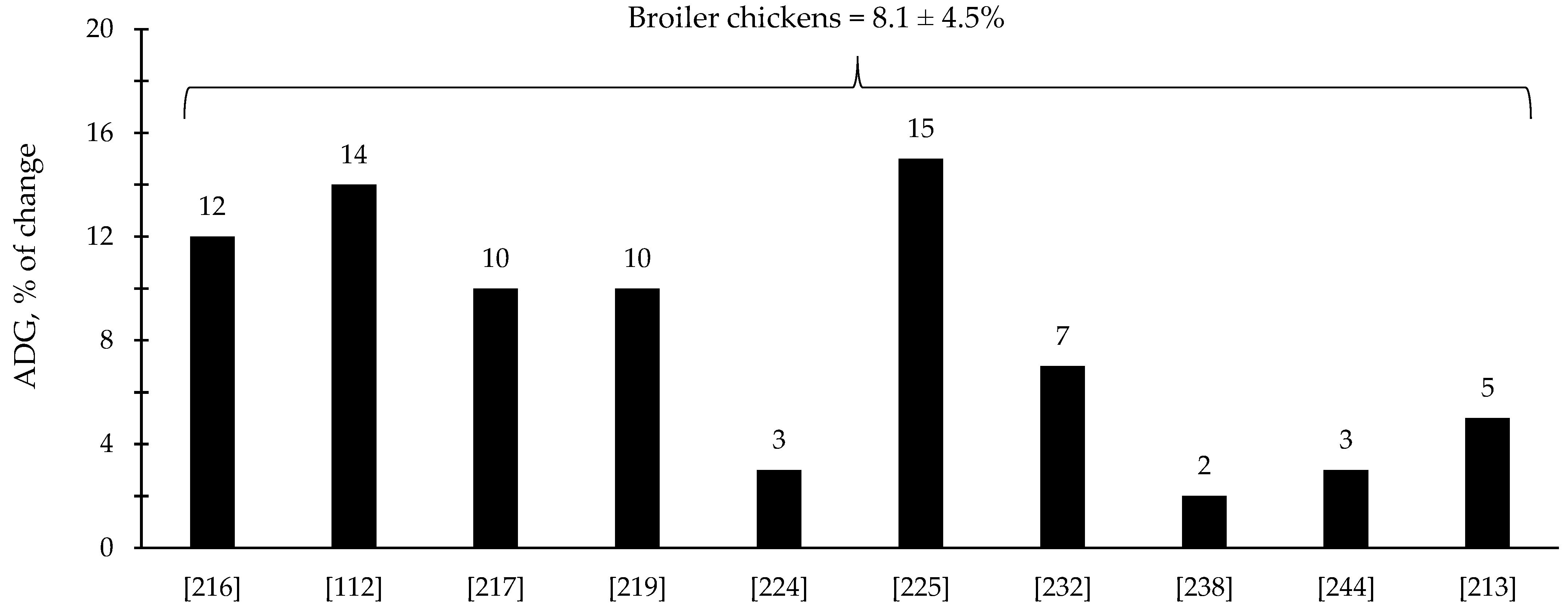
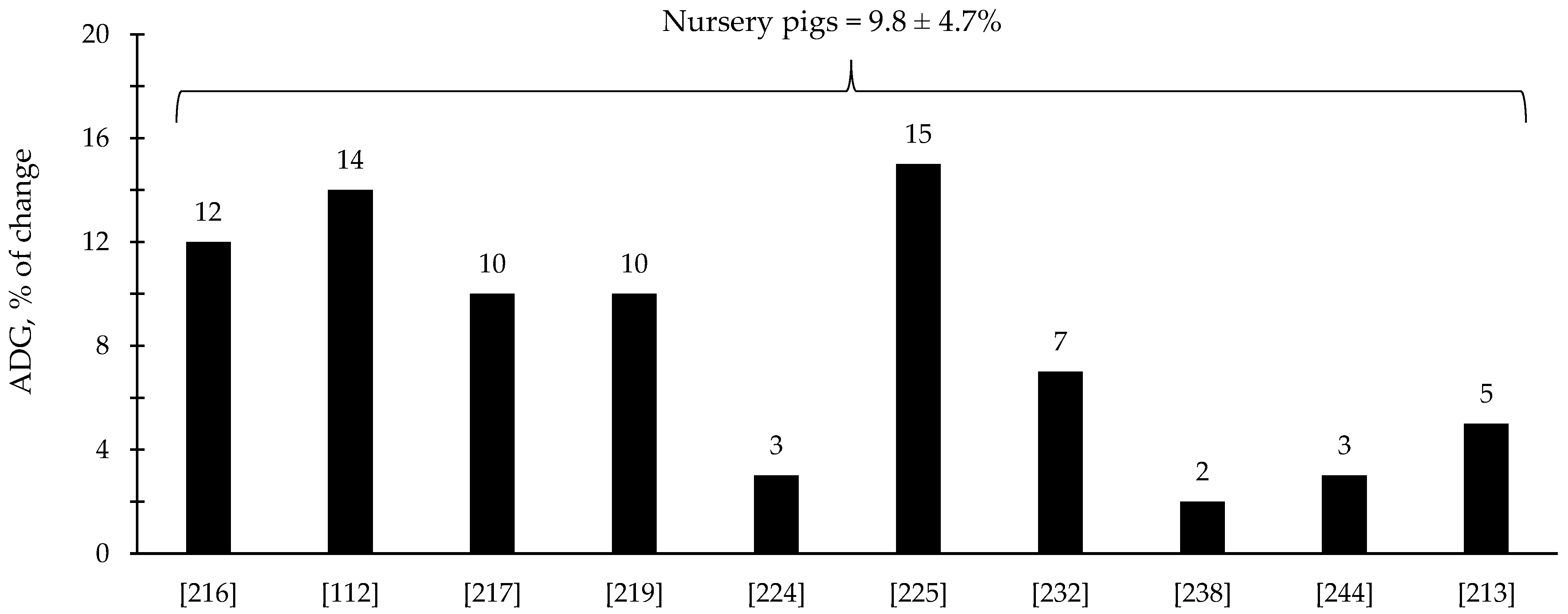
The efficacy of xylanase supplementation in swine and poultry diets has been relatively variable in comparison with phytase with some studies reporting improvements in the reduction in digesta viscosity and growth performance as well as positive alterations in intestinal health and microbiota; however, other studies reported no effects (Table 3 and Table 4). These inconsistent results from xylanase supplementation could be due to a myriad of factors such as animal species and growth stage, diet composition, supplementation level, and xylanase properties. Based on the compiled data from Table 3 and Table 4, xylanase supplementation showed on average 8.1% improvement in the ADG of broiler chickens (Figure 5) and 9.8% improvement in the ADG of nursery pigs (Figure 6).
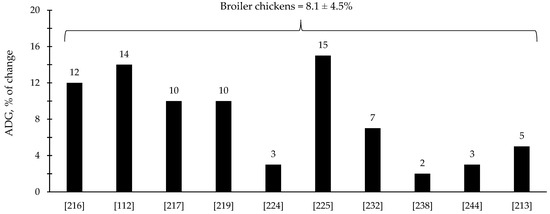
Figure 5.
Changes in the average daily gain (ADG) of broiler chickens by xylanase supplementation among the studies displayed in Table 3 that showed changes in the ADG. The studies were selected from peer-reviewed literature available after 2000. The percentage of change refers to a statistically significant (p < 0.05) and tendency (0.05 ≤ p < 0.10) effects of xylanase individually on the ADG reported from each respective study. The described effects were in comparison with treatments containing no xylanase. The average changes in the ADG of broiler chickens by xylanase supplementation were calculated excluding the studies that showed no effect.
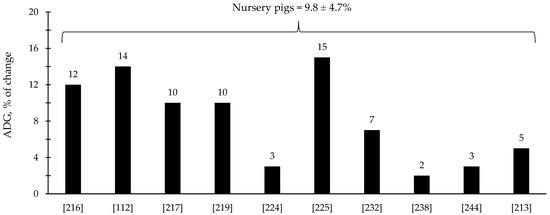
Figure 6.
Changes in the average daily gain (ADG) of nursery pigs by xylanase supplementation among the studies displayed in Table 3 that showed changes in the ADG. The studies were selected from peer-reviewed literature available after 2000. The percentage of change refers to a statistically significant (p < 0.05) and tendency (0.05 ≤ p < 0.10) effects of xylanase individually on the ADG reported from each respective study. The described effects were in comparison with treatments containing no xylanase. The average changes in the ADG of nursery pigs by xylanase supplementation were calculated excluding the studies that showed no effect.

Table 3.
A list of studies describing effects of xylanase supplemented individually at different levels in diets for broiler chickens.
Table 3.
A list of studies describing effects of xylanase supplemented individually at different levels in diets for broiler chickens.
| Duration, Day of Age | Activity | % Change * | Reference ** |
|---|---|---|---|
| 1–42 | ND 1 | Final body weight (2%), ADFI 2 (−2%), tissue protein content (14%), gizzard weight (−8%), duodenum-jejunum weight (−8%), ileal digesta viscosity (ND), ileum lactic acid bacteria (4%) | [217] |
| 1–25 | 0–200 FXU/kg feed | ADG 3 (12%), FCR 4 (−9%), excreta moisture (−5%), jejunum arabinose (80%), jejunum xylose (95%), ileum arabinose (88%), ileum xylose (97%), duodenum digesta viscosity (−33%), jejunum digesta viscosity (−49%) | [218] |
| 1–22 | 0–1000 XU/kg feed | ADG (14%), ADFI (10%), FCR (−5%), duodenum digesta viscosity (−29%), jejunum digesta viscosity (−23%), ileum digesta viscosity (−39%), jejunum weight (−16%), jejunum length (−16%), jejunum crypt depth (−13%) | [113] |
| 1–41 | 0–2000 U/kg feed | ADG (10%), FCR (−6%), in-vitro intrinsic viscosity (−38%) | [219] |
| 1–28 | 0–500 U/kg feed | Jejunum digesta viscosity (−85%) | [220] |
| 1–18 | ND | ADG (10%), ADFI (16%), ileum length (−30%), jejunum crypt depth (−19%) | [221] |
| 1–21 | 0–1000 XU/kg feed | FCR (−2%), AME 5 (3%) | [222] |
| 7–28 | 0–500 U/kg feed | G:F 6 (8–16%), jejunum digesta viscosity (−18%), DM 7 digestibility (27–37%) | [223] |
| 1–28 | 0–2500 GXU/kg feed | ADFI (9%), jejunum digesta viscosity (−11%), cecum arabinose concentration (59%) | [224] |
| 1–43 | 0–16,000 U/kg feed | FCR (−3%), N 8 digestibility (3%), ileal digestible energy digestibility (3%), threonine digestibility (5%), lysine digestibility (3%) | [225] |
| 1–24 | 0–200 FXU/kg feed | ADG (3%), G:F (4%), jejunum digesta viscosity (−21%), DM retention (10%), CP 9 retention (13%), energy retention (9%) | [226] |
| 1–42 | 0–2000 U/kg feed | ADG (15%), FCR (−11%), cecum Salmonella prevalence (−62%) | [227] |
| 1–35 | ND | Feed conversion ratio (−3%), starch digestibility (4%), fat digestibility (3%), AME retention (4%) | [228] |
| 1–43 | 0–16,000 BXU/kg feed | FCR (−3%) | [229] |
| 1–43 | 0–16,000 XU/kg feed | Serum insulin (%), serum peptide YY (61%), cecal VFA 10 (−4%) | [230] |
| 1–35 | 0–250 FXU/kg feed | Ileum viscosity (−20%), ileum xylose concentration (−14%), jejunum protein content (28%) | [231] |
| 1–32 | 0–32,000 BXU/kg feed | No effects on the evaluated parameters | [232] |
| 1–50 | 0–16,000 BXU/kg feed | FCR (−7%), DM digestibility (9%), ileal digestible energy (7%), N digestibility (4%), cecum temperature (4%) | [233] |
| 1–43 | 0–1250 XU/kg feed | ADG (7%), FCR (−5%), jejunum viscosity (−49%), cecum acetic acid concentration (35%), fat digestibility (5%), CP digestibility (4%), DM retention 3%), fat retention (6%), P 11 retention (6%), NDF 12 retention (14%), ADF 13 retention (42%), AME (2%) | [234] |
| 1–21 | 0–5500 U/kg feed | FCR (−7%) | [235] |
| 1–49 | 0–32,000 BXU/kg feed | FCR (−6%), energy digestibility (6%), gizzard weight (16%), cecum propionic acid concentration (22%), cecum caproic acid concentration (24%) | [236] |
| 7–21 | 0–2000 U/kg feed | AME (1%), DM retention (2%), fat digestibility (2%) | [237] |
| 1–42 | 0–160,000 BXU/kg feed | Gizzard weight (17%), gizzard length (19%) | [238] |
| 1–42 | 0–2000 XU/kg feed | FCR (−2%), ileal digestible energy (8%), starch digestibility (1%), N digestibility (5%), GE digestibility (8%), ileum total NSP 14 concentration (−26%) | [239] |
| 1–36 | 0–5625 XU/kg feed | ADG (2%), GE 15 digestibility (3%), CP digestibility (4%), DM digestibility (1%), RA 16 of ileal Lactobacillus (12%), cecal Lactobacillus (11%), ileal Escherichia coli (−15%), and cecal Escherichia coli (−11%), duodenum villus height (8%), jejunum villus height (10%), ileum villus height (12%) | [240] |
| 1–42 | 0–16,000 BXU/kg feed | FCR (−4%) | [241] |
| 1–41 | 0–24,000 BXU/kg feed | Mortality (−54%) | [242] |
| 1–42 | 0–16,000 BXU/kg feed | FCR (−3%), duodenum acetic acid concentration (12%) | [243] |
| 1–42 | 0–200 FXU/kg feed | FCR (−1%), jejunum digesta viscosity (−40%), ileum digesta viscosity (−58%) | [244] |
| 1–29 | 0–160,000 BXU/kg feed | FCR (−8%), copper digestibility (44%) | [245] |
| 1–30 | 0–32,000 BXU/kg feed | Ileal frutose (31%), ileal arabinose (29%), ileal galactose (33%) | [215] |
| 1–33 | 0–11,250 XU/kg feed | ADG (3%), FCR (−4%), DM digestibility (4%), GE digestibility (4%), ileum digesta viscosity (−12%), duodenum villus height (8%), jejunum villus height (9%) | [246] |
| 1–43 | 0–16,000 BXU/kg feed | ADG (5%), final body weight (6%), acetate (27%), total SCFA 17 (24%) | [214] |
* The described effects were in comparison with treatments without xylanase. Described effects were considered significant with p < 0.05 and tendency with 0.05 ≤ p < 0.10. ** The references were selected from peer-reviewed literature available after 2000. 1 No data. 2 Average daily feed intake. 3 Average daily gain. 4 Feed conversion ratio. 5 Apparent metabolizable energy. 6 Gain to feed ratio. 7 Dry matter. 8 Nitrogen. 9 Crude protein. 10 Volatile fatty acids. 11 Phosphorus. 12 Neutral detergent fiber. 13 Acid detergent fiber. 14 Non-starch polysaccharides. 15 Gross energy. 16 Relative abundance. 17 Short chain fatty acids.

Table 4.
A list of studies describing effects of xylanase supplemented individually at different levels in diets for nursery pigs.
Table 4.
A list of studies describing effects of xylanase supplemented individually at different levels in diets for nursery pigs.
| Duration, Day of Age | Activity | % Change * | Reference ** |
|---|---|---|---|
| 35–70 | 0–4000 XU/kg feed | DM 1 fecal digestibility (1%), N 2 digestibility (2%) | [247] |
| 7–28 | 0–5600 EXU/kg feed | No effects on the evaluated parameters | [248] |
| 21–56 | ND 3 | FCR 4 (−3%), CP 5 digestibility (4%), fat digestibility (2%), starch digestibility (1%), total amino acids digestibility (3%), leucine digestibility (2%), isoleucine digestibility (4%), jejunum digesta viscosity (−76%), colon digesta viscosity (−81%), jejunum acetate concentration (27%), jejunum propionate concentration (30%), total conjugated/deconjugated bile acids (33%) | [249] |
| ND | 0–1500 U/kg feed | CP digestibility (2%), crude ash digestibility (2%), Ca 6 digestibility (3%), P 7 digestibility (1%), ADF 8 digestibility (9%) | [250] |
| ND1 | 0–1400 LXU/kg feed | NDF 9 digestibility (36%), DM digestibility (17%), GE 10 digestibility (15%), OM 11 digestibility (15%), jejunum digesta viscosity (−14%) | [29] |
| ND | 0–0.01% | ADG 12 (5%), G:F 13 (4%), DM digestibility (3%), N digestibility (4%), GE digestibility (3%), fecal Lactobacillus (1%) | [69] |
| 28–56 | 0–4000 XU/kg feed | DM digestibility (2%), NDF digestibility (23%), P digestibility (29%) | [251] |
| 21–49 | 0–4000 XU/kg feed | ADG (14%), DM digestibility (4%), CP digestibility (7%), NDF digestibility (19%), ADF digestibility (15%), Ca digestibility (22%), P digestibility (14%), fecal Lachnospiraceae (−50%) | [252] |
| 23–43 | 0–1500 EPU/kg feed | SCFA 14 (20%), acetate (32%), propionate (19%), total NSP 15 digestibility coefficient (15%), arabinoxylan digestibility coefficient (38%), GE digestibility coefficient (8%), duodenum villus height (11%), jejunum crypt proliferation rate (17%), jejunum claudin (57%), jejunum occludin (75%), jejunum zonula occludens 1 (80%), jejunum digesta viscosity (−26%) | [30] |
| 21–45 | 0–45,000 XU/kg feed | ADG (6%), jejunal digesta viscosity (−13%), jejunum mucosal MDA 16 (−17%), jejunum crypt depth (−10%), jejunum crypt cell proliferation (−15%) | [23] |
| 18–53 | 0–16,000 BXU/kg feed | Ileum pH (6%), colon pH (3%), CP digestibility (7%), DM digestibility (3%), Ca digestibility (16%), P digestibility (8%), colon propionic acid concentration (10%) | [253] |
| 21–41 | 0–1500 EPU/kg feed | ADG (7%), jejunal digesta viscosity (−14%), plasma TNF-α 17 (−36%), GE digestibility (6%), NDF digestibility (22%), duodenum crypt depth (11%) | [58] |
| 23–65 | 0–16,000 BXU/kg feed | Ileum Clostridiaceae (−10%), cecum Lactobacillaceae count (36%) | [254] |
| 21–63 | 0–16,000 XU/kg feed | ADG (9%), DM digestibility (4%), GE digestibility (3%), N digestibility (3%) and P digestibility (15%), RA 18 fecal Veillonella spp. (−33%) | [255] |
| 21–59 | 0–1760 XU/kg feed | Jejunal digesta viscosity (−23%), jejunum mucosal MDA (−39%), jejunum mucosal PC 19 (−15%), jejunum villus height (13%), NDF digestibility (6%), EE 20 digestibility (6%), RA of jejunum Cupriavidus (−43%), Megasphaera (−82%), Succinivibrio (73%), and Pseudomonas (63%) | [5] |
| 28–63 | 0–135,000 U/kg feed | ADG (18%), ADFI (−2%), G:F (20%), diarrhea rate (−61%), DM digestibility (4%), CP digestibility (6%), EE digestibility (8%), NDF digestibility (2%), ADF digestibility (7%), GE digestibility (30%), starch digestibility (27%), N digestibility (26%), jejunum villus height (15%), feces propionate concentration (41%), fecal butyrate concentration (35%), feces ammonia concentration −26%) | [256] |
* The described effects were in comparison with treatments without xylanase. Described effects were considered significant with p < 0.05 and tendency with 0.05 ≤ p < 0.10. ** The references were selected from peer-reviewed literature available after 2000. 1 Dry matter. 2 Nitrogen. 3 No data. 4 Feed conversion ratio. 5 Crude protein. 6 Calcium. 7 Phosphorus. 8 Acid detergent fiber. 9 Neutral detergent fiber. 10 Gross energy. 11 Organic matter. 12 Average daily gain. 13 Gain to feed ratio. 14 Short chain fatty acids. 15 Non-starch polysaccharides. 16 Malondealdehyde. 17 Tumor necrosis factor alpha. 18 Relative abundance. 19 Protein carbonyl. 20 Ether extract.
3.2.2. Effects of Supplementing Xylanase on the Intestinal Health of Nursery Pigs and Broiler Chickens
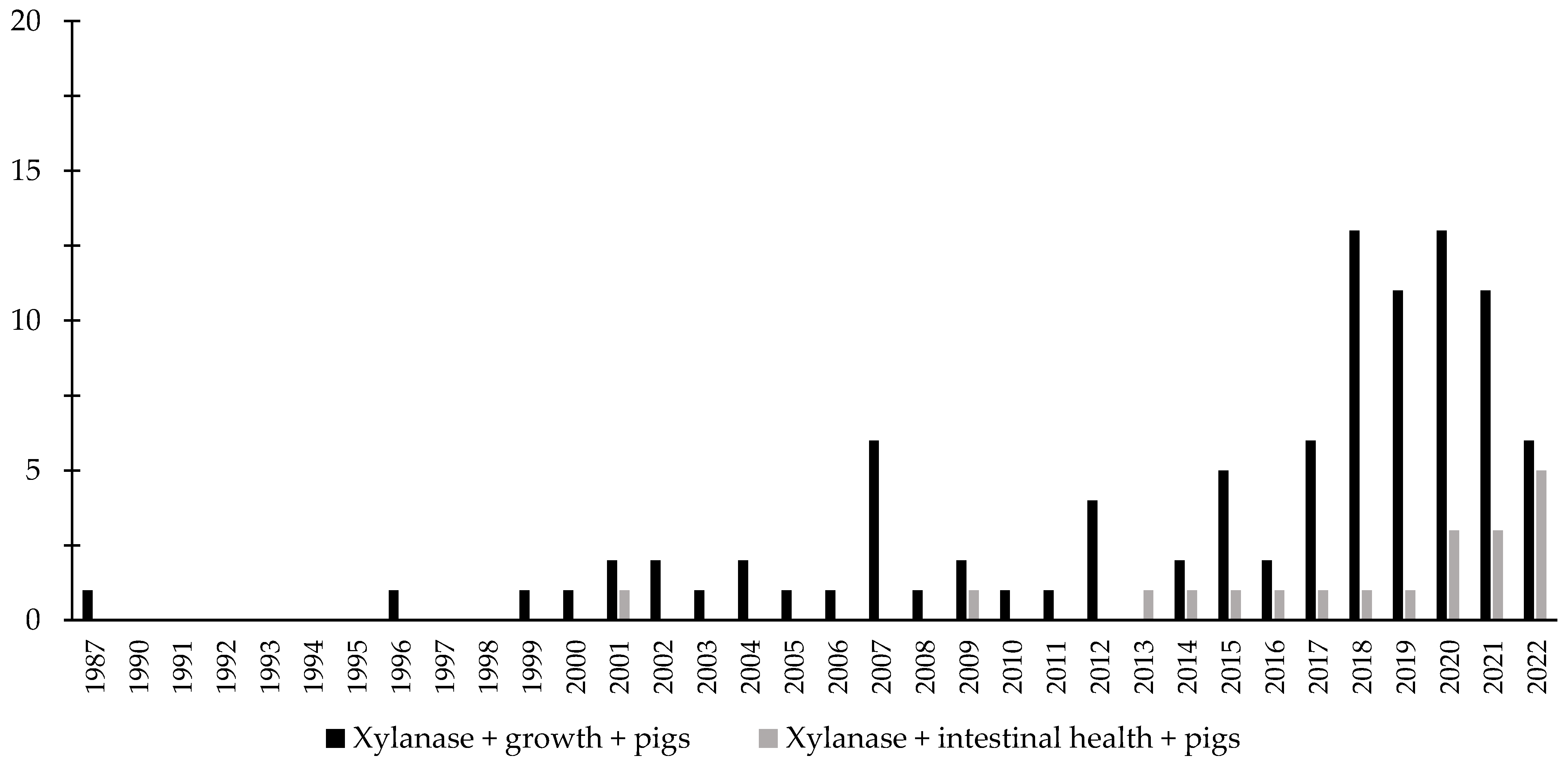
Throughout the last few years, there has been an increase in the interest in the investigation of the effects of xylanase related with the intestinal health of broiler chickens and nursery pigs (Figure 7 and Figure 8). Several studies were conducted investigating the effects of xylanase on the intestinal health and microbiota of nursery and broiler chickens [5,15,213,214,215,242]. The authors reported a wide range of positive effects of xylanase supplementation not only related to growth performance and nutrient digestibility, but especially related to intestinal health.
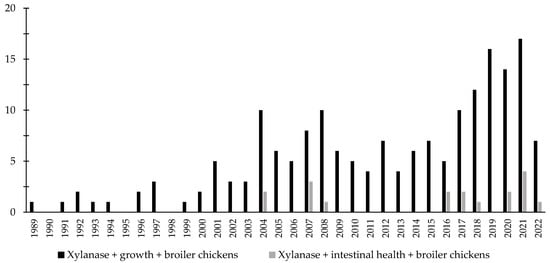
Figure 7.
A list of the number of peer-reviewed papers found in the PubMed using different keywords. Black bars: xylanase, growth, broiler chickens as key words; grey bars: xylanase, intestinal health, broiler chickens as keywords.
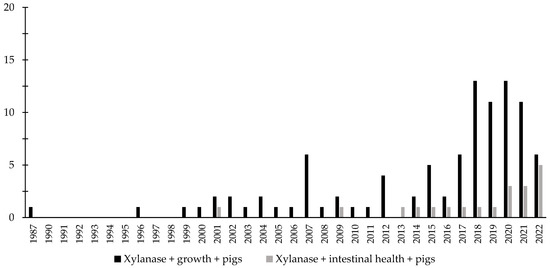
Figure 8.
A list of the number of peer-reviewed papers found in the PubMed using different keywords. Black bars: xylanase, growth, pigs as key words; grey bars: xylanase, intestinal health, pigs as keywords.
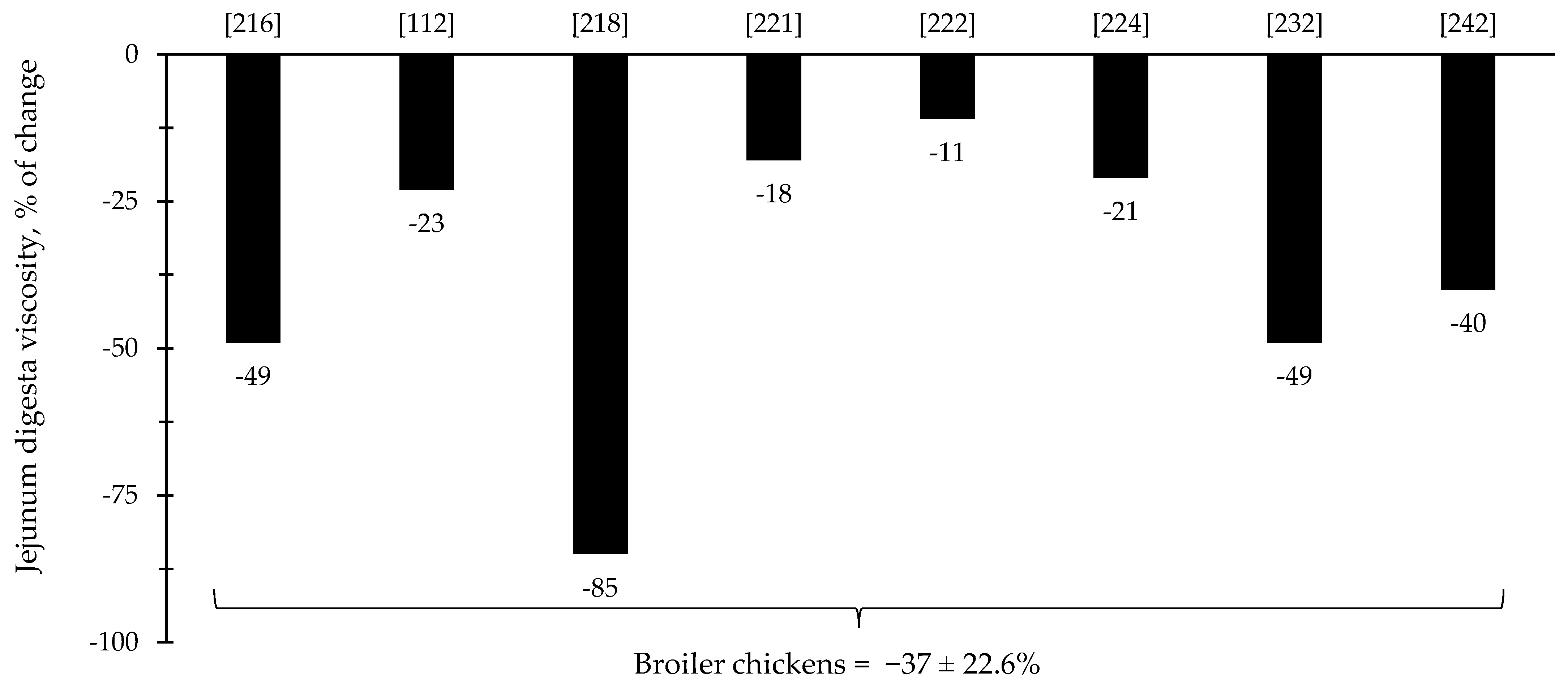
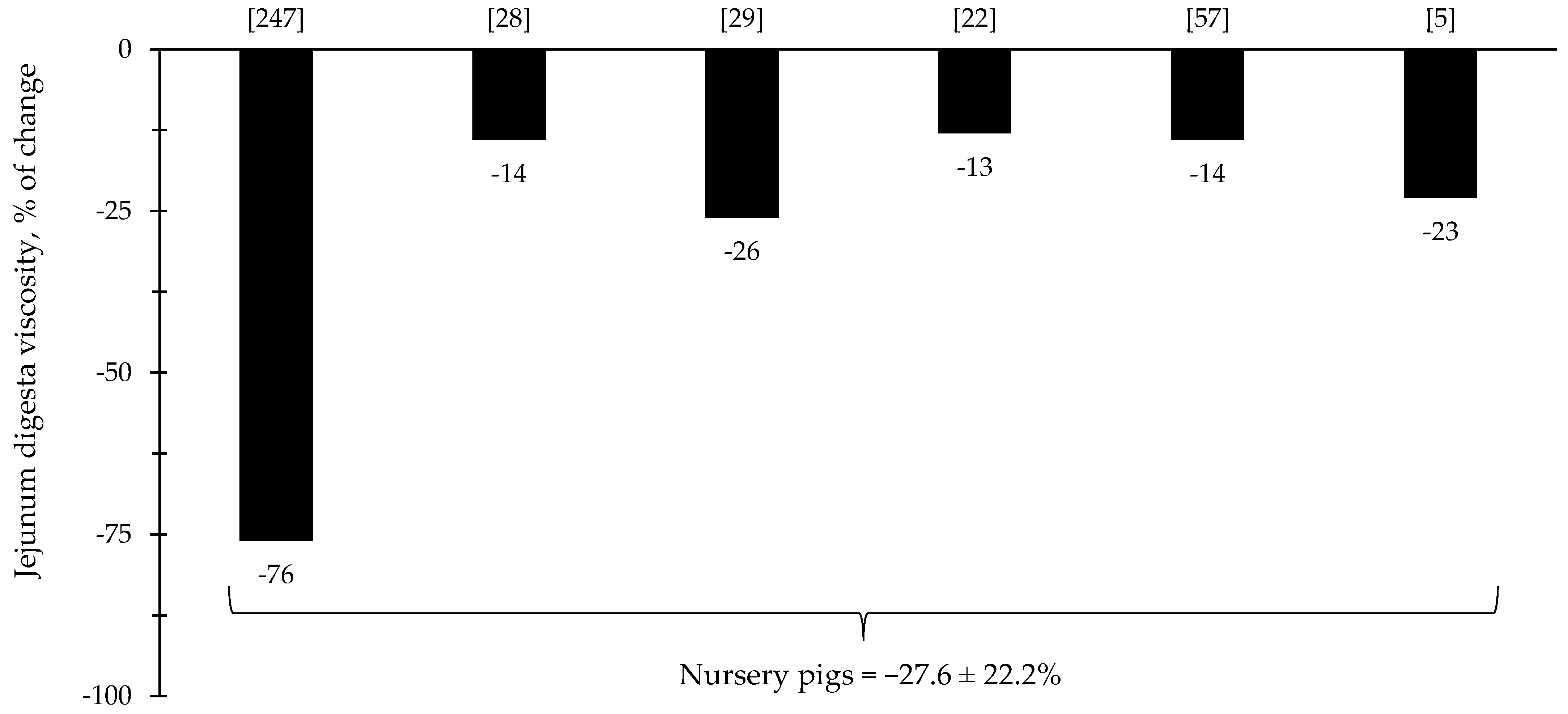
The reduction in digesta viscosity and increase in nutrient digestibility are described as some of the primarily established benefits of xylanase supplementation for pigs and broiler chickens. Most of the studies for broiler chickens showed consistent improvements related to the reduction in jejunal digesta viscosity (=37%), which may account for other benefits reported in each of the respective described studies (Figure 9). For nursery pigs, xylanase also showed potential improvements related to the reduction in jejunal digesta viscosity (27.6%; Figure 10). These benefits may contribute for the expression of other possible benefits, such as, reduction in the oxidative stress, positive modulation of the intestinal microbiota, and enhancement of growth performance. However, there were studies that even reporting a reduction in the digesta viscosity did not report improvements on the growth performance [5,29].
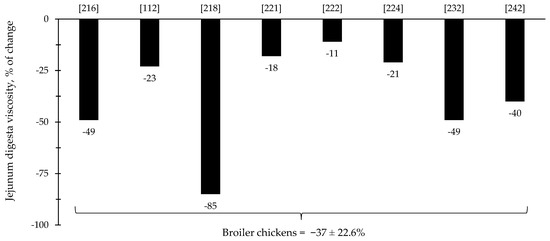
Figure 9.
Changes in the jejunum digesta viscosity of broiler chickens by xylanase supplementation among the studies displayed in Table 3 that showed changes in the jejunal digesta viscosity. The studies were selected from peer-reviewed literature available after 2000. The percentage of change refers to a statistically significant (p < 0.05) and tendency (0.05 ≤ p < 0.10) effects of xylanase on the jejunal digesta viscosity reported from each respective study. The described effects were in comparison with treatments containing no xylanase. The average changes in the jejunal digesta viscosity of broiler chickens by xylanase supplementation were calculated excluding the studies that showed no effect.
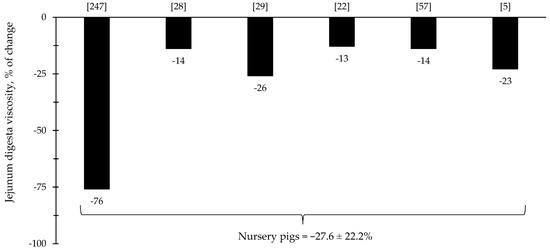
Figure 10.
Changes in the jejunum digesta viscosity of nursery pigs by xylanase supplementation among the studies displayed in Table 4 that showed changes in the jejunal digesta viscosity. The studies were selected from peer-reviewed literature available after 2000. The percentage of change refers to a statistically significant (p < 0.05) and tendency (0.05 ≤ p < 0.10) effects of xylanase on the jejunal digesta viscosity reported from each respective study. The described effects were in comparison with treatments containing no xylanase. The average changes in the jejunal digesta viscosity of nursery pigs by xylanase supplementation were calculated excluding the studies that showed no effect.
The authors of [5] reported reduced digesta viscosity and increased apparent ileal digestibility (AID) of neutral detergent fiber (NDF), crude protein (CP), and ether extract (EE) of nursery pigs supplemented with increasing levels of xylanase (0, 220, 440, 880, 1760 XU/kg feed), whereas no effects on the overall growth performance. Firstly, the authors attributed these benefits to the depolymerization of the β-1,4 xylan bonds into shorter chain that possibly led to alterations in the physicochemical properties of the digesta and consequently reducing its viscosity and bulkiness. A higher digesta viscosity can alter the passage rate and reduce the interaction between digesta and other endogenous enzymes and lipid emulsifiers. Secondly, they also believed that the depolymerization of the xylan bonds led to an increase in the release trapped nutrients and fermentable NSP-released compounds. The results from [5] agree with other studies that also reported supplemental effects of xylanase reducing digesta viscosity and increasing digestibility of both nursery pigs and broiler chickens [23,58,240]. A reduction in digesta viscosity and bulkiness, and subsequent generation of trapped nutrients and fermentable NSP-released compounds bioavailability may contribute to the positive results observed in growth performance and nutrient digestibility, but especially on the modulation of mucosa-associated microbiota in the jejunum and jejunal oxidative stress and morphology parameters.
Moreover, a reduction was reported in the MDA and PC concentrations in the jejunal mucosa as indicators of reduced oxidative stress. These results are in agreement with [23] and [213], whereas [30] and [58] did not observe any xylanase-related effects to oxidative stress status. Overall, there is still no consensus about the specific mechanisms of xylanase modulating the oxidative stress and enhancing antioxidant capacity. The authors of [140] suggested that a potential mechanism could be due to an increasing fragmentation of the xylan structure and subsequent generation of fermentable NSP-released compounds, more specifically comprised of phenolic compounds such as, ferulic acid. Ferulic acid can play antioxidant [257] and antimicrobial functions [258] and was shown to be correlated with oxidative stress status of pigs [259] and with a reduction in pathogenic bacteria concentration in the feces [260].
The potential mechanism of xylanase associated with the reduction in inflammatory response parameters remains unclear. The authors of [58] reported decreased plasma concentration of TNF-α and peptide YY (PYY) of nursery pigs. The authors believed that the active hydrolysis of the β-1,4 xylan bonds generating oligosaccharides coupled with the observed reduction in digesta viscosity may contributed to the reduction in pro-inflammatory cytokines such as TNF-α, that are known as mediators of the immune response. The reduction in the plasma concentration of PYY by xylanase in nursery pigs by [261]; however, it did not show the same effects for broiler chickens [230]. The supplementation of xylanase has shown consistent results regarding the reduction digesta viscosity and increasing nutrient digestibility between nursery pigs and broiler chickens [5,23,70,215,219]. On the other hand, nursery pigs and broiler chickens may express different responses to the use of xylanase on the intestinal health parameters.
According to [4], the intestinal microbiota can be altered at different taxonomic levels based on the given fermentable substrate. In this context, the short chain fatty acids, XOS, and AXOS potentially released from the xylan structure would be rapidly fermented by beneficial bacteria populations such as, lactic acid bacterial, and may exert prebiotic functions [13]. The results from [5] showed a tendency on the reduction in the relative abundance (RA) of Cupriavidus and Megasphera and on the increase in Succinivibrio and Pseudomonas in jejunal mucosa of nursery pigs. The authors of [262] found a correlation between a low abundance of Succinivibrio with gastrointestinal disorders and loss of intestinal integrity in the colon of humans, and [263] reported this genus degrading cellulose and hemicellulose. These shifts in the jejunal bacterium population may indicate changes in the intestinal environment and substrates towards a healthier state, as a consequence of the positive effects observed in the nutrients digestibility and reduced viscosity. The authors of [15] also observed positive effects of xylanase supplementation in the modulation of the microbiota by increasing the RA of Lactobacillus and Bifidobacterium, and decreasing Streptococcus and Turicibacter in the ileum digesta; and increasing Bifidobacterium and decreasing Escherichia-shigella in the ileum mucosa of growing pigs. Regarding the effects of xylanase on the intestinal microbiota of broiler chickens [240], observed a decrease in the RA of Escherichia coli and an increase in Lacbotobacillus in the cecum, whilst [214] reported an increase in Rumonococcaceae also in the cecum.
Although the effects of xylanase positively modulating the intestinal microbiota of nursery pigs and broiler chickens show great potential, further investigation is still desired in order to elucidate the specific mechanisms of action. A positive modulation of the intestinal microbiota even at different levels and sections can contribute to enhance positive effects associated with other parameters and overall biological response. An increase in the RA of selected beneficial bacteria may increase the endogenous secretion of other degrading enzymes and short chain fatty acids, collectively enhancing nutrient digestibility, intestinal health, and growth performance of nursery pigs and broiler chickens. Even showing variable results, the supplementation of xylanase also shows positive results associated with the intestinal health of nursery pigs and broiler chickens. However, there is still no consensus among authors regarding the different mechanisms of action of xylanase among the evaluated parameters, animal species, and inclusion levels.
4. Conclusions
In conclusion, 6-phytase seems to be more effective and to provide a wider range of benefits compared to 3-phytase, especially related to growth performance. The supplementation of 6-phytase for nursery pigs and broiler chickens reaffirmed the positive results related to enhancement of nutrient digestibility, bone health, and growth performance, but also showed functional roles reducing oxidative stress parameters, and positively modulating the intestinal microbiota. Supplementing phytase beyond traditional levels (500 to 1000 FTU/kg feed), so-called “super-dosing” (above 1000 FTU/kg feed), showed a more active hydrolysis of phytic acid providing “extra-phosphoric effects” that were further reflected in improvements in intestinal and bone health, nutrient digestibility, growth performance of nursery pigs and broiler chickens.
The supplementation of xylanase can effectively reduce the digesta viscosity of nursery pigs and broiler chickens, which in turn could lead to potential benefits associated with the reduction in immune and oxidative stress parameters, positive modulation of intestinal microbiota, and enhancement of nutrient digestibility. Therefore, the growth performance of the animals can be potentially enhanced by supplementing xylanase.
Overall, the supplementation of phytase and xylanase in diets for nursery pigs and broiler chickens reaffirmed their nutritional roles enhancing nutrient digestibility and growth performance, whilst also playing functional roles reducing the oxidative stress response, and possibly through the modulation of the mucosal microbiota in the small intestine. As a result, it could contribute to a reduction in the feed costs by allowing the use of a wider range of feedstuffs without compromising the optimal performance of the animals, as well as the environmental concerns associated with a poor hydrolysis of antinutritional factors present in the diets for nursery pigs and broilers chickens.
Author Contributions
Conceptualization, V.H.C.M. and S.W.K.; methodology, S.W.K.; formal analysis, S.W.K.; investigation, V.H.C.M.; resources, S.W.K.; data curation, V.H.C.M. and S.W.K.; writing—original draft preparation, V.H.C.M. and S.W.K.; writing—review and editing, V.H.C.M. and S.W.K.; supervision, S.W.K.; project administration, S.W.K.; funding acquisition, S.W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by North Carolina Agricultural Foundation (#660101) and USDA National Institute of Food and Agriculture (Hatch #02893).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, S.W.; Duarte, M.E. Understanding intestinal health in nursery pigs and the relevant nutritional strategies. Anim. Biosci. 2021, 34, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.B.; Kim, S.W. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly weaned pigs. J. Anim. Sci. 2019, 97, 4875–4882. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Duarte, M.E.; Kim, S.W. Effects of Yarrowia lipolytica supplementation on growth performance, intestinal health and apparent ileal digestibility of diets fed to nursery pigs. Anim. Biosci. 2021, 35, 605–613. [Google Scholar] [CrossRef]
- Duarte, M.E.; Kim, S.W. Intestinal microbiota and its interaction to intestinal health in nursery pigs. Anim. Nutr. 2021, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Moita, V.H.C.; Duarte, M.E.; Kim, S.W. Functional roles of xylanase enhancing intestinal health and growth performance of nursery pigs by reducing the digesta viscosity and modulating the mucosa-associated microbiota in the jejunum. J. Anim. Sci. 2022, 100, skac116. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Moeser, A.J.; Ryan, K.A.; Nighot, P.K.; Blikslager, A.T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Liver Physiol. 2007, 293, G413–G421. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; van Heugten, E.; Ji, F.; Lee, C.H.; Mateo, R.D. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 2010, 88, 214–224. [Google Scholar] [CrossRef]
- Taliercio, E.; Kim, S.W. Epitopes from two soybean glycinin subunits are antigenic in pigs. J. Sci. Food Agric. 2013, 93, 2927–2932. [Google Scholar] [CrossRef]
- Deng, Z.; Duarte, M.E.; Jang, K.B.; Kim, S.W. Soy protein concentrate replacing animal protein supplements and its impacts on intestinal immune status, intestinal oxidative stress status, nutrient digestibility, mucosa-associated microbiota, and growth performance of nursery pigs. J. Anim. Sci. 2022, 100, skac255. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Duarte, M.E.; Holanda, D.M.; Kim, S.W. Friend or Foe? Impacts of Dietary Xylans, Xylooligosaccharides, and Xylanases on Intestinal Health and Growth Performance of Monogastric Animals. Animals 2021, 11, 609. [Google Scholar] [CrossRef]
- Ptak, A.; Bedford, M.R.; Światkiewicz, S.; Zyła, K.; Józefiak, D. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS ONE 2015, 10, e0119770. [Google Scholar] [CrossRef]
- Moita, V.H.C.; Duarte, M.E.; Kim, S.W. Supplemental effects of phytase on modulation of mucosa-associated microbiota in the jejunum and the impacts on nutrient digestibility, intestinal morphology, and bone parameters in broiler chickens. Animals 2021, 11, 3351. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.L.; Patience, J.F.; Koester, L.R.; Huntley, N.F.; Bedford, M.R.; Schmitz-Esser, S. Xylanase modulates the microbiota of ileal mucosa and digesta of pigs fed corn-based arabinoxylans likely through both a stimbiotic and prebiotic mechanism. PLoS ONE 2021, 16, e0246144. [Google Scholar] [CrossRef] [PubMed]
- Böhme, H. Enzymes in farm animal nutrition. Anim. Feed Sci. Technol. 2001, 91, 241–242. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th. ed.; W.H. Freeman Co.: New York, NY, USA, 2017; p. 2. [Google Scholar]
- Ravindran, V. Feed enzymes: The science, practice, and metabolic realities. J. Appl. Poult. Res. 2013, 22, 628–636. [Google Scholar] [CrossRef]
- Choct, M. Enzymes for the feed industry: Past, present and future. Worlds. Poult. Sci. J. 2006, 62, 5–16. [Google Scholar] [CrossRef]
- Slominski, B.A. Recent advances in research on enzymes for poultry diets. Poult. Sci. 2011, 90, 2013–2023. [Google Scholar] [CrossRef]
- Adeola, O.; Cowieson, A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef] [PubMed]
- Karadas, F.; Pirgozliev, V.; Pappas, A.C.; Acamovic, T.; Bedford, M.R. Effects of different dietary phytase activities on the concentration of antioxidants in the liver of growing broilers. J. Anim. Physiol. Anim. Nutr. 2010, 94, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Zhou, F.X.; Dutra, W.M.; Kim, S.W. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. 2019, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Porres, J.M. Phytase enzymology, applications, and biotechnology. Biotechnol. Lett. 2003, 25, 1787–1794. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef]
- Mach, N.; Berri, M.; Estellé, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.-J.; Chevaleyre, C.; Billon, Y.; Doré, J.; et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W.; et al. The Dynamic Distribution of Porcine Microbiota across Different Ages and Gastrointestinal Tract Segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef] [PubMed]
- Passos, A.A.; Park, I.; Ferket, P.; von Heimendahl, E.; Kim, S.W. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim. Nutr. 2015, 1, 19–23. [Google Scholar] [CrossRef]
- Tiwari, U.P.; Chen, H.; Kim, S.W.; Jha, R. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim. Feed Sci. Technol. 2018, 245, 77–90. [Google Scholar] [CrossRef]
- Rodica, C.; Adrian, C.; Julean, C. Biochemical Aspects of Non-Starch Polysaccharides. Anim. Sci. Biotechnol. 2010, 43, 368–375. [Google Scholar]
- Kiarie, E.; Romero, L.F.; Nyachoti, C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013, 26, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Selle, P.H.; Ravindran, V. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 2008, 113, 99–122. [Google Scholar] [CrossRef]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- McCance, R.A.; Widdowson, E.M. Mineral metabolism of healthy adults on white and brown bread dietaries. J. Physiol. 1942, 101, 44–85. [Google Scholar] [CrossRef]
- McCance, R.A.; Walsham, C.M. The Digestibility and Absorption of the Calories, Proteins, Purines, Fat and Calcium in Wholemeal Wheaten Bread. Br. J. Nutr. 1948, 2, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Halsted, J.A.; Ronaghy, H.A.; Abadi, P.; Haghshenass, M.; Amirhakemi, G.H.; Barakat, R.M.; Reinhold, J.G. Zinc deficiency in man. The Shiraz experiment. Am. J. Med. 1972, 53, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Costa-Bauza, A. Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules 2019, 24, 4434. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Tyler, C. The reaction between phytate and protein. J. Agric. Sci. 1954, 44, 324–326. [Google Scholar] [CrossRef]
- Singh, M.; Krikorian, A.D. Inhibition of trypsin activity in vitro by phytate. J. Agric. Food Chem. 1982, 30, 799–800. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Cheryan, M. Effects of Phytic Acid, Divalent Cations, and Their Interactions on α-Amylase Activity. J. Food Sci. 1984, 49, 516–519. [Google Scholar] [CrossRef]
- Reddy, N.R.; Sathe, S.K.; Salunkhe, D.K. Phytates in Legumes and Cereals. In Advances in Food Research; Academic Press: Cambridge, MA, USA, 1982; Volume 28, pp. 1–92. [Google Scholar]
- Pallauf, J.; Rimbach, G. Nutritional significance of phytic acid and phytase. Arch. Tierernaehrung 1997, 50, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Cap, U.P.; Stark, C.; Carolina, N. Feed manufacturing to lower feed cost. Allen D. Leeman Swine Conf. 2012, 39, 127–133. [Google Scholar]
- Hölzel, C.S.; Müller, C.; Harms, K.S.; Mikolajewski, S.; Schäfer, S.; Schwaiger, K.; Bauer, J. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ. Res. 2012, 113, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Misiura, M.M.; Filipe, J.A.N.; Walk, C.L.; Kyriazakis, I. How do pigs deal with dietary phosphorus deficiency? Br. J. Nutr. 2020, 124, 256–272. [Google Scholar] [CrossRef]
- Cheryan, M.; Rackis, J.J. Phytic acid interactions in food systems. C R C Crit. Rev. Food Sci. Nutr. 1980, 13, 297–335. [Google Scholar] [CrossRef]
- Angel, R.; Tamim, N.M.; Applegate, T.J.; Dhandu, A.S.; Ellestad, L.E. Phytic acid chemistry: Influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 2002, 11, 471–480. [Google Scholar] [CrossRef]
- Yi, Z.; Kornegay, E.T. Sites of phytase activity in the gastrointestinal tract of young pigs. Anim. Feed Sci. Technol. 1996, 61, 361–368. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Ruckebusch, J.P.; Knap, I.; Guggenbuhl, P.; Fru-Nji, F. Phytate-free nutrition: A new paradigm in monogastric animal production. Anim. Feed Sci. Technol. 2016, 222, 180–189. [Google Scholar] [CrossRef]
- Kim, S.W.; Knabe, D.A.; Hong, K.J.; Easter, R.A. Use of carbohydrases in corn–soybean meal-based nursery diets1. J. Anim. Sci. 2003, 81, 2496–2504. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Dalsgaard, S.; Knudsen, K.E.E.B.; Yu, S.; Lærke, H.N. Compositional profile and variation of Distillers Dried Grains with Solubles from various origins with focus on non-starch polysaccharides. Anim. Feed Sci. Technol. 2014, 197, 130–141. [Google Scholar] [CrossRef]
- Jaworski, N.W.; Lærke, H.N.; Bach Knudsen, K.E.; Stein, H.H. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains1. J. Anim. Sci. 2015, 93, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.E.; Pethick, D.W.; Mullan, B.P.; Hampson, D.J. Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth, and stimulates proliferation of enterotoxigenic Escherichia coli in newly-weaned pigs. Br. J. Nutr. 2001, 86, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Berrocoso, J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef]
- Bakker, G.C.M.; Dekker, R.A.; Jongbloed, R.; Jongbloed, A.W. Non-starch polysaccharides in pig feeding. Vet. Q. 1998, 20, 59–64. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Kim, S.W. Effects of supplemental xylanase on health of the small intestine in nursery pigs fed diets with corn distillers’ dried grains with solubles. J. Anim. Sci. 2020, 98, skaa185. [Google Scholar] [CrossRef]
- Choct, M. Feed non-starch polysaccharides for monogastric animals: Classification and function. Anim. Prod. Sci. 2015, 55, 1360. [Google Scholar] [CrossRef]
- Linhardt, R.J. Polysaccharides I: Structure, Characterization and Use. Advances in Polymer Science, 186 Edited by Thomas Heinze (Friedrich-Schiller-Universität Jena, Germany). Springer: Berlin, Heidelberg, New York. 2005. xii + 282 pp. $249.00. ISBN 3-540-26112-5. J. Am. Chem. Soc. 2006, 128, 6268. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Köhnke, T.; Östlund, Å.; Brelid, H. Adsorption of arabinoxylan on cellulosic surfaces: Influence of degree of substitution and substitution pattern on adsorption characteristics. Biomacromolecules 2011, 12, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Hoebler, C.; Guillon, F.; Darcy-Vrillon, B.; Vaugelade, P.; Lahaye, M.; Worthington, E.; Duée, P.H.; Barry, J.L. Supplementation of pig diet with algal fibre changes the chemical and physicochemical characteristics of digesta. J. Sci. Food Agric. 2000, 80, 1357–1364. [Google Scholar] [CrossRef]
- Gutierrez, N.A.; Serão, N.V.L.; Kerr, B.J.; Zijlstra, R.T.; Patience, J.F. Relationships among dietary fiber components and the digestibility of energy, Dietary fiber, And amino acids and energy content of nine corn coproducts fed to growing pigs. J. Anim. Sci. 2014, 92, 4505–4517. [Google Scholar] [CrossRef] [PubMed]
- Petry, A.L.; Patience, J.F. Xylanase supplementation in corn-based swine diets: A review with emphasis on potential mechanisms of action. J. Anim. Sci. 2020, 98, skaa318. [Google Scholar] [CrossRef] [PubMed]
- Tervilä-Wilo, A.; Parkkonen, T.; Morgan, A.; Hopeakoski-Nurminen, M.; Poutanen, K.; Heikkinen, P.; Autio, K. In vitro digestion of wheat microstructure with xylanase and cellulase from Trichoderma reesei. J. Cereal Sci. 1996, 24, 215–225. [Google Scholar] [CrossRef]
- O’Neill, H.V.M.; Liu, N.; Wang, J.P.; Diallo, A.; Hill, S. Effect of xylanase on performance and apparent metabolisable energy in starter broilers fed diets containing one maize variety harvested in different regions of China. Asian-Australas. J. Anim. Sci. 2012, 25, 515–523. [Google Scholar] [CrossRef]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Pearson Education Limited: Harlow, UK, 2011; ISBN 0582219272. [Google Scholar]
- Lan, R.; Li, T.; Kim, I. Effects of xylanase supplementation on growth performance, nutrient digestibility, blood parameters, fecal microbiota, fecal score and fecal noxious gas emission of weaning pigs fed corn-soybean meal-based diet. Anim. Sci. J. 2017, 88, 1398–1405. [Google Scholar] [CrossRef]
- García, M.; Lázaro, R.; Latorre, M.A.; Gracia, M.I.; Mateos, G.G. Influence of enzyme supplementation and heat processing of barley on digestive traits and productive performance of broilers. Poult. Sci. 2008, 87, 940–948. [Google Scholar] [CrossRef]
- Hayakawa, T.; Suzuki, K.; Miura, H.; Ohno, T.; Igaue, I. Myo-inositol Polyphosphate Intermediates in the Dephosphorylation of Phytic Acid by Acid Phosphatase with Phytase Activity from Rice Bran. Agric. Biol. Chem. 1990, 54, 279–286. [Google Scholar] [CrossRef]
- Konietzny, U.; Greiner, R. Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int. J. Food Sci. Technol. 2002, 37, 791–812. [Google Scholar] [CrossRef]
- McDowell, L.R. Minerals in Animal and Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9780444513670. [Google Scholar]
- Cromwell, G.L. Phosphorus and Swine Nutrition. In Phosphorus: Agriculture and the Environment; Wiley: Hoboken, NJ, USA, 2015; pp. 607–634. [Google Scholar]
- Létourneau-Montminy, M.P.; Narcy, A.; Lescoat, P.; Magnin, M.; Bernier, J.F.; Sauvant, D.; Jondreville, C.; Pomar, C. Modeling the fate of dietary phosphorus in the digestive tract of growing pigs. J. Anim. Sci. 2011, 89, 3596–3611. [Google Scholar] [CrossRef]
- Adeola, O.; Sands, J.S.; Simmins, P.H.; Schulze, H. The efficacy of an Escherichia coli-derived phytase preparation. J. Anim. Sci. 2004, 82, 2657–2666. [Google Scholar] [CrossRef]
- De Jong, J.A.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D.; Tokach, M.D.; Dritz, S.S.; Stark, C.R.; Jones, C.K. Stability of four commercial phytase products under increasing thermal conditioning temperatures. Transl. Anim. Sci. 2017, 1, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.; Lantzsch, H.J.; Langbein, U.; Drochner, W. Determination of phytase activity in cereal grains by direct incubation. J. Anim. Physiol. Anim. Nutr. 2002, 86, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Rojas, O.J.; Stein, H.H. Processing of ingredients and diets and effects on nutritional value for pigs. J. Anim. Sci. Biotechnol. 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Mrudula Vasudevan, U.; Jaiswal, A.K.; Krishna, S.; Pandey, A. Thermostable phytase in feed and fuel industries. Bioresour. Technol. 2019, 278, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.J.; Bedford, M.R.; Selle, P.H.; Ravindran, V. Phytate and microbial phytase: Implications for endogenous nitrogen losses and nutrient availability. Worlds. Poult. Sci. J. 2009, 65, 401–418. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Lu, H.; Cowieson, A.J.; Wilson, J.W.; Ajuwon, K.M.; Adeola, O. Extra-phosphoric effects of super dosing phytase on growth performance of pigs is not solely due to release of myo-inositol. J. Anim. Sci. 2019, 97, 3898–3906. [Google Scholar] [CrossRef]
- Moita, V.H.C.; Duarte, M.E.; Kim, S.W. 93 Efficacy of bacterial 6-phytase and its optimal supplementation level on bone parameters, nutrient digestibility, and growth performance of nursery pigs. J. Anim. Sci. 2022, 100, 36. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Wilcock, P.; Bedford, M.R. Super-dosing effects of phytase in poultry and other monogastrics. Worlds. Poult. Sci. J. 2011, 67, 225–236. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Kwakernaak, C. Comparative effects of two phytases versus increasing the inorganic phosphorus content of the diet, on nutrient and amino acid digestibility in boilers. Anim. Feed Sci. Technol. 2019, 253, 166–180. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Damodaran, S. Effect of Phytate on Solubility, Activity and Conformation of Trypsin and Chymotrypsin. J. Food Sci. 1989, 54, 695–699. [Google Scholar] [CrossRef]
- Cosgrove, D.; Irving, C. Inositol Phosphates: Their Chemistry, Biochemistry and Physiology; Elsevier Scientific: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Sandström, B.; Sandberg, A.S. Inhibitory effects of isolated inositol phosphates on zinc absorption in humans. J. Trace Elem. Electrolytes Health Dis. 1992, 6, 99–103. [Google Scholar]
- Augspurger, N.R.; Webel, D.M.; Lei, X.G.; Baker, D.H. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J. Anim. Sci. 2003, 81, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.; Olukosi, O.A.; Jendza, J.A.; Dilger, R.N.; Bedford, M.R. Response of growing pigs to Peniophora lycii- and Escherichia coli -derived phytases or varying ratios of calcium to total phosphorus. Anim. Sci. 2006, 82, 637–644. [Google Scholar] [CrossRef]
- Sands, J.S.; Ragland, D.; Dilger, R.N.; Adeola, O. Responses of pigs to Aspergillus niger phytase supplementation of low-protein or high-phytin diets. J. Anim. Sci. 2009, 87, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Goodband, R.D.; Langbein, K.B.; Tokach, M.D.; DeRouchey, J.M.; Dritz, S.S. Influence of a superdose of phytase (Optiphos) on finishing pig performance and carcass characteristics. Kansas Agric. Exp. Stn. Res. Rep. 2013, 116–120. [Google Scholar] [CrossRef]
- Guggenbuhl, P.; Calvo, E.P.; Fru, F. Effect of high dietary doses of a bacterial 6-phytase in piglets fed a corn-soybean meal diet. J. Anim. Sci. 2016, 94, 307–309. [Google Scholar] [CrossRef]
- Cambra-López, M.; Cerisuelo, A.; Ferrer, P.; Ródenas, L.; Aligué, R.; Moset, V.; Pascual, J.J. Age influence on effectiveness of a novel 3-phytase in barley-wheat based diets for pigs from 12 to 108 kg under commercial conditions. Anim. Feed Sci. Technol. 2020, 267, 114549. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Kornegay, E.T.; Radcliffe, J.S.; Denbow, D.M.; Veit, H.P.; Larsen, C.T. Comparison of genetically engineered microbial and plant phytase for young broilers. Poult. Sci. 2000, 79, 709–717. [Google Scholar] [CrossRef]
- Ravindran, V.; Selle, P.H.; Ravindran, G.; Morel, P.C.H.; Kies, A.K.; Bryden, W.L. Microbial phytase improves performance, apparent metabolizable energy, and ileal amino acid digestibility of broilers fed a lysine-deficient diet. Poult. Sci. 2001, 80, 338–344. [Google Scholar] [CrossRef]
- Yan, F.; Kersey, J.H.; Waldroup, P.W. Phosphorus requirements of broiler chicks three to six weeks of age as influenced by phytase supplementation. Poult. Sci. 2001, 80, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.Q.; Abdullah, N.; Jalaludin, S.; Ho, Y.W. Efficacy of supplementation of a phytase-producing bacterial culture on the performance and nutrient use of broiler chickens fed corn-soybean meal diets. Poult. Sci. 2002, 81, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Brenes, A.; Arija, I.; Centeno, C. Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus. Poult. Sci. 2002, 81, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Viveros, A.; Arija, I.; Centeno, C.; Pizarro, M.; Bravo, C. The effect of citric acid and microbial phytase on mineral utilization in broiler chicks. Anim. Feed Sci. Technol. 2003, 110, 201–219. [Google Scholar] [CrossRef]
- Shirley, R.B.; Edwards, H.M. Graded levels of phytase past industry standards improves broiler performance. Poult. Sci. 2003, 82, 671–680. [Google Scholar] [CrossRef]
- Jendza, J.A.; Dilger, R.N.; Sands, J.S.; Adeola, O. Efficacy and equivalency of an Escherichia coli-derived phytase for replacing inorganic phosphorus in the diets of broiler chickens and young pigs. J. Anim. Sci. 2006, 84, 3364–3374. [Google Scholar] [CrossRef]
- Payne, R.L.; Lavergne, T.K.; Southern, L.L. A comparison of two sources of phytase in liquid and dry forms in broilers. Poult. Sci. 2005, 84, 265–272. [Google Scholar] [CrossRef]
- Watson, B.C.; Matthews, J.O.; Southern, L.L.; Shelton, J.L. The effects of phytase on growth performance and intestinal transit time of broilers fed nutritionally adequate diets and diets deficient in calcium and phosphorus. Poult. Sci. 2006, 85, 493–497. [Google Scholar] [CrossRef]
- Powell, S.; Johnston, S.; Gaston, L.; Southern, L.L. The effect of dietary phosphorus level and phytase supplementation on growth performance, bone-breaking strength, and litter phosphorus concentration in broilers. Poult. Sci. 2008, 87, 949–957. [Google Scholar] [CrossRef]
- Rousseau, X.; Létourneau-Montminy, M.P.; Même, N.; Magnin, M.; Nys, Y.; Narcy, A. Phosphorus utilization in finishing broiler chickens: Effects of dietary calcium and microbial phytase. Poult. Sci. 2012, 91, 2829–2837. [Google Scholar] [CrossRef]
- Taheri, H.R.; Heidari, A.; Shahir, M.H. Effect of high-dose phytase supplementation in broilers from 22 to 42 days post-hatch given diets severely limited in available phosphorus. Br. Poult. Sci. 2015, 56, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Taheri, H.R.; Jabbari, Z.; Adibnia, S.; Shahir, M.H.; Hosseini, S.A. Effect of high-dose phytase and citric acid, alone or in combination, on growth performance of broilers given diets severely limited in available phosphorus. Br. Poult. Sci. 2015, 56, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Srikanthithasan, K.; Macelline, S.P.; Wickramasuriya, S.S.; Tharangani, H.; Li-Ang; Jayasena, D.D.; Heo, J.M. Effects of adding phytase from aspergillus Niger to a low phosphorus diet on growth performance, Tibia characteristics, phosphorus excretion, and meat quality of broilers 35 days after hatching. J. Poult. Sci. 2020, 57, 28–36. [Google Scholar] [CrossRef]
- Ravindran, V.; Gabahug, S.; Ravindran, G.; Selle, P.H.; Bryden, W.L. Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorous levels. II. Effects on apparent metabolisable energy, nutrient digestibility and nutrient retention. Br. Poult. Sci. 2000, 41, 193–200. [Google Scholar] [CrossRef]
- Dilger, R.N.; Onyango, E.M.; Sands, J.S.; Adeola, O. Evaluation of microbial phytase in broiler diets. Poult. Sci. 2004, 83, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Ravindran, V.; Thomas, D.G.; Birtles, M.J.; Hendriks, W.H. Influence of phytase and xylanase, individually or in combination, on performance, apparent metabolisable energy, digestive tract measurements and gut morphology in broilers fed wheat-based diets containing adequate level of phosphorus. Br. Poult. Sci. 2004, 45, 76–84. [Google Scholar] [CrossRef]
- Onyango, E.M.; Bedford, M.R.; Adeola, O. Efficacy of an evolved Escherichia coli phytase in diets of broiler chicks. Poult. Sci. 2005, 84, 248–255. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Acamovic, T.; Bedford, M.R. Supplementation of corn-soy-based diets with an Eschericia coli-derived phytase: Effects on broiler chick performance and the digestibility of amino acids and metabolizability of minerals and energy. Poult. Sci. 2006, 85, 1389–1397. [Google Scholar] [CrossRef]
- Pillai, P.B.; O’Connor-Dennie, T.; Owens, C.M.; Emmert, J.L. Efficacy of an Escherichia coli phytase in broilers fed adequate or reduced phosphorus diets and its effect on carcass characteristics. Poult. Sci. 2006, 85, 1737–1745. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Oduguwa, O.; Acamovic, T.; Bedford, M.R. Diets containing Escherichia coli-derived phytase on young chickens and turkeys: Effects on performance, metabolizable energy, endogenous secretions, and intestinal morphology. Poult. Sci. 2007, 86, 705–713. [Google Scholar] [CrossRef]
- Walk, C.L.; Bedford, M.R.; Mcelroy, A.P. Influence of limestone and phytase on broiler performance, gastrointestinal pH, and apparent ileal nutrient digestibility. Poult. Sci. 2012, 91, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Pirgozliev, V.; Oduguwa, O.; Acamovic, T.; Bedford, M.R. Effects of dietary phytase on performance and nutrient metabolism in chickens. Br. Poult. Sci. 2008, 49, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ru, Y.J.; Cowieson, A.J.; Li, F.D.; Cheng, X.C. Effects of Phytate and Phytase on the Performance and Immune Function of Broilers Fed Nutritionally Marginal Diets. Poult. Sci. 2008, 87, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ru, Y.J.; Li, F.D.; Wang, J.P.; Lei, X.Q. Effect of dietary phytate and phytase on proteolytic digestion and growth regulation of broilers. Arch. Anim. Nutr. 2009, 63, 292–303. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Slominski, B.A.; Jones, R.O. Growth performance and nutrient utilization of broiler chickens fed diets supplemented with phytase alone or in combination with citric acid and multicarbohydrase. Poult. Sci. 2010, 89, 2221–2229. [Google Scholar] [CrossRef]
- Tiwari, S.P.; Gendley, M.K.; Pathak, A.K.; Gupta, R. Influence of an enzyme cocktail and phytase individually or in combination in Ven Cobb broiler chickens. Br. Poult. Sci. 2010, 51, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Olukosi, O.A.; Cowieson, A.J.; Adeola, O. Broiler responses to supplementation of phytase and admixture of carbohydrases and protease in maize-soyabean meal diets with or without maize Distillers’ Dried Grain with Solubles. Br. Poult. Sci. 2010, 51, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Pirgozliev, V.; Karadas, F.; Pappas, A.; Acamovic, T.; Bedford, M.R. The effect on performance, energy metabolism and hepatic carotenoid content when phytase supplemented diets were fed to broiler chickens. Res. Vet. Sci. 2010, 89, 203–205. [Google Scholar] [CrossRef]
- Kong, C.; Adeola, O. Protein utilization and amino acid digestibility of canola meal in response to phytase in broiler chickens. Poult. Sci. 2011, 90, 1508–1515. [Google Scholar] [CrossRef]
- Powell, S.S.; Bidner, T.D.; Southern, L.L. Phytase supplementation improved growth performance and bone characteristics in broilers fed varying levels of dietary calcium. Poult. Sci. 2011, 90, 604–608. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Bedford, M.R.; Acamovic, T.; Mares, P.; Allymehr, M. The effects of supplementary bacterial phytase on dietary energy and total tract amino acid digestibility when fed to young chickens. Br. Poult. Sci. 2011, 52, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Chung, T.K.; Thomas, D.V.; Zou, M.L.; Moughan, P.J. Effect of a novel phytase on growth performance, apparent metabolizable energy, and the availability of minerals and amino acids in a low-phosphorus corn-soybean meal diet for broilers. Poult. Sci. 2012, 91, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Walk, C.L.; Addo-Chidie, E.K.; Bedford, M.R.; Adeola, O. Evaluation of a highly soluble calcium source and phytase in the diets of broiler chickens. Poult. Sci. 2012, 91, 2255–2263. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Bedford, M.R. Energy utilisation and growth performance of chicken fed diets containing graded levels of supplementary bacterial phytase. Br. J. Nutr. 2013, 109, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.; Walk, C.L. Linking ileal digestible phosphorus and bone mineralization in broiler chickens fed diets supplemented with phytase and highly soluble calcium. Poult. Sci. 2013, 92, 2109–2117. [Google Scholar] [CrossRef]
- Paiva, D.M.; Walk, C.L.; McElroy, A.P. Influence of dietary calcium level, calcium source, and phytase on bird performance and mineral digestibility during a natural necrotic enteritis episode. Poult. Sci. 2013, 92, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Olukosi, O.A.; Kong, C.; Fru-Nji, F.; Ajuwon, K.M.; Adeola, O. Assessment of a bacterial 6-phytase in the diets of broiler chickens. Poult. Sci. 2013, 92, 2101–2108. [Google Scholar] [CrossRef]
- Singh, A.; Walk, C.L.; Ghosh, T.K.; Bedford, M.R.; Haldar, S. Effect of a novel microbial phytase on production performance and tibia mineral concentration in broiler chickens given low-calcium diets. Br. Poult. Sci. 2013, 54, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.J.; Ptak, A.; Maćkowiak, P.; Sassek, M.; Pruszyńska-Oszmałek, E.; Zyła, K.; Światkiewicz, S.; Kaczmarek, S.; Józefiak, D. The effect of microbial phytase and myo-inositol on performance and blood biochemistry of broiler chickens fed wheat/corn-based diets. Poult. Sci. 2013, 92, 2124–2134. [Google Scholar] [CrossRef] [PubMed]
- Walk, C.L.; Santos, T.T.; Bedford, M.R. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 2014, 93, 1172–1177. [Google Scholar] [CrossRef]
- Kiarie, E.; Woyengo, T.; Nyachoti, C.M. Efficacy of new 6-phytase from buttiauxella spp. on growth performance and nutrient retention in broiler chickens fed corn soybean meal-based diets. Asian-Australas. J. Anim. Sci. 2015, 28, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Pekel, A.Y.; Horn, N.L.; Adeola, O. The efficacy of dietary xylanase and phytase in broiler chickens fed expeller-extracted camelina meal. Poult. Sci. 2017, 96, 98–107. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.; Walk, C.L.; Wyatt, C.L.; Adeola, O. Phosphorus utilization response of pigs and broiler chickens to diets supplemented with antimicrobials and phytase. Anim. Nutr. 2017, 3, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Cowieson, A.J.; Selle, P.H. The influence of meat-and-bone meal and exogenous phytase on growth performance, bone mineralisation and digestibility coefficients of protein (N), amino acids and starch in broiler chickens. Anim. Nutr. 2016, 2, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Manobhavan, M.; Elangovan, A.V.; Sridhar, M.; Shet, D.; Ajith, S.; Pal, D.T.; Gowda, N.K.S. Effect of super dosing of phytase on growth performance, ileal digestibility and bone characteristics in broilers fed corn-soya-based diets. J. Anim. Physiol. Anim. Nutr. 2016, 100, 93–100. [Google Scholar] [CrossRef]
- Pieniazek, J.; Smith, K.A.; Williams, M.P.; Manangi, M.K.; Vazquez-Anon, M.; Solbak, A.; Miller, M.; Lee, J.T. Evaluation of increasing levels of a microbial phytase in phosphorus deficient broiler diets via live broiler performance, tibia bone ash, apparent metabolizable energy, and amino acid digestibility. Poult. Sci. 2017, 96, 370–382. [Google Scholar] [CrossRef]
- Beeson, L.A.; Walk, C.L.; Bedford, M.R.; Olukosi, O.A. Hydrolysis of phytate to its lower esters can influence the growth performance and nutrient utilization of broilers with regular or super doses of phytase. Poult. Sci. 2017, 96, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, D.; Karimi, A.; Sadeghi, G.; Rostamzadeh, J.; Bedford, M.R. Effects of a high dose of microbial phytase and myo-inositol supplementation on growth performance, tibia mineralization, nutrient digestibility, litter moisture content, and foot problems in broiler chickens fed phosphorus-deficient diets. Poult. Sci. 2017, 96, 3664–3675. [Google Scholar] [CrossRef] [PubMed]
- Momeneh, T.; Karimi, A.; Sadeghi, G.; Vaziry, A.; Bedford, M.R. Evaluation of dietary calcium level and source and phytase on growth performance, serum metabolites, and ileum mineral contents in broiler chicks fed adequate phosphorus diets from one to 28 days of age. Poult. Sci. 2018, 97, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Walk, C.L.; Rao, S.V.R. High doses of phytase on growth performance and apparent ileal amino acid digestibility of broilers fed diets with graded concentrations of digestible sulfur amino acids. Poult. Sci. 2018, 97, 3610–3621. [Google Scholar] [CrossRef]
- Gautier, A.E.; Walk, C.L.; Dilger, R.N. Effects of a high level of phytase on broiler performance, bone ash, phosphorus utilization, and phytate dephosphorylation to inositol. Poult. Sci. 2018, 97, 211–218. [Google Scholar] [CrossRef]
- Scholey, D.V.; Morgan, N.K.; Riemensperger, A.; Hardy, R.; Burton, E.J. Effect of supplementation of phytase to diets low in inorganic phosphorus on growth performance and mineralization of broilers. Poult. Sci. 2018, 97, 2435–2440. [Google Scholar] [CrossRef]
- Walk, C.L.; Bedford, M.R.; Olukosi, O.A. Effect of phytase on growth performance, phytate degradation and gene expression of myo-inositol transporters in the small intestine, liver and kidney of 21 day old broilers. Poult. Sci. 2018, 97, 1155–1162. [Google Scholar] [CrossRef]
- Walk, C.L.; Rao, S.V.R. High doses of phytase on growth performance and apparent ileal amino acid digestibility of broilers fed diets with graded concentrations of digestible lysine. J. Anim. Sci. 2019, 97, 698–713. [Google Scholar] [CrossRef]
- Ajuwon, K.M.; Sommerfeld, V.; Paul, V.; Däuber, M.; Schollenberger, M.; Kühn, I.; Adeola, O.; Rodehutscord, M. Phytase dosing affects phytate degradation and Muc2 transporter gene expression in broiler starters. Poult. Sci. 2020, 99, 981–991. [Google Scholar] [CrossRef]
- Akter, M.; Graham, H.; Iji, P.A. Response of broiler chickens to diets containing different levels of sodium with or without microbial phytase supplementation. J. Anim. Sci. Technol. 2019, 61, 87–97. [Google Scholar] [CrossRef]
- Kriseldi, R.; Walk, C.L.; Bedford, M.R.; Dozier, W.A. Inositol and gradient phytase supplementation in broiler diets during a 6-week production period: 1. effects on growth performance and meat yield. Poult. Sci. 2021, 100, 964–972. [Google Scholar] [CrossRef]
- Sens, R.F.; Bassi, L.S.; Almeida, L.M.; Rosso, D.F.; Teixeira, L.V.; Maiorka, A. Effect of different doses of phytase and protein content of soybean meal on growth performance, nutrient digestibility, and bone characteristics of broilers. Poult. Sci. 2021, 100, 100917. [Google Scholar] [CrossRef]
- Marchal, L.; Bello, A.; Sobotik, E.B.; Archer, G.; Dersjant-Li, Y. A novel consensus bacterial 6-phytase variant completely replaced inorganic phosphate in broiler diets, maintaining growth performance and bone quality: Data from two independent trials. Poult. Sci. 2021, 100, 100962. [Google Scholar] [CrossRef]
- Song, T.; Yu, C.; Zhao, X.; Chen, F.; Liu, Y.; Yang, C.; Yang, Z. Effects of thermostable phytase supplemented in diets on growth performance and nutrient utilization of broilers. Anim. Sci. J. 2021, 92, e13513. [Google Scholar] [CrossRef]
- Babatunde, O.O.; Bello, A.; Dersjant-Li, Y.; Adeola, O. Evaluation of the responses of broiler chickens to varying concentrations of phytate phosphorus and phytase. I. Starter phase (day 1–11 post hatching). Poult. Sci. 2021, 100, 101396. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, O.O.; Bello, A.; Dersjant-Li, Y.; Adeola, O. Evaluation of the responses of broiler chickens to varying concentrations of phytate phosphorus and phytase. II. Grower phase (day 12–23 post hatching). Poult. Sci. 2022, 101, 101616. [Google Scholar] [CrossRef]
- Stahl, C.H.; Roneker, K.R.; Thornton, J.R.; Lei, X.G. A new phytase expressed in yeast effectively improves the bioavailability of phytate phosphorus to weanling pigs. J. Anim. Sci. 2000, 78, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Kornegay, E.T.; Radcliffe, J.S.; Wilson, J.H.; Veit, H.P. Comparison of phytase from genetically engineered Aspergillus and canola in weanling pig diets. J. Anim. Sci. 2000, 78, 2868–2878. [Google Scholar] [CrossRef] [PubMed]
- Gentile, J.M.; Roneker, K.R.; Crowe, S.E.; Pond, W.G.; Lei, X.G. Effectiveness of an experimental consensus phytase in improving dietary phytate-phosphorus utilization by weanling pigs. J. Anim. Sci. 2003, 81, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Omogbenigun, F.O.; Nyachoti, C.M.; Slominski, B.A. The effect of supplementing microbial phytase and organic acids to a corn-soybean based diet fed to early-weaned pigs. J. Anim. Sci. 2003, 81, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.M.; Hill, G.M.; Link, J.E.; Raney, N.E.; Tempelman, R.J.; Ernst, C.W. Pharmacological zinc and phytase supplementation enhance metallothionein mRNA abundance and protein concentration in newly weaned pigs. J. Nutr. 2004, 134, 538–544. [Google Scholar] [CrossRef]
- Jondreville, C.; Hayler, R.; Feuerstein, D. Replacement of zinc sulphate by microbial phytase for piglets given a maize-soya-bean meal diet. Anim. Sci. 2005, 81, 77–83. [Google Scholar] [CrossRef]
- Revy, P.S.; Jondreville, C.; Dourmad, J.Y.; Nys, Y. Assessment of dietary zinc requirement of weaned piglets fed diets with or without microbial phytase. J. Anim. Physiol. Anim. Nutr. 2006, 90, 50–59. [Google Scholar] [CrossRef]
- James, B.W.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Dritz, S.S.; Lynch, G.L. Effect of phytase dosage and source on growth performance and bone development of nursery pigs. Prof. Anim. Sci. 2008, 24, 88–94. [Google Scholar] [CrossRef]
- Pengu, R.; Delia, E.; Nepravishta, A. Microbial phytase as a way to improve growth performance of weaned piglets and to reduce phosphorus excretion. Albanian J. Agric. Sci. 2016, 15, 35–37. [Google Scholar]
- Woyengo, T.A.; Guenter, W.; Sands, J.S.; Nyachoti, C.M.; Mirza, M.A. Nutrient utilisation and performance responses of broilers fed a wheat-based diet supplemented with phytase and xylanase alone or in combination. Anim. Feed Sci. Technol. 2008, 146, 113–123. [Google Scholar] [CrossRef]
- Sands, J.S.; Ragland, D.; Baxter, C.; Joern, B.C.; Sauber, T.E.; Adeola, O. Phosphorus bioavailability, growth performance, and nutrient balance in pigs fed high available phosphorus corn and phytase. J. Anim. Sci. 2001, 79, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.B.; Southern, L.L.; Bidner, T.D. Effects of supplemental dietary phytase and pharmacological concentrations of zinc on growth performance and tissue zinc concentrations of weanling pigs. J. Anim. Sci. 2005, 83, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.M.; Link, J.E.; Hill, G.M. Dietary pharmacological or excess zinc and phytase effects on tissue mineral concentrations, metallothionein, and apparent mineral retention in the newly weaned pig. Biol. Trace Elem. Res. 2005, 105, 97–115. [Google Scholar] [CrossRef]
- Kies, A.K.; Kemme, P.A.; Šebek, L.B.J.; van Diepen, J.T.M.; Jongbloed, A.W. Effect of graded doses and a high dose of microbial phytase on the digestibility of various minerals in weaner pigs. J. Anim. Sci. 2006, 84, 1169–1175. [Google Scholar] [CrossRef]
- Veum, T.L.; Bollinger, D.W.; Buff, C.E.; Bedford, M.R. A genetically engineered Escherichia coli phytase improves nutrient utilization, growth performance, and bone strength of young swine fed diets deficient in available phosphorus. J. Anim. Sci. 2006, 84, 1147–1158. [Google Scholar] [CrossRef]
- Pagano, A.R.; Yasuda, K.; Roneker, K.R.; Crenshaw, T.D.; Lei, X.G. Supplemental Escherichia coli phytase and strontium enhance bone strength of young pigs fed a phosphorus-adequate diet. J. Nutr. 2007, 137, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.L.; Landero, J.L.; Owusu-Asiedu, A.; Cervantes, M.; Zijlstra, R.T. Growth performance, diet nutrient digestibility, and bone mineralization in weaned pigs fed pelleted diets containing thermostable phytase. J. Anim. Sci. 2013, 91, 745–754. [Google Scholar] [CrossRef]
- Walk, C.L.; Srinongkote, S.; Wilcock, P. Influence of a microbial phytase and zinc oxide on young pig growth performance and serum minerals. J. Anim. Sci. 2013, 91, 286–291. [Google Scholar] [CrossRef][Green Version]
- Zeng, Z.K.; Wang, D.; Piao, X.S.; Li, P.F.; Zhang, H.Y.; Shi, C.X.; Yu, S.K. Effects of Adding Super Dose Phytase to the Phosphorus-deficient Diets of Young Pigs on Growth Performance, Bone Quality, Minerals and Amino Acids Digestibilities. Asian-Australas. J. Anim. Sci. 2014, 27, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Li, Q.; Tian, Q.; Zhao, P.; Xu, X.; Yu, S.; Piao, X. Super high dosing with a novel Buttiauxella phytase continuously improves growth performance, nutrient digestibility, and mineral status of weaned pigs. Biol. Trace Elem. Res. 2015, 168, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Walk, C.L.; Wilcock, P.; Magowan, E. Evaluation of the effects of pharmacological zinc oxide and phosphorus source on weaned piglet growth performance, plasma minerals and mineral digestibility. Animal 2015, 9, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Gourley, K.M.; Woodworth, J.C.; Derouchey, J.M.; Dritz, S.S.; Tokach, M.D.; Goodband, R.D. Determining the available phosphorus release of natuphos E 5,000 g phytase for nursery pigs. J. Anim. Sci. 2018, 96, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Gourley, K.M.; Woodworth, J.C.; Derouchey, J.M.; Dritz, S.S.; Tokach, M.D.; Goodband, R.D. Effect of high doses of natuphos E 5,000 G phytase on growth performance of nursery pigs. J. Anim. Sci. 2018, 96, 570–578. [Google Scholar] [CrossRef]
- Lee, S.A.; Dunne, J.; Febery, E.; Wilcock, P.; Mottram, T.; Bedford, M.R. Superdosing phytase reduces real-time gastric pH in broilers and weaned piglets. Br. Poult. Sci. 2018, 59, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Broomhead, J.N.; Lessard, P.A.; Raab, R.M.; Lanahan, M.B. Effects of feeding corn-expressed phytase on the live performance, bone characteristics, and phosphorus digestibility of nursery pigs. J. Anim. Sci. 2019, 97, 1254–1261. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Dusel, G. Increasing the dosing of a Buttiauxella phytase improves phytate degradation, mineral, energy, and amino acid digestibility in weaned pigs fed a complex diet based on wheat, corn, soybean meal, barley, and rapeseed meal. J. Anim. Sci. 2019, 97, 2524–2533. [Google Scholar] [CrossRef]
- Lu, H.; Kühn, I.; Bedford, M.R.; Whitfield, H.; Brearley, C.; Adeola, O.; Ajuwon, K.M. Effect of phytase on intestinal phytate breakdown, plasma inositol concentrations, and glucose transporter type 4 abundance in muscle membranes of weanling pigs. J. Anim. Sci. 2019, 97, 3907–3919. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Woodworth, J.C.; Tokach, M.D.; Dritz, S.S.; Derouchey, J.M.; Goodband, R.D.; Bergstrom, J.R. Standardized total tract digestible phosphorus requirement of 6 to 13 kg pigs fed diets without or with phytase. Animal 2019, 13, 2473–2482. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Villca, B.; Sewalt, V.; de Kreij, A.; Marchal, L.; Velayudhan, D.E.; Sorg, R.A.; Christensen, T.; Mejldal, R.; Nikolaev, I.; et al. Functionality of a next generation biosynthetic bacterial 6-phytase in enhancing phosphorus availability to weaned piglets fed a corn-soybean meal-based diet without added inorganic phosphate. Anim. Nutr. 2020, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Shin, S.; Kuehn, I.; Bedford, M.; Rodehutscord, M.; Adeola, O.; Ajuwon, K.M. Effect of phytase on nutrient digestibility and expression of intestinal tight junction and nutrient transporter genes in pigs. J. Anim. Sci. 2020, 98, skaa206. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Blavi, L.; González-Vega, C.; Liu, Y.; Hancock, D.; Vazquez-Añón, M.; Almeida, F.N.; Stein, H.H. Effects of a novel E. coli phytase expressed in Pseudomonas fluorescens on growth, bone mineralization, and nutrient digestibility in pigs fed corn-soybean meal diets. Transl. Anim. Sci. 2020, 4, txaa201. [Google Scholar] [CrossRef] [PubMed]
- Shili, C.N.; Broomhead, J.N.; Spring, S.C.; Lanahan, M.B.; Pezeshki, A. A Novel Corn-Expressed Phytase Improves Daily Weight Gain, Protein Efficiency Ratio and Nutrients Digestibility and Alters Fecal Microbiota in Pigs Fed with Very Low Protein Diets. Animals 2020, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Vanessa Lagos, L.; Lee, S.A.; Bedford, M.R.; Stein, H.H. Reduced concentrations of limestone and monocalcium phosphate in diets without or with microbial phytase did not influence gastric pH, fecal score, or growth performance, but reduced bone ash and serum albumin in weanling pigs. Transl. Anim. Sci. 2021, 5, txab115. [Google Scholar] [CrossRef] [PubMed]
- Tous, N.; Tarradas, J.; Francesch, M.; Font-I-furnols, M.; Ader, P.; Torrallardona, D. Effects of exogenous 6-phytase (Ec 3.1.3.26) supplementation on performance, calcium and phosphorous digestibility, and bone mineralisation and density in weaned piglets. Animals 2021, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.L.; Boyd, R.D.; Koehler, D.; Gould, S.A.; Li, Q.; Patience, J.F. The impact of “super-dosing” phytase in pig diets on growth performance during the nursery and grow-out periods. Transl. Anim. Sci. 2019, 3, 419–428. [Google Scholar] [CrossRef]
- Hawthorne, J.N.; White, D.A. Myo-inositol Lipids. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 1976; Volume 33, pp. 529–573. [Google Scholar]
- Holub, B. Metabolism and Function of myo-Inositol and Inositol Phospholipids. Annu. Rev. Nutr. 1986, 6, 563–597. [Google Scholar] [CrossRef]
- Liu, N.; Ru, Y.; Wang, J.; Xu, T. Effect of dietary sodium phytate and microbial phytase on the lipase activity and lipid metabolism of broiler chickens. Br. J. Nutr. 2010, 103, 862–868. [Google Scholar] [CrossRef]
- Lee, S.A.; Bedford, M.R. Inositol—An effective growth promotor? Worlds. Poult. Sci. J. 2016, 72, 743–760. [Google Scholar] [CrossRef]
- Walk, C.L.; Kühn, I.; Stein, H.H.; Kidd, M.T.; Rodehutscord, M. Phytate Destruction—Consequences for Precision Animal Nutrition; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; ISBN 9789086868360. [Google Scholar]
- Walk, C.L.; Bedford, M.R.; Santos, T.S.; Paiva, D.; Bradley, J.R.; Wladecki, H.; Honaker, C.; McElroy, A.P. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poult. Sci. 2013, 92, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Duarte, M.E.; Kim, S.W. 297 Super dosing effects of corn-expressed phytase on growth performance, bone characteristics, and nutrient digestibility in nursery pigs fed diets deficient in phosphorus and calcium. J. Anim. Sci. 2017, 95, 144. [Google Scholar] [CrossRef]
- Moran, K.; Wilcock, P.; Elsbernd, A.; Zier-Rush, C.; Boyd, R.D.; van Heugten, E. Effects of super-dosing phytase and inositol on growth performance and blood metabolites of weaned pigs housed under commercial conditions. J. Anim. Sci. 2019, 97, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Borda-Molina, D.; Vital, M.; Sommerfeld, V.; Rodehutscord, M.; Camarinha-Silva, A. Insights into Broilers’ Gut Microbiota Fed with Phosphorus, Calcium, and Phytase Supplemented Diets. Front. Microbiol. 2016, 7, 2033. [Google Scholar] [CrossRef]
- Lee, J.K.; Chen, H.; Park, I.; Kim, S.W. 0931 Effects of corn-expressed phytase on growth performance and gut health of nursery pigs. J. Anim. Sci. 2016, 94, 448. [Google Scholar] [CrossRef]
- Mancabelli, L.; Ferrario, C.; Milani, C.; Mangifesta, M.; Turroni, F.; Duranti, S.; Lugli, G.A.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016, 18, 4727–4738. [Google Scholar] [CrossRef]
- Nari, N.; Ghasemi, H.A.; Hajkhodadadi, I.; Farahani, A.H.H.K. Intestinal microbial ecology, immune response, stress indicators, and gut morphology of male broiler chickens fed low-phosphorus diets supplemented with phytase, butyric acid, or Saccharomyces boulardii. Livest. Sci. 2020, 234, 103975. [Google Scholar] [CrossRef]
- Karadas, F.; Pirgozliev, V.; Acamovic, T.; Bedford, M.R. The effects of dietary phytase activity on the concentration of Coenzyme Q 10 in the liver of young turkeys and broilers. Br. Poult. Abstr. 2005, 1, 1–74. [Google Scholar] [CrossRef]
- Geng, A.L.; Guo, Y.M.; Yang, Y. Reduction of ascites mortality in broilers by coenzyme Q10. Poult. Sci. 2004, 83, 1587–1593. [Google Scholar] [CrossRef]
- Surai, K.P.; Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Antioxidant-prooxidant balance in the intestine: Food for thought 2. Antioxidants. Curr. Top. Nutraceutical Res. 2004, 2, 27–46. [Google Scholar]
- Dang, N.T.; Mukai, R.; Yoshida, K.I.; Ashida, H. D-pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci. Biotechnol. Biochem. 2010, 74, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M. Use of pure xylanases in pig and poultry nutrition. Lohmann Inf. 2001, 25, 1–5. [Google Scholar]
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Petry, A.L.; Huntley, N.F.; Bedford, M.R.; Patience, J.F. Xylanase increased the energetic contribution of fiber and improved the oxidative status, gut barrier integrity, and growth performance of growing pigs fed insoluble corn-based fiber. J. Anim. Sci. 2020, 98, skaa233. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Mishra, B.; Bedford, M.R.; Jha, R. Effects of supplemental xylanase and xylooligosaccharides on production performance and gut health variables of broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 98. [Google Scholar] [CrossRef]
- Craig, A.D.; Khattak, F.; Hastie, P.; Bedford, M.R.; Olukosi, O.A. Xylanase and xylo- oligosaccharide prebiotic improve the growth performance and concentration of potentially prebiotic oligosaccharides in the ileum of broiler chickens. Br. Poult. Sci. 2020, 61, 70–78. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. hua. Microbial exo-xylanases: A mini review. Appl. Biochem. Biotechnol. 2014, 174, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Engberg, R.M.; Hedemann, M.S.; Steenfeldt, S.; Jensen, B.B. Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult. Sci. 2004, 83, 925–938. [Google Scholar] [CrossRef]
- Choct, M.; Kocher, A.; Waters, D.L.E.; Pettersson, D.; Ross, G. A comparison of three xylanases on the nutritive value of two wheats for broiler chickens. Br. J. Nutr. 2004, 92, 53–61. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Hruby, M.; Faurschou Isaksen, M. The effect of conditioning temperature and exogenous xylanase addition on the viscosity of wheat-based diets and the performance of broiler chickens. Br. Poult. Sci. 2005, 46, 717–724. [Google Scholar] [CrossRef]
- Murphy, T.C.; Bedford, M.R.; McCracken, K.J. 2004 Spring Meeting of the WPSA UK Branch Posters. Br. Poult. Sci. 2004, 45, S61–S62. [Google Scholar] [CrossRef]
- Parsaie, S.; Shariatmadari, F.; Zamiri, M.J.; Khajeh, K. Influence of wheat-based diets supplemented with xylanase, bile acid and antibiotics on performance, digestive tract measurements and gut morphology of broilers compared with a maize-based diet. Br. Poult. Sci. 2007, 48, 594–600. [Google Scholar] [CrossRef]
- Amerah, A.M.; Ravindran, V.; Lentle, R.G.; Thomas, D.G. Influence of particle size and xylanase supplementation on the performance, energy utilisation, digestive tract parameters and digesta viscosity of broiler starters. Br. Poult. Sci. 2008, 49, 455–462. [Google Scholar] [CrossRef]
- Murphy, T.C.; McCracken, J.K.; McCann, M.E.E.; George, J.; Bedford, M.R. Broiler performance and in vivo viscosity as influenced by a range of xylanases, varying in ability to effect wheat in vitro viscosity. Br. Poult. Sci. 2009, 50, 716–724. [Google Scholar] [CrossRef]
- Denstadli, V.; Westereng, B.; Biniyam, H.G.; Ballance, S.; Knutsen, S.H.; Svihus, B. Effects of structure and xylanase treatment of brewers’ spent grain on performance and nutrient availability in broiler chickens. Br. Poult. Sci. 2010, 51, 419–426. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Bedford, M.R.; Ravindran, V. Interactions between xylanase and glucanase in maize-soy-based diets for broilers. Br. Poult. Sci. 2010, 51, 246–257. [Google Scholar] [CrossRef]
- Esmaeilipour, O.; Shivazad, M.; Moravej, H.; Aminzadeh, S.; Rezaian, M.; van Krimpen, M.M. Effects of xylanase and citric acid on the performance, nutrient retention, and characteristics of gastrointestinal tract of broilers fed low-phosphorus wheat-based diets. Poult. Sci. 2011, 90, 1975–1982. [Google Scholar] [CrossRef]
- Amerah, A.M.; Mathis, G.; Hofacre, C.L. Effect of xylanase and a blend of essential oils on performance and Salmonella colonization of broiler chickens challenged with Salmonella Heidelberg. Poult. Sci. 2012, 91, 943–947. [Google Scholar] [CrossRef]
- Kalmendal, R.; Tauson, R. Effects of a xylanase and protease, individually or in combination, and an ionophore coccidiostat on performance, nutrient utilization, and intestinal morphology in broiler chickens fed a wheat-soybean meal-based diet. Poult. Sci. 2012, 91, 1387–1393. [Google Scholar] [CrossRef]
- Masey O’Neill, H.V.; Mathis, G.; Lumpkins, B.S.; Bedford, M.R. The effect of reduced calorie diets, with and without fat, and the use of xylanase on performance characteristics of broilers between 0 and 42 days. Poult. Sci. 2012, 91, 1356–1360. [Google Scholar] [CrossRef]
- Singh, A.; O’Neill, H.V.M.; Ghosh, T.K.; Bedford, M.R.; Haldar, S. Effects of xylanase supplementation on performance, total volatile fatty acids and selected bacterial population in caeca, metabolic indices and peptide YY concentrations in serum of broiler chickens fed energy restricted maize-soybean based diets. Anim. Feed Sci. Technol. 2012, 177, 194–203. [Google Scholar] [CrossRef]
- Barekatain, M.R.; Choct, M.; Iji, P.A. Xylanase supplementation improves the nutritive value of diets containing high levels of sorghum distillers’ dried grains with solubles for broiler chickens. J. Sci. Food Agric. 2013, 93, 1552–1559. [Google Scholar] [CrossRef]
- Gehring, C.K.; Bedford, M.R.; Dozier, W.A. Extra-phosphoric effects of phytase with and without xylanase in corn-soybean meal-based diets fed to broilers. Poult. Sci. 2013, 92, 979–991. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Masey O’neill, H.V. Effects of exogenous xylanase on performance, nutrient digestibility and caecal thermal profiles of broilers given wheat-based diets. Br. Poult. Sci. 2013, 54, 346–354. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Ravindran, V. Growth performance, nutrient utilization, and digesta characteristics in broiler chickens fed corn or wheat diets without or with supplemental xylanase. Poult. Sci. 2014, 93, 1186–1196. [Google Scholar] [CrossRef]
- Guo, S.; Liu, D.; Zhao, X.; Li, C.; Guo, Y. Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens. Poult. Sci. 2014, 93, 94–103. [Google Scholar] [CrossRef]
- Masey-O’neill, H.V.; Singh, M.; Cowieson, A.J. Effects of exogenous xylanase on performance, nutrient digestibility, volatile fatty acid production and digestive tract thermal profiles of broilers fed on wheat- or maize-based diet. Br. Poult. Sci. 2014, 55, 351–359. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Rose, S.P.; Pellny, T.; Amerah, A.M.; Wickramasinghe, M.; Ulker, M.; Rakszegi, M.; Bedo, Z.; Shewry, P.R.; Lovegrove, A. Energy utilization and growth performance of chickens fed novel wheat inbred lines selected for different pentosan levels with and without xylanase supplementation. Poult. Sci. 2015, 94, 232–239. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Ortiz, G.; Sola-Oriol, D.; Martinez-Mora, M.; Perez, J.F.; Bedford, M.R. Response of broiler chickens fed wheat-based diets to xylanase supplementation. Poult. Sci. 2017, 96, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Amerah, A.M.; Romero, L.F.; Awati, A.; Ravindran, V. Effect of exogenous xylanase, amylase, and protease as single or combined activities on nutrient digestibility and growth performance of broilers fed corn/soy diets. Poult. Sci. 2017, 96, 807–816. [Google Scholar] [CrossRef]
- Liu, W.C.; Kim, I.H. Metabolism and nutrition: Effects of dietary xylanase supplementation on performance and functional digestive parameters in broilers fed wheat-based diets. Poult. Sci. 2017, 96, 566–573. [Google Scholar] [CrossRef]
- Lee, S.A.; Dunne, J.; Febery, E.; Brearley, C.A.; Mottram, T.; Bedford, M.R. Exogenous phytase and xylanase exhibit opposing effects on real-time gizzard pH in broiler chickens. Br. Poult. Sci. 2018, 59, 568–578. [Google Scholar] [CrossRef]
- McCafferty, K.W.; Bedford, M.R.; Kerr, B.J.; Dozier, W.A. Effects of cereal grain source and supplemental xylanase concentrations on broiler growth performance and cecal volatile fatty acid concentrations from 1 to 40 d of age. Poult. Sci. 2019, 98, 2866–2879. [Google Scholar] [CrossRef]
- González-Ortiz, G.; dos Santos, T.T.; Vienola, K.; Vartiainen, S.; Apajalahti, J.; Bedford, M.R. Response of broiler chickens to xylanase and butyrate supplementation. Poult. Sci. 2019, 98, 3914–3925. [Google Scholar] [CrossRef]
- Arczewska-Wlosek, A.; Światkiewicz, S.; Bederska-Lojewska, D.; Orczewska-Dudek, S.; Szczurek, W.; Boros, D.; Fras, A.; Tomaszewska, E.; Dobrowolski, P.; Muszynski, S.; et al. The efficiency of xylanase in broiler chickens fed with increasing dietary levels of rye. Animals 2019, 9, 46. [Google Scholar] [CrossRef]
- Olukosi, O.A.; González-Ortiz, G.; Whitfield, H.; Bedford, M.R. Comparative aspects of phytase and xylanase effects on performance, mineral digestibility, and ileal phytate degradation in broilers and turkeys. Poult. Sci. 2020, 99, 1528–1539. [Google Scholar] [CrossRef]
- Lee, S.H.; Hosseindoust, A.; Laxman Ingale, S.; Rathi, P.C.; Yoon, S.Y.; Choi, J.W.; Kim, J.S. Thermostable xylanase derived from Trichoderma citrinoviride increases growth performance and non-starch polysaccharide degradation in broiler chickens. Br. Poult. Sci. 2020, 61, 57–62. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Schulze, H.; Schrama, J.W.; Verreth, J.A.; Verstegen, M.W.A. Feed intake, growth, digestibility of dry matter and nitrogen in young pigs as affected by dietary cation-anion difference and supplementation of xylanase 1. J. Anim. Physiol. Anim. Nutr. 2001, 85, 101–109. [Google Scholar] [CrossRef]
- Diebold, G.; Mosenthin, R.; Sauer, W.C.; Dugan, M.E.R.; Lien, K.A. Supplementation of xylanase and phospholipase to wheat-based diets for weaner pigs. J. Anim. Physiol. Anim. Nutr. 2005, 89, 316–325. [Google Scholar] [CrossRef]
- Vahjen, W.; Osswald, T.; Schäfer, K.; Simon, O. Comparison of a xylanase and a complex of non starch polysaccharide- degrading enzymes with regard to performance and bacterial metabolism in weaned piglets. Arch. Anim. Nutr. 2007, 61, 90–102. [Google Scholar] [CrossRef]
- He, J.; Yin, J.; Wang, L.; Yu, B.; Chen, D. Functional characterisation of a recombinant xylanase from Pichia pastoris and effect of the enzyme on nutrient digestibility in weaned pigs. Br. J. Nutr. 2010, 103, 1507–1513. [Google Scholar] [CrossRef]
- Tsai, T.; Dove, C.R.; Cline, P.M.; Owusu-Asiedu, A.; Walsh, M.C.; Azain, M. The effect of adding xylanase or β-glucanase to diets with corn distillers dried grains with solubles (CDDGS) on growth performance and nutrient digestibility in nursery pigs. Livest. Sci. 2017, 197, 46–52. [Google Scholar] [CrossRef]
- Dong, B.; Liu, S.; Wang, C.; Cao, Y. Effects of xylanase supplementation to wheat-based diets on growth performance, nutrient digestibility and gut microbes in weanling pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 1491–1499. [Google Scholar] [CrossRef]
- Mejicanos, G.A.; González-Ortiz, G.; Nyachoti, C.M. Effect of dietary supplementation of xylanase in a wheat-based diet containing canola meal on growth performance, nutrient digestibility, organ weight, and short-chain fatty acid concentration in digesta when fed to weaned pigs. J. Anim. Sci. 2020, 98, skaa064. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Callegari, M.A.; Wilcock, P.; Melo-Duran, D.; Bedford, M.R.; Oliveira, H.R.V.; da Silva, M.A.A.; Pierozan, C.R.; da Silva, C.A. Dietary xylanase and live yeast supplementation influence intestinal bacterial populations and growth performance of piglets fed a sorghum-based diet. Anim. Nutr. 2020, 6, 457–466. [Google Scholar] [CrossRef]
- Lu, H.; Yan, H.; Masey O’Neill, H.M.; Bradley, C.; Bedford, M.R.; Wilcock, P.; Nakatsu, C.H.; Adeola, O.; Ajuwon, K.M. Effect of timing of postweaning xylanase supplementation on growth performance, nutrient digestibility, and fecal microbial composition in weanling pigs. Can. J. Anim. Sci. 2020, 100, 27–36. [Google Scholar] [CrossRef]
- Boontiam, W.; Phaenghairee, P.; van Hoeck, V.; Vasanthakumari, B.L.; Somers, I.; Wealleans, A. Xylanase impact beyond performance: Effects on gut structure, faecal volatile fatty acid content and ammonia emissions in weaned piglets fed diets containing fibrous ingredients. Animals 2022, 12, 3043. [Google Scholar] [CrossRef]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002, 22, 2711–2717. [Google Scholar]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Yu, J.; Chen, H.; He, J.; Luo, Y.; Zheng, P. Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 2020, 12, 3811. [Google Scholar] [CrossRef]
- Arzola-Alvarez, C.; Hume, M.E.; Anderson, R.C.; Latham, E.A.; Ruiz-Barrera, O.; Castillo-Castillo, Y.; Olivas-Palacios, A.L.; Felix-Portillo, M.; Armendariz-Rivas, R.L.; Arzola-Rubio, A.; et al. Influence of sodium chlorate, ferulic acid, and essential oils on Escherichia coli and porcine fecal microbiota. J. Anim. Sci. 2020, 98, skaa059. [Google Scholar] [CrossRef]
- May, K.; O’Sullivan, S.E.; Brameld, J.M.; Masey O’Neill, H.V.; Parr, T.; Wiseman, J. Xylanase supplementation in feed reduces incretin and PYY levels in piglets. Proc. Nutr. Soc. 2015, 74, E294. [Google Scholar] [CrossRef]
- Hespell, R.B. The Genera Succinivibrio and Succinimonas. In The Prokaryotes; Springer New York: New York, NY, USA, 1992; pp. 3979–3982. [Google Scholar]
- Pu, X.; Guo, X.; Shahzad, K.; Wang, M.; Jiang, C.; Liu, J.; Zhang, X.; Zhang, S.; Cheng, L. Effects of dietary non-fibrous carbohydrate (Nfc) to neutral detergent fiber (ndf) ratio change on rumen bacteria in sheep based on three generations of full-length amplifiers sequencing. Animals 2020, 10, 192. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).