Simple Summary
Brazil, with its remarkably diverse marine habitats, harbour one of the world’s richest fish diversities. Consequently, the diversity of their trematode parasites is also expected to be extremely rich. However, our current knowledge on this group of animals is incomplete and there are many unknown trematode species that await discovery and genetic characterisation. The Hemiuridae (Digenea) is the second most speciose trematode family in marine fishes from Brazil; however, to date, it remains understudied. We examined forty-three specimens of nine fish species belonging to eight families (Carangidae, Clupeidae, Haemulidae, Muraenidae, Percophidae, Pinguipedidae, Trichiuridae, and Triglidae) collected from the coastal zone off Rio de Janeiro, Brazil and found hemiurid trematodes in the stomach of 14 fishes. Using morphological and molecular analyses, we identified eight species from four genera of the family Hemiuridae. One of these species is reported in Brazil for the first time, four are reported from new fish hosts, and four were genetically characterised for the first time. Our novel data contributes to the knowledge on marine biodiversity in Brazil and will further contribute to the classification of the family Hemiuridae.
Abstract
Brazil is a tropical country with remarkably diverse marine habitats that harbour a rich diversity of fish. Only a small portion of this fish diversity has been investigated for parasites, and thus the diversity of their trematode parasites remains unexplored. Moreover, only 5 out of 184 known digenean trematode species of marine fish in Brazil have been genetically characterised. The Hemiuridae Looss, 1899 is the second most speciose trematode family in marine fishes from Brazil but, in many ways, it remains a neglected group. Forty-three trematode specimens from nine fish species were collected from the coastal zone off Rio de Janeiro, Brazil. Trematodes were found in the stomach of 14 specimens of 9 fish species belonging to 8 families (Carangidae, Clupeidae, Haemulidae, Muraenidae, Percophidae, Pinguipedidae, Trichiuridae, and Triglidae). Trematode specimens were studied using morphological and molecular genetic analyses. A total of eight hemiurid species from four genera, Ectenurus, Lecithochirium, Myosaccium, and Parahemiurus were identified. This paper reports on new host records for four species of hemiurids, adds a new record on the geographical distribution for one species, and provides the first DNA sequence data supplemented with the detailed description of morphology for five species. Phylogenetic analyses supported that the subfamily classifications of the Hemiuridae—based entirely on morphological characters—needs to be reconsidered, taking into account a wider range of information sources.
1. Introduction
Brazil is a tropical country with remarkably diverse marine habitats that harbour one of the world’s highest diversities of fish [1,2]. Based on the assumption that parasite diversity is positively correlated with host diversity [3], parasites ought to be one of the specious components of Brazilian marine fauna. Since the first report on helminth parasites in marine fishes in Brazil in 1819, the discovery of these parasites continues to accelerate, with digenean trematodes shown to be one of the most diverse parasite groups [4,5]. However, our knowledge on the actual species diversity of digenean trematodes, their host associations, life cycles, distributions, and phylogenetic relationships remains limited [6,7]. Furthermore, and despite continued interest in this group of parasites, the diversity of digenean trematodes is not reflected in the available genomic data from Brazil. To date, there are only five species for which DNA sequence data is available [7].
The Hemiuroidea is possibly the most ubiquitously distributed superfamily of cosmopolitan and morphologically diverse digeneans that predominantly parasitise marine teleost fish [8]. In Brazil, members of 11 hemiuroid families were reported from phylogenetically and ecologically diverse marine teleosts, belonging to 32 families of 20 orders [4,9]. Out of 16 currently recognised families within the Hemiuroidea, the Hemiuridae Looss, 1899 is one of the most speciose but, in many ways, remains a neglected group. Despite numerous described species of hemiurids, the morphological descriptions of many are inadequate and numerous species therefore require re-evaluation and redescription. Although hemiuroids were one of the first superfamilies to be phylogenetically analysed based on DNA sequences data, the limited number of taxa used in historic and recent studies [10,11,12] do not allow for the clarification of the statuses of several families and subfamilies. Additionally, the identification of some isolates used for sequence generation remain questionable and there are no vouchers available to re-evaluate the material. Similarly, the lack of DNA sequences for numerous taxa within the Hemiuridae hinders the elucidation of systematics of the family based on molecular phylogenetic analysis. Therefore, the taxonomic identity of many taxa within the family are still based entirely on morphological data [9].
Hemiurid life cycles are complex and often involve the exploitation of three to four hosts. However, to date, complete or fragmentary data is only available for 13 species [13]. In Brazil, the life cycle was partially elucidated for a species from the genus Parahemiurus, Parahemiurus merus [14].
The Hemiuridae is the second most species-rich family among fish digeneans in marine ecosystems in Brazil [9]. A total of 28 species from 10 genera are known to parasitise fishes from 28 families of 17 orders in Brazil [7,9]. The majority of species were reported from fishes of the family Carangidae (Carangiformes) collected off the Rio de Janeiro coast [9,15,16]. Only recently were the first DNA sequences of Brazilian marine digenean trematodes published, with three of them being of hemiurids [7].
Parasitological examination of fish for the present study was carried out in the State of Rio de Janeiro, Brazil in January 2021. Overall, the most frequent digeneans collected were species of the family Hemiuridae. They were found in nine species of marine fishes. Here, we provide detailed descriptions of the morphology of the recorded hemiurids, together with newly generated DNA sequences. Furthermore, in order to assess the subfamilial/generic affiliations and relationships of the studied species, we carried out phylogenetic analyses using newly generated sequences of the 28S ribosomal RNA gene. Based on novel data generated in this study, we assessed the phylogenetic affinities for three hemiuroid genera, namely, Ectenurus, Myosaccium, and Parahemiurus, for the first time.
2. Materials and Methods
2.1. Host and Parasite Collection and Morphological Evaluation
In January 2021, forty-three specimens of nine species of fish, namely, Anisotremus virginicus (Linnaeus) (Haemulidae), Decapterus punctatus (Cuvier) (Carangidae), Gymnothorax vicinus (Castelnau) (Muraenidae), Harengula clupeola (Cuvier) (Clupeidae), Percophis brasiliensis Quoy and Gaimard, 1825 (Percophidae), Prionotus punctatus (Bloch) (Triglidae), Pseudopercis numida Miranda Ribeiro (Pinguipedidae), Sardinella brasiliensis (Steindachner) (Clupeidae), and Trichiurus lepturus (Linnaeus) (Trichiuridae) were received from local fisherman from the coastal zone of Cabo Frio (22°52′46″ S, 42°01′07″ W), State of Rio de Janeiro, Brazil. Fish taxonomy and names were used in accordance with Froese and Pauly [2]. Fish were examined for the presence of infection with helminth parasites. Trematode individuals collected from examined fish were washed in 0.9% saline, heat killed, and fixed in 96% ethanol and in 4% formalin (further hologenophores and paragenophores sensu Pleijel et al. [17]). Specimens selected for molecular analyses (i.e., hologenophores) were processed as described in Faltýnková et al. [18], i.e., a small piece of the body of each specimen was excised and used for DNA extraction and the remaining voucher (hologenophore) was used for morphological evaluation. Specimens fixed in formalin (i.e., paragenophores) and the remaining vouchers were stained in Mayer’s hydrochloric carmine solution and mounted as described in Pantoja et al. [7], and thereafter, used for morphological evaluation. Drawings were made by use of a drawing attachment to a light microscope Olympus BX 51. Measurements were taken using AxioCam image analysis software adapted to a Zeiss Primo Star light microscope and, unless otherwise stated, are given in micrometres (μm). Voucher specimens are lodged in the Helminthological Collection of the Oswaldo Cruz Institute (CHIOC), Rio de Janeiro, Brazil.
2.2. DNA Sequence Generation and Phylogenetic Analyses
Total genomic DNA was extracted from trematodes and fish (from a fin clipping) following Georgieva et al. [19]. For trematodes, DNA amplification of two partial fragments of the mitochondrial cox1 gene was performed using the primers JB3 (forward; 5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) (Bowles et al. [20]) and CO1-R-trema (reverse; 5′-CAA CAA ATC ATG ATG CAA AAG G-3′) (Koehler et al. [21]), and Dig_cox1Fa (forward; 5′-ATG ATW TTY TTY TTY YTD ATG CC-3′) and Dig_cox1R (reverse; 5′-TCN GGR TGH CCR AAR AAY CAA AA-3′) (Wee et al. [22]) following the polymerase chain reaction (PCR) protocols as described in Koehler et al. [21] and Wee et al. [22], respectively. The D1–D3 region of the large ribosomal subunit 28S rDNA gene was amplified using the primers digl2 (forward; 5′-AAG CAT ATC ACT AAG CGG-3′) and 1500R (reverse; 5′-GCTA TCC TGA GGG AAA CTT CG-3′) (Snyder and Tkach, [23]), as well as of the ribosomal ITS1-5.8S-ITS2 region were generated using the primers D1 (forward; 5′-AGG AAT TCC TGGTAA GTG CAA G-3′) and D2 (reverse; 5′-CGT TAC TGA GGG AAT CCT GGT-3′) (Galazzo et al. [24]) following the PCR protocols as described in Tkach et al. [25] and Galazzo et al. [24], respectively. The ribosomal ITS2 region was amplified using the primers 3S (forward; 5′-GGT ACC GGT GGA TCA CGT GGC TAG TG-3′) (Bowles et al. [26]) and ITS2.2 (reverse; 5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′) (Cribb et al. [27]) following the PCR protocol as described in Cutmore et al. [28]. For fish, DNA amplification of the partial mitochondrial cox1 gene was performed using the primers VF1 (forward; 5′-TCT CAA CCA ACC ACA AAG ACA TTGG-3′) and VR1 (reverse; 5′-TAG ACT TCT GGG TGG CCA AAG AAT CA-3′) [29] following the PCR protocol of Ward et al. [29]. PCR amplicons were purified with the Exo-SAP-IT KitTM Express Reagent (Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania) and sequenced using the Big Dye Terminator V3.1 Cycle Sequencing kit and ABI 3730 (XL) DNA analyzer capillary sequencing robot (Applied Biosystems, Foster City, CA, USA). The original PCR primers were used for sequencing. Two additional internal primers 300F (forward; 5′-CAA GTA CCG TGA GGG AAA GTT G-3′) (Littlewood et al. [30]) and ECD2 (reverse; 5′-CCT TGG TCC GTG TTT CAA GAC GGG-3′) (Littlewood et al. [31]) were used for sequencing of the 28S rDNA amplicons. Geneious ver. 11 (Biomatters, Auckland, New Zealand) was used to assemble and edit sequences. The newly generated sequences (cox1, 445–500 nt and 756 nt for trematodes; cox1 620–677 for fish; ITS1-5.8S-ITS2, 1676 nt; ITS2, 779–782 nt; and 28S rDNA, 1181–1268 nt) were deposited in GenBank with accession numbers OP918119–OP918132; OP918136–OP918139; OP918021–OP918026; OP948302–OP948305 for trematodes and OP905634; OP925860 for fish.
Three alignments, including novel and previously published sequences, for the species of the Hemiuridae were built using ClustalW implemented in Geneious ver. 11. All alignments were used for comparative sequence analysis (p-distance and nucleotide [nt] difference) and Alignment 1 was further used for phylogenetic analyses. Alignment 1 (957 nt long) included 37 28S rDNA sequences of the species from the Hemiuridae; 14 sequences of 7 species generated in the present study. Alignment 2 (416 nt long) included six ITS2 sequences of Lecithochirium spp.; three sequences of three species generated in the present study. Alignment 3 (443 nt long) included eight cox1 sequences of four species of Lecithochirium; six sequences of four species generated in the present study. Pairwise genetic distances (uncorrected p-distance and no. of differences) for the three datasets were calculated in MEGA ver. 11 [32] using the following conditions: “Variance Estimation Method = None”, “Model/Method = p-distance or no. of differences”, “Substitutions to Include = d: Transitions + Transversions” and “Gaps/Missing Data Treatment = Pairwise deletion”.
Phylogenetic relationships of the taxa in Alignment 1 were assessed using Bayesian inference (BI) and maximum likelihood (ML) analyses. Sequences of Isoparochis eurytremus (MH628315), a parasite of Silurus asotus Linnaeus from Takashima, Japan, was used as the outgroup based on the topology in the phylogenetic tree of the superfamily Hemiuroidea by Louvard et al. [13]. The analyses were conducted using the GTR + I + G model, which was predicted as the best model by the Akaike Information Criterion in jModelTest 2.1.2 [33]. BI analysis was performed using MrBayes software (ver. 3.2.3) [34] through the CIPRES Science Gateway ver. 3.3 [35] accessed on 22 September 2022. Markov Chain Monte Carlo chains were run for 10,000,000 generations, log-likelihood scores were plotted and only the final 75% of trees were used to build the consensus tree. ML analysis was performed using PhyML ver. 3.0 [36] and run on the ATGC bioinformatics platform (http://www.atgc-montpellier.fr, accessed on 22 September 2022) with a nonparametric bootstrap value of 100 pseudoreplicates.
To avoid ambiguity for some generic names, the following abbreviations were used: D., Decapterus; Di., Dinurus; H., Harengula; He., Hemiurus; O., Oligoplites; Op., Opisthonema; P., Parahemiurus; Pe., Percophis; Pr., Prionotus; Ps., Pseudopercis; Pu., Pulmovermis; S., Sardinella; and Sa., Sardinops.
3. Results
Evaluation of the specimens of hemiurid trematodes obtained in the present study via morphological and molecular methods confirmed the presence of eight trematode species in the examined fishes. Out of the 43 specimens of 9 fish species examined, 14 (32.5%) were found to be infected with at least 1 species of hemiurid and 4 (9.3%) were infected with 2 species of hemiurids. All specimens of hemiurids were detected in the fish stomach. Thirty sequences were newly generated; twenty-eight sequences for seven out of eight recorded species of trematodes: 28S rDNA (n = 14), ITS1-5.8S-ITS2 (n = 1), ITS2 (n = 3) and cox1 (n = 10). Sequences of the unidentified species of Lecithochirium were not generated. Two cox1 sequences were generated for the fish hosts, Pseudopercis numida and Trichiurus lepturus. The photographs of the fish specimens that were used to generate sequences are provided in Figure S1. Fish hosts were identified following Menezes and Figueiredo [37]. The cox1 sequences for fish obtained in the present study were compared with sequences available in GenBank. The sequence divergence between our specimen of T. lepturus (OP905634) and specimens of T. lepturus (GU702467; JX124915) collected in São Paulo, Brazil was low and ranged from 0.2 to 0.3% (1–2 nt). Thus, we consider these isolates as conspecific. The sequence divergence between our specimen of Ps. numida (OP925860) and specimens of Ps. semifasciata (JQ365526; EU074573) from São Paulo, Brazil and from Argentina was low and ranged from 0 to 1.6% (0–8 nt). Although the genetic divergence between our specimen identified as Ps. numida and specimens of Ps. semifasciata available in GenBank suggests that these specimens are conspecific, morphologically our specimens belong to Ps. numida.
3.1. Morphological Evaluation
- Aphanurinae Skrjabin and Guschanskaja, 1954
- Myosaccium Montgomery, 1957
- Myosaccium ecaude Montgomery, 1957
- Host: Sardinella brasiliensis (Steindachner) (Clupeidae).
- Infection rates: two out of two; three–four specimens per fish; seven specimens in total.
- Representatives DNA sequences: OP918123 (28S); OP948303 (cox1).
- Voucher material: CHIOC 39908 a–e.
Description (Figure 1a–c). (Based on four paragenophores and one hologenophore; measurements of paragenophores in Table 1 and hologenophore in description): Body elongate, dorso-ventrally flattened. Maximum width at level of ventral sucker, 189. Tegument covered with conspicuous plications to level of vitellarium. Forebody short 217 long.
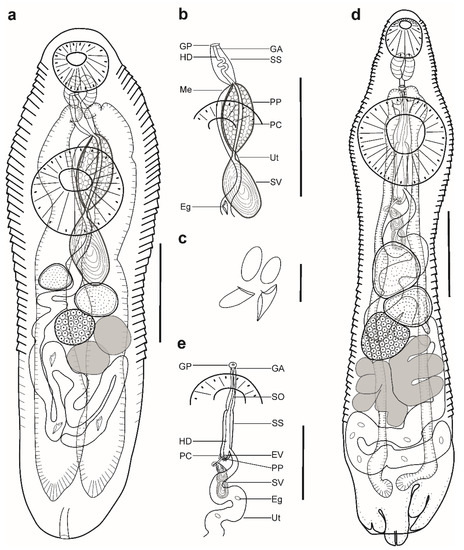
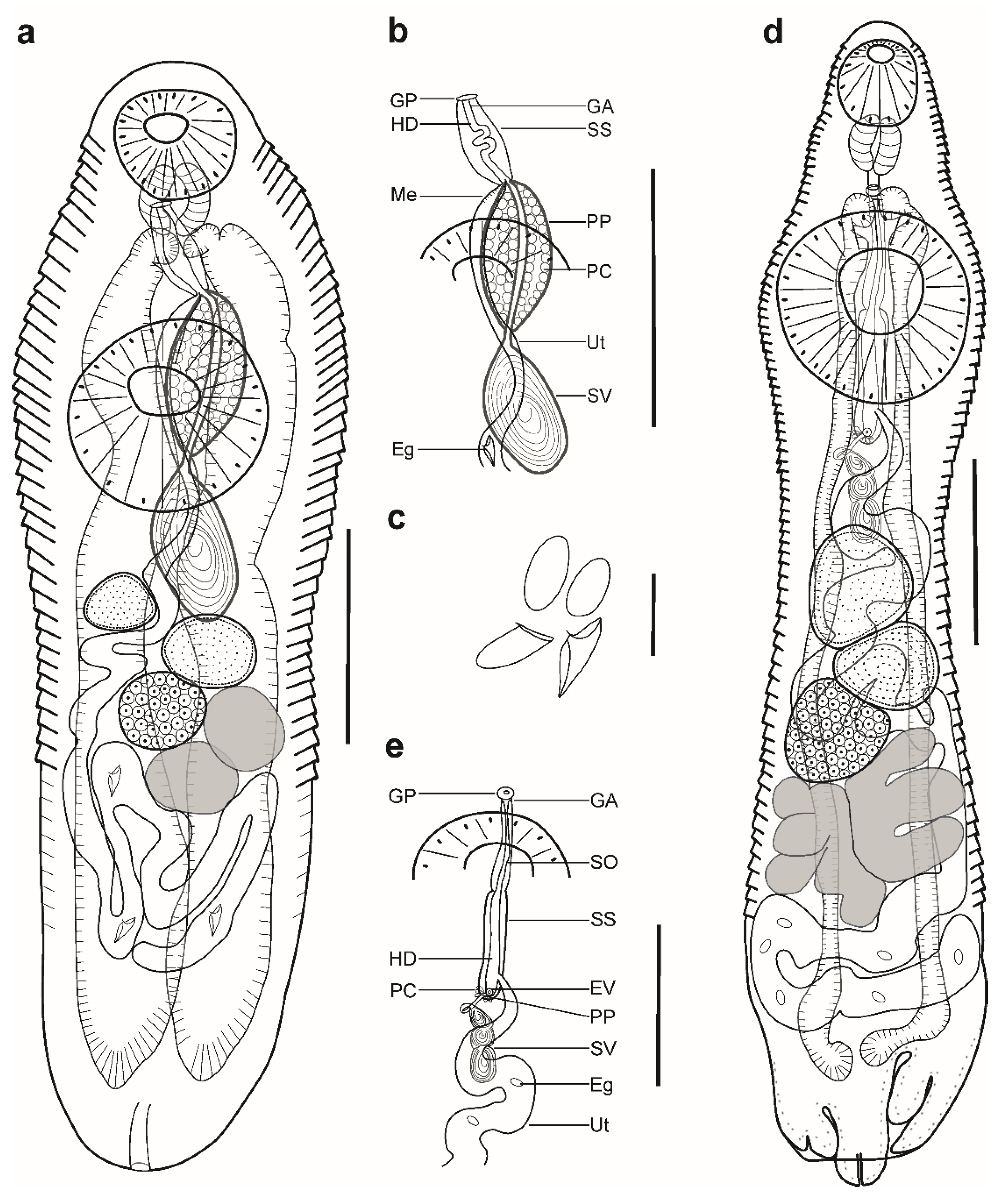
Figure 1.
Hemiurid parasites of marine fishes from Brazil. (a–c) Adult of Myosaccium ecaude ex Harengula clupeola: (a) complete specimen, ventral view; (b) detail of the terminal genitalia, ventral view; (c) eggs; (d,e) adult of Ectenurus virgula ex Decapterus puntatus: (d) complete specimen, ventral view; (e) detail of the terminal genitalia, ventral view. Scale bars (a,b,d,e): 200 µm; (c): 50 µm. Abbreviations: Eg, eggs; GA, genital atrium; GP, genital pore; HD, hermaphroditic duct; PC, prostatic cells; PP, pars prostatica; SO, sinus-organ; SS, sinus-sac; SV, seminal vesicle; Ut, uterus.
Pre-oral lobe distinct, 25 long. Oral sucker muscular, well developed, subspherical or transversely oval, ventro-subterminal, 76 long, 86 wide. Prepharynx absent. Pharynx muscular, well developed, spherical or subspherical, 45 long, 45 wide. Oesophagus absent. “Drüsenmagen” present. Intestinal bifurcation just posterior to pharynx. Caeca blind, with thin walls and wide lumen, terminates in posterior body extremity. Ventral sucker muscular, well developed, spherical or subspherical, 129 long, 139 wide, larger than oral sucker (1:1.62), pre-equatorial.
Testes 2, obliquely symmetrical, separated, entire, pre-ovarian, in anterior hindbody, separated from ventral sucker; dextral testis subspherical to transversely oval, 32 long, 28 wide, sinistral testis transversely oval, 38 long, 55 wide. Distance between ventral sucker and dextral testis 50. Seminal vesicle thick-walled, saccular, elongate-oval, 128 long, 109 wide (Figure 1a,b). Seminal vesicle entirely in anterior hindbody or extends only to posterior margin of ventral sucker, connected to pars prostatica by aglandular duct. Pars prostatica elongate-oval, vesicular, densely invested by prostatic cells, antero-dorsal to ventral sucker (Figure 1b). Sinus-sac elongate, between suckers, with muscular wall, 100 long, 28 wide. Hermaphroditic duct straight or coiled within sinus-sac, opens directly through genital pore. Genital pore median or sub-median just posterior to oral sucker.
Ovary median, entire, subspherical (n = 3) or transversely oval (n = 2), 48 long, 68 wide, in anterior half or in middle of hindbody, contiguous with sinistral testis. Vitellarium in two compact oblique masses, subspherical; dextral mass posterior to ovary, 37 long, 41 wide, sinistral mass lateral to ovary, 41 long, 39 wide. Juel’s organ and Mehlis’ gland not observed. Uterus coiled, occupies post-ovarian region. Metraterm not differentiated, terminal part of uterus passes into sinus-sac ventrally, joins male duct forming hermaphroditic duct. Eggs numerous, 25–26 × 11–13 (n = 4) (Figure 1c).
Excretory vesicle not observed; excretory pore terminal.
Remarks: Specimens found in the present study correspond well to the generic diagnosis of Myosaccium Montgomery, 1957 provided by Gibson [8] in having a saccular and elongate-oval seminal vesicle posterior to the middle of the ventral sucker, a vesicular pars prostatica with a muscular wall, a tubular sinus-sac enclosing the hermaphrodite duct, and vitellarium composed of two distinct masses.
Our specimens correspond in their morphology to the type-species of the genus, M. ecaude Montgomery, 1957, described from the stomach of South American pilchard, Sardinops sagax (=S. caerulea) (Jenyns) by Montgomery [38] in La Jolla, CA, USA, north-eastern Pacific Ocean. Specimens corresponded in body shape as well as by possessing plicated tegument, a subspherical pharynx, obliquely symmetrical testes, and a similar sucker ratio (1:1.66–1.79 vs. 1:1.57–1.75). However, the maxima for all dimensions in our specimens was lower except for the eggs (Table 1). Except for the sucker ratio (1:1.66–1.79 vs. 1:2.30) (Table 1), all dimensions of our specimens overlap with specimens of M. ecaude collected from Opisthonema libertae (Günther) in the Chamela Bay, Jalisco, Mexico, north-eastern Pacific Ocean by León-Règagnon et al. [39].
Myosaccium ecaude is known as a parasite of the stomach of fishes from the family Clupeidae in the Pacific and Atlantic oceans. After the original description, the species was reported in Op. libertate and Harengula thrissima (Jordan and Gilbert) in the Chamela Bay, Jalisco, Mexico [39,40], in the type host Sa. sagax in California, USA, and Baja California, Mexico, north-eastern Pacific Ocean [41,42,43] and in H. clupeola and S. brasiliensis in Rio de Janeiro, Brazil, southwestern Atlantic Ocean [44,45].
Myosaccium ecaude was originally described as possessing filamented eggs. Overstreet [46] and León-Règagnon et al. [39] re-evaluated the type material and provided different conclusions. The first author concluded that “specimens from Sardinella anchovia do not have filaments or spines on the eggs, although a look at collapsed specimens on a fixed plane strongly suggests their presence”. Additionally, the same author did not observe morphological differences between M. ecaude and the only other species of the genus, M. opisthonemae (Siddiqi and Cable, 1960) described from Op. oglinum (Lesueur) in Playa Mani, Puerto Rico, by Siddiqi and Cable [47], north-western Atlantic Ocean, and suggested that M. opisthonemae might be a small or progenetic form of M. ecaude. León-Règagnon with co-authors [39] observed the presence of filamented eggs in the type material and in their newly collected specimens from fish in Mexico. These authors suggested that the presence of filamented eggs is a strong characteristic to distinguish M. ecaude from M. opisthonemae. Thereafter, Kohn and Buhrnheim [48] collected specimens of M. ecaude in S. aurita in the same region to that of the present study and described their specimens as having filamented and spined eggs. However, later Kohn et al. [4] corrected their species identification in Kohn and Buhrnheim [48] to M. opisthonemae without further explanation.
In our material, the hologenophore possess collapsed and non-collapsed eggs. The collapsed eggs may look as if they bear filaments and spines; however, after detailed examination, we concluded that these structures are absent (Figure 1c). Therefore, we agree with Overstreet [46] who, after re-examining the type material of M. ecaude, did not report on filaments or spines on the eggs. Although Overstreet [46] did not observe morphological differences between M. ecaude and M. opisthonemae, there are morphometric differences that can be noticed (Table 1). Newly collected material of M. ecaude from S. brasiliensis in the present study was used to provide a detailed description of the species together with novel DNA sequence.


Table 1.
Comparative metrical data of Myosaccium spp.
Table 1.
Comparative metrical data of Myosaccium spp.
| Species | Myosaccium ecaude Montgomery, 1957 | Myosaccium opisthonemae (Siddiqi & Cable, 1960) | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | Present study | Montgomery [38] | León-Règagnon et al. [39] | Overstreet [46] | Siddiqi and Cable [47] | Kohn and Buhruheim [48] | ||
| Locality | Atlantic Ocean, Rio de Janeiro, Brazil | Pacific Ocean, California, USA | Pacific Ocean, La Chamela Bay, Mexico | Atlantic Ocean, Florida, USA | Atlantic Ocean, Playa Mani, Puerto Rico | Atlantic Ocean, Rio de Janeiro, Brazil | ||
| Host | Sardinella brasiliensis | Sardinops sagax | Opisthonema libertae | Sardinella aurita | Opisthonema oglinum | Sardinella aurita | ||
| Range (n = 4) | Mean | Range (n = 10) | Range (n = 30) | Mean | Range (n = 12) | Range (n = 40) | Range (n = NP) | |
| Body length | 924–1009 | 906 | 1380–1530 | 920–1120 | 1020 | 440–900 | 534–827 | 650–1040 |
| Body width | 221–248 | 232 | 360–400 | 280–350 | 320 | – | 153–193 | 120–200 |
| Forebody length | 182−224 | 204 | – | – | – | – | – | – |
| Hindbody length | 449−629 | 538 | – | – | – | – | – | – |
| Pre-oral lobe length | 17−26 | 21 | – | – | – | – | – | – |
| Oral sucker length | 71−88 | 79 | 90–100 | 60–80 | 70 | – | 52–69 | 40–80 |
| Oral sucker width | 78−102 | 88 | 110–120 | 80–140 | 90 | – | 68–79 | 50–90 |
| Pharynx length | 40−51 | 47 | 60–70 | – | – | – | 31–37 | 30–50 |
| Pharynx width | 47−54 | 50 | 60–70 | – | – | – | – | 40–60 |
| Ventral sucker length | 126−169 | 143 | – | 150–190 | 170 | – | 94–120 | 170–170 |
| Ventral sucker width | 140−169 | 154 | – | 140–210 | 180 | – | – | 70–170 |
| DIBAE | 118−168 | 139 | – | – | – | – | – | – |
| DTVS | 44−106 | 62 | – | – | – | – | – | – |
| Anterior testis length | 29−53 | 40 | – | – | – | – | 29–69 | 30–80 |
| Anterior testis width | 47−51 | 49 | – | – | – | – | – | 20–70 |
| Posterior testis length | 31–50 | 40 | – | – | – | – | – | 30–90 |
| Posterior testis width | 51–67 | 62 | – | – | – | – | – | 30–70 |
| Post-testicular region length | 322–410 | 381 | – | – | – | – | – | – |
| Seminal vesicle length | 99−152 (n = 3) | 131 | – | – | – | – | – | 30–180 |
| Seminal vesicle width | 57−74 (n = 3) | 63 | – | – | – | – | – | 30–60 |
| Sinus-sac length | 73–105 | 90 | 130–170 | – | – | – | – | 60 |
| Sinus-sac width | 21–26 | 24 | 30–40 | – | – | – | – | – |
| Ovary length | 43−66 | 57 | – | – | – | – | 39–69 | 30–50 |
| Ovary width | 64−80 | 69 | – | – | – | – | – | 20–50 |
| Vitellarium length | 81–117 | 107 | – | – | – | – | – | – |
| Vitellarium width | 85–111 | 101 | – | – | – | – | – | – |
| Egg length | 23–30 (n = 10) * | 28 | 15–18 | 26–29 | 28 | 21–26 | 29–32 | 30–41 |
| Egg width | 8–13 (n = 10)* | 9 | 9 | 10–13 | 11 | 9–11 | 12–15 | 9–13 |
| Body length/body width | 1:3.35–4.19 | 1:3.90 | – | – | – | – | – | – |
| Oral/ventral sucker width | 1:1.66−1.79 | 1:1.75 | 1:1.57–1.75 | 1:2.30 | 1:230 | 1:1.60–1.80 | 1:160 | 1:1.45–2.32 |
| Forebody/body length, % | 21−24 | 23 | – | – | – | – | – | – |
| Post-testicular region/body length, % | 39–44 | 42 | – | – | – | – | – | – |
* Measurements of collapsed eggs. DIBAE = distance from intestinal bifurcation to anterior extremity, DTVS = distance from testes to ventral sucker, NP = not provided.
- Dinurinae Looss, 1907
- Ectenurus Looss, 1907
- Ectenurus virgula Linton, 1910
- Hosts: Anisotremus virginicus (Linnaeus) (Haemulidae), Decapterus punctatus (Cuvier) (Carangidae), Prionotus punctatus (Bloch) (Triglidae).
- Infection rates: A. virginicus, two out of five; one–five specimens per fish; six specimens in total; D. punctatus, one out of one; two specimens in total; P. punctatus 1 out of 23; two specimens in total.
- Representatives DNA sequences: OP918121, OP918122, OP918126 (28); OP918136 (ITS1-5.8S-ITS2); OP948304 (cox1).
- Voucher material: CHIOC 39798; CHIOC 39799 a–e; CHIOC 39800 a,b.
Description (Figure 1d,e). (Based on five paragenophores and one hologenophore; measurements of paragenophores in Table 2 and hologenophore in description): Body elongate, dorso-ventrally flattened. Maximum width at level of ventral sucker or close to posterior body extremity, 361. Tegument covered with conspicuous plications to level of vitellarium. Forebody short, 208. Ecsoma well developed, withdrawn (n = 5) or partially extruded (n = 1).
Pre-oral lobe distinct, 11 long. Oral sucker muscular, well developed, spherical or subspherical, ventro-subterminal, 104 long, 100 wide. Prepharynx absent. Pharynx muscular, well developed, spherical or subspherical, 65 long, 72 wide. Oesophagus short, 20 long. “Drüsenmagen” present. Intestinal bifurcation just anterior to ventral sucker. Caeca blind, with thick walls and narrow lumen, usually terminates in body or inside of ecsoma when it is extruded (n = 1). Ventral sucker muscular, well developed, subspherical, elongate-oval or transversely oval, 300 long, 319 wide, larger than oral sucker (1:3.19), pre-equatorial.
Testes 2, oblique to tandem, contiguous, entire, pre-ovarian, median, in anterior hindbody, separated from ventral sucker (contiguous in hologenophore); subspherical (n = 2) to subtriangular (n = 4), anterior testis, 132 long, 158 wide, posterior testis, 137 long, 150 wide. Seminal vesicle thin walled, 260 long, 96 wide; tripartite, anterior portion pyriform, 53 long, 44 wide; middle portion subspherical, 71 long, 61 wide; posterior portion elongate-oval, 132 long, 96 wide (Figure 1e). Seminal vesicle in anterior hindbody, connected to pars prostatica by aglandular duct. Pars prostatica very short, tubular, invested by prostatic cells, in anterior hindbody. Ejaculatory vesicle conspicuous, spherical, enclosed within sinus-sac. Sinus-sac elongate, tubular, dorsal to ventral sucker, with muscular wall, 145 long, 22 wide. Hermaphroditic duct straight, enclosed within sinus-sac and sinus-organ. Permanent sinus-organ elongate, tubular and muscular. Genital atrium short. Genital pore median, just anterior to intestinal bifurcation (Figure 1e).
Ovary median (n = 3), dextral (n = 2) or sinistral (n = 1), entire, subtriangular (n = 4) to transversely oval (n = 2), 71 long, 183 wide, in posterior half of hindbody, contiguous with posterior testis. Vitellarium seven elongate digitiform lobes (three sinistral and four dextral), contiguous with ovary, 243 long, 292 wide. Juel’s organ and Mehlis’ gland not observed. Uterus coiled, restricted to body or extends up to one-fifth length of ecsoma (n = 1) when it is extruded. Metraterm not differentiated, terminal part of uterus passes into sinus-sac ventrally, joins male duct forming hermaphroditic duct. Eggs numerous, 17–19 × 9–12 (n = 10).
Excretory vesicle not observed; excretory pore terminal.
Remarks: Specimens obtained in this study agree well with the generic diagnosis of Ectenurus Looss, 1907 provided by Gibson [8] in having a plicated body surface, a seminal vesicle divided into three portions in the anterior hindbody, the presence of a sinus-sac, a permanent sinus-organ, and a short pars prostatica connected to the seminal vesicle by a distinct, long, and occasionally convoluted aglandular duct.
Morphologically, the present specimens most closely resemble E. virgula described from the stomach of round sardinella, Sardinella aurita Valenciennes (=Clupanodon pseudohispanica) (type host) in Dry Tortugas, Florida and from Selar (=Trachurops) crumenophthalmus (Bloch) at Woods Hole, Massachusetts, USA, north-western Atlantic Ocean by Linton [49]. Specimens corresponded in body shape, plicated tegument, by possessing an elongate sinus-sac which reaches to the mid-level of the ventral sucker or more posteriorly and not lying in the forebody, a tripartite seminal vesicle, contiguous testes, an ovary contiguous with the posterior testis, and in egg size (16–19 × 8–12 vs. 17–8). However, the maxima for all dimensions was lower in our specimens, except for the sinus-sac length (125–194 vs. 180–260) and eggs (16–19 × 8–12 vs. 17–8). In comparison with specimens collected from Caranx hippos (Linnaeus), Trachinotus rhodopus Gill and Ophioscion scierus (Jordan and Gilbert) in the Chamela Bay, Jalisco, Mexico, north-eastern Pacific Ocean by León-Règagnon et al. [39], the maxima for all dimensions was lower except for the sucker ratio (1:2.26–3.22 vs. 1:2.3) (Table 2).
Ectenurus virgula parasitises in the stomach of a variety of fish from the Atlantic and Pacific oceans. After the original description, the species was reported parasitising fishes from the Bothidae, Carangidae, Clupeidae, Phycidae, Sciaenidae, Percophidae, and Priacanthidae in the Atlantic Ocean [50,51,52,53,54,55,56,57,58], and from the Carangidae and Sciaenidae in the Pacific Ocean [27].
In Brazil, Travassos et al. [59] reported E. lepidus from Oligoplites saurus (Bloch and Schneider) in Espírito Santo. However, based on their morphological description, Gibson and Bray [60] claimed that Travassos et al. [59] found E. virgula. Thereafter, Pereira et al. [61] reported E. virgula from the phycid U. brasiliensis (Kaup) in Rio de Janeiro. Our record of E. virgula infecting A. virginicus, D. punctatus, and Pr. punctatus collected off the Brazilian coast represent a new host record. Specimens of E. virgula collected in our study contributed to a novel detailed description of the species and to generation of DNA sequences.


Table 2.
Comparative metrical data of Ectenurus virgula Linton, 1910.
Table 2.
Comparative metrical data of Ectenurus virgula Linton, 1910.
| Source | Present Study | León-Règagnon et al. [39] | Linton [49] | ||
|---|---|---|---|---|---|
| Locality | Atlantic Ocean, Rio de Janeiro, Brazil | Pacific Ocean, Jalisco, Mexico | Atlantic Ocean, Florida, USA | ||
| Host | Anisotremus virginicus, Prionotus punctatus | Caranx hippos, Ophioscion scierus, Trachinotus rhodopus | Sardinella aurita | ||
| Range (n = 5) | Mean | Range (n = 9) | Mean | Range (n = 1) | |
| Body length | 1061–1278 | 1157 | 1450–1600 | 1520 | 3000 |
| Body width | 198–310 | 253 | 440–510 | 470 | 500 |
| Ecsoma length | 252 (n = 1) | 252 | 560–880 | 720 | – |
| Total length | 1342 (n = 1) | 1342 | – | – | – |
| Forebody length | 163−239 | 189 | – | – | – |
| Hindbody length | 684−818 | 743 | – | – | – |
| Pre-oral lobe length | 13−19 | 17 | – | – | – |
| Oral sucker length | 74−94 | 82 | 150 | – | 150 |
| Oral sucker width | 73−95 | 82 | – | – | – |
| Pharynx length | 43−56 | 49 | – | – | 80 |
| Pharynx width | 42−54 | 50 | – | – | – |
| Oesophagus length | 20–41 | 32 | – | – | – |
| Ventral sucker length | 186−276 | 212 | 320 | – | 380 |
| Ventral sucker width | 172−281 | 225 | – | – | – |
| DIBAE | 112−179 | 156 | – | – | – |
| DTVS | 32−118 | 66 | – | – | – |
| Anterior testis length | 96−171 | 121 | – | – | – |
| Anterior testis width | 91−114 | 102 | – | – | – |
| Posterior testis length | 95–162 | 114 | – | – | – |
| Posterior testis width | 77–120 | 102 | – | – | – |
| Post-testicular region | 412–547 | 488 | – | – | – |
| Seminal vesicle length | 85−183 | 126 | – | – | – |
| Seminal vesicle width | 29−88 | 53 | – | – | – |
| Sinus-sac length | 125–194 | 154 | – | – | 180–260* |
| Sinus-sac width | 20–32 | 27 | – | – | – |
| Ovary length | 61−92 | 79 | – | – | – |
| Ovary width | 99−125 | 111 | – | – | – |
| Vitellarium length | 99–151 | 123 | – | – | – |
| Vitellarium width | 149–242 | 191 | – | – | – |
| Egg length | 16–19 | 17 | – | – | 17 |
| Egg width | 8–12 | 10 | – | – | 8 |
| Body length/body width | 1:4.61–5.51 | 1:4.66 | – | – | – |
| Oral/ventral sucker width | 1:2.26−3.22 | 1:2.75 | 1:2.3 | – | – |
| Ecsoma/body length, % | 23 (n = 1) | 23 | – | – | – |
| Forebody/body length, % | 15−20 | 16 | – | – | – |
| Post-testicular region/body length, % | 39–45 | 42 | – | – | – |
* Reported by Bray [62] after examination of the type material. DIBAE = distance from intestinal bifurcation to anterior extremity, DTVS = distance from testes to ventral sucker.
- Hemiurinae Looss, 1899
- Parahemiurus Vaz and Pereira, 1930
- Parahemiurus merus (Linton, 1910)
- Hosts: Harengula clupeola (Cuvier), Sardinella brasiliensis (Steindachner) (Clupeidae).
- Infection rates: H. clupeola, two out of three; two–six specimens per fish; eight in total; S. brasiliensis, one out of two; two specimens in total.
- Representatives DNA sequences: OP918124, OP918125 (28S).
- Voucher material: CHIOC 39909; CHIOC 39910 a–e.
Description (Figure 2a,b). (Based on four paragenophores and two hologenophores; measurements of paragenophores in Table 3 and hologenophores in description): Body elongate, dorso-ventrally flattened, 993–1174 long. Maximum width close to posterior body extremity, 345–393. Tegument covered with conspicuous plications to level of posterior margin of ovary. Forebody short, 138–158, representing 13–14% of body length. Ecsoma well developed, withdrawn (n = 2), protruded (n = 3) or partially extruded (n = 1).
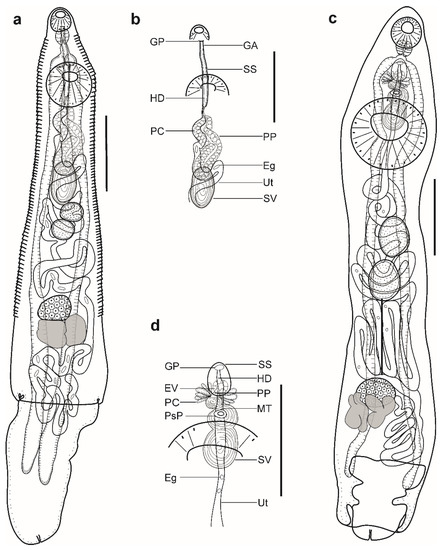
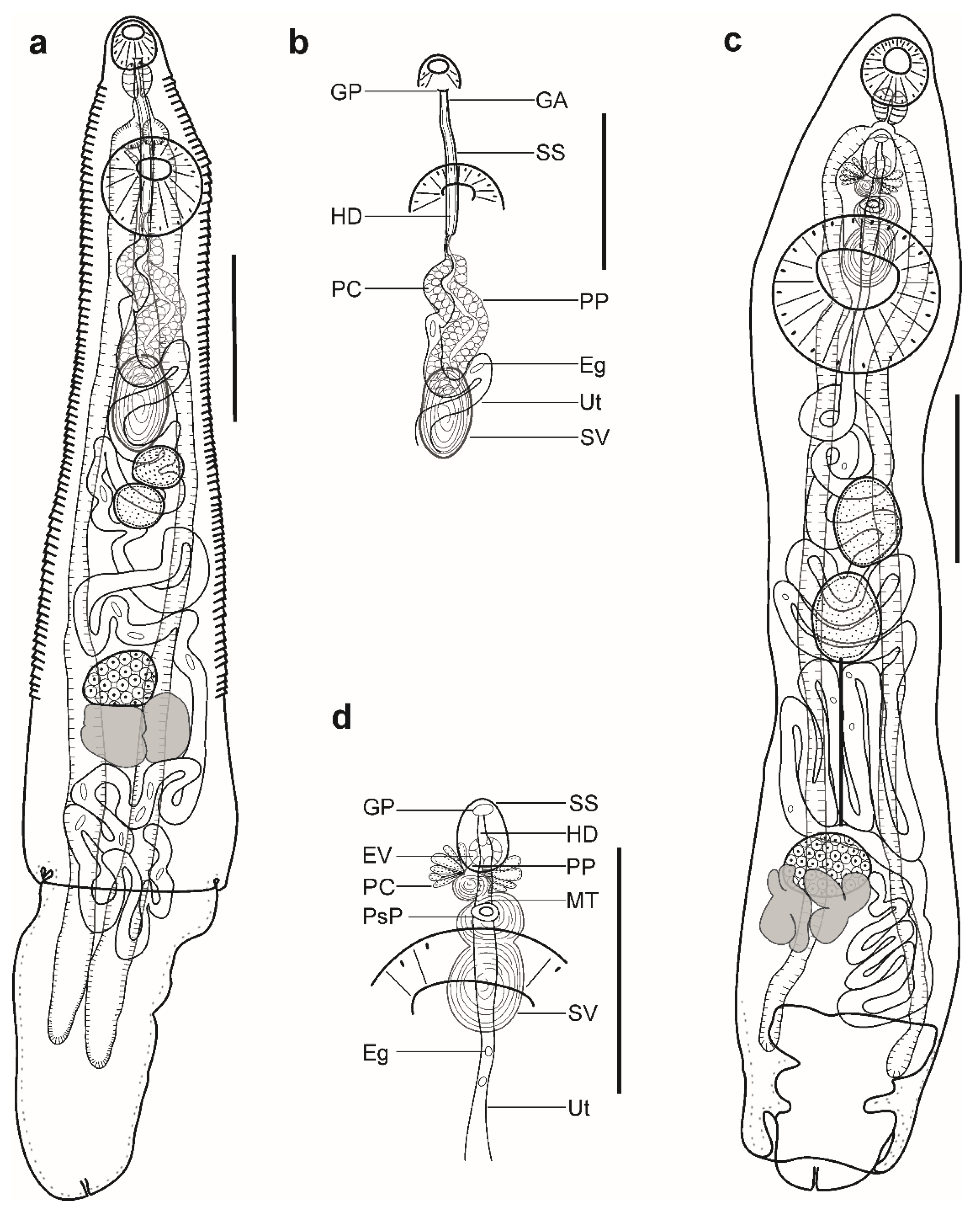
Figure 2.
Hemiurid parasites of marine fishes from Brazil. (a,b) Adult of Parahemiurus merus ex Harengula clupeola: (a) complete specimen, ventral view; (b) detail of the terminal genitalia, ventral view. (c,d) Adult of Lecithochirium microstomum ex Trichiurus lepturus: c complete specimen, ventral view; (d) detail of the terminal genitalia, ventral view. Scale bars (a,b): 400 µm, (c,d): 500 µm. Abbreviations: Eg, eggs; EV, ejaculatory vesicle; GA, genital atrium; GP, genital pore; HD, hermaphroditic duct; PC, prostatic cells; PP, pars prostatica; SS, sinus-sac; SV, seminal vesicle; Ut, uterus.

Table 3.
Comparative metrical data of Parahemiurus merus (Linton, 1910).
Pre-oral lobe distinct, 15–28 long. Oral sucker muscular, well developed, spherical or subspherical, ventro-subterminal, 80–89 long, 90–100 wide. Prepharynx absent. Pharynx muscular, well developed, spherical or subspherical, 55–56 long, 53–58 wide. Oesophagus short, 22–42 long. “Drüsenmagen” present. Intestinal bifurcation just anterior to ventral sucker. Caeca blind, with thick walls and wide lumen, terminates in body or inside of ecsoma when it is extruded (n = 4). Ventral sucker muscular, well developed, subspherical, 172–182 long, 189–194 wide, larger than oral sucker (1:1.94–2.10), pre-equatorial.
Testes 2, oblique to tandem, contiguous, entire, pre-ovarian, median, in middle of hindbody, separated from ventral sucker; anterior testis subspherical, elongate-oval or transversely oval, 81–91 long, 87–120 wide, posterior testis subspherical, elongate-oval or transversely oval, 81–95 long, 113–116 wide. Distance between ventral sucker and anterior testis 114–162. Post-testicular field 511–519, 44–51% of body length. Seminal vesicle thick-walled, saccular, 142–165 long, 67–97 wide. Seminal vesicle in anterior hindbody, connected to pars prostatica by aglandular duct. Pars prostatica long, tubular, convoluted, densely invested by prostatic cells, in anterior hindbody (Figure 2b). Sinus-sac elongate, tubular, muscular, dorsal to ventral sucker, with muscular wall, 178–220 long, 14–16 wide. Hermaphroditic duct straight, enclosed within sinus-sac. Genital atrium short. Genital pore median, ventral to oral sucker.
Ovary median (n = 2), dextral (n = 3), or sinistral (n =1), entire, subspherical or transversely oval, 67–93 long, 111–140 wide, in posterior half of hindbody, always separated from posterior testis by uterine coils. Distance between posterior testis and ovary 95–131. Vitellarium in 2 lateral subsymmetrical oval masses with irregular margins, dextral mass 125–130 long, 88–93 wide, sinistral mass 81–118 long, 77–139 wide. Juel’s organ and Mehlis’ gland not observed. Uterine coils posterior to vitellarium, restricted to body or extends to one-fourth length of ecsoma when it is extruded. Metraterm not differentiated, terminal part of uterus passes into sinus-sac ventrally, joins male duct forming hermaphroditic duct. Eggs numerous, large, 22–26 × 9–10 (n = 10).
Excretory vesicle not observed; excretory pore terminal.
Remarks: Specimens found in the present study correspond well to the generic diagnosis of Parahemiurus Vaz and Pereira, 1930 provided by Gibson [8] in having a well-developed ecsoma, the absence of an ejaculatory vesicle, in possessing a tubular sinus-sac, a vitellarium composed of two distinct oval masses, a plicated body surface, an oval seminal vesicle with a muscular wall, and a tubular pars prostatica.
Our specimens correspond in their morphology to Parahemiurus merus (Linton, 1910) described as Hemiurus merus from the stomach of Sardinella aurita (=Clupanodon pseudohispaniciis) in Tortugas, Florida, USA, north-western Atlantic Ocean by Linton [49]. Specimens corresponded in body shape, having plicated body surface to level of the vitellarium, in possessing a narrow and elongate sinus-sac, an oval seminal vesicle, and contiguous testes. However, except for the egg size (22–27 × 7–11 vs. 27 × 10) (Table 3), the maxima for all dimensions in our specimens was lower. The features and dimensions of our specimens overlap with specimens collected from Sardinella aurita Valenciennes in São Paulo, Brazil, specimens collected from Engraulis anchoita Hubbs and Marini off the Argentinean and Uruguayan coasts, southwestern Atlantic Ocean by Vaz and Pereira [63] and Timi et al. [64], specimens collected from several fish hosts of the families Carangidae, Clupeidae, Haemulidae, Merlucciidae, Pomatomidae, Salmonidae, Scorpaenidae, and Sparidae in different localities from the Atlantic, Indian, and Pacific oceans in a review published by Bray [65], and specimens collected from several fish hosts of the families Balistidae, Clupeidae, Engraulidae, and Haemulidae in Chamela Bay, Jalisco, Mexico, north-eastern Pacific Ocean by León-Règagnon et al. [39] (Table 3).
Parahemiurus merus is reported mainly from clupeid, carangid, salmonid, and engraulid fishes from the Atlantic, Indian, and Pacific oceans [65]. In Brazil, this species has been reported several times in fishes from at least 12 families (Belonidae, Carangidae, Clupeidae, Dactylopteridae, Engraulidae, Haemulidae, Ophidiidae, Phycidae, Pinguipedidae, Pomatomidae, Sciaenidae, and Sparidae) [9]. Wallet and Kohn [66] and Luque et al. [31] reported P. merus in H. clupeola, and Luque et al. [44] and Moreira et al. [45] reported it in S. brasiliensis. Benicio et al. [67] reported it in Cetengraulis edentulus (Cubier) in the same region as in our study. Newly collected specimens of P. merus have allowed us to provide a detailed description of the species and generate the first DNA sequence data.
- Lecithochirinae Lühe, 1901
- Lecithochirium floridense (Manter, 1934)
- Host: Percophis brasiliensis Quoy and Gaimard (Percophidae).
- Infection rates: one out of five; five specimens in total.
- Representatives DNA sequences: OP918131 (28S); OP918025 (cox1).
- Voucher material: CHIOC 39902 a–c.
Remarks: Specimens found in the present study correspond well to the generic diagnosis of Lecithochirium Luhe, 1901 provided by Gibson and Bray [60] and Gibson [8] in having a well-developed ecsoma, a pre-oral lobe, a tubular pars prostatica, a vitellarium of two lateral masses divided into three and four short lobes, and eggs without polar filaments. Comparative sequence analyses (see below) demonstrated that the 28S rDNA and cox1 sequences of our isolate from the percophid P. brasiliensis identified as L. floridense was identical to sequences of the same species reported in Auxis thazard (Lacépède) in Rio de Janeiro, Brazil by Pantoja et al. [7].
Lecithochirium floridense is a parasite of the stomach of a variety of marine fish species. To date, L. floridense has been reported from fishes from at least 17 families, including the present data, with the majority of records coming from the western Atlantic Ocean (Pantoja et al. [7]). In Brazil, L. floridense was reported from A. thazard in Rio de Janeiro, southwestern Atlantic Ocean. Our record of L. floridense infecting Pe. brasiliensis collected off the Brazilian coast represents a new host record for this species. We do not provide a description of the specimens of this species because a detailed morphological description of this species with DNA sequence data was provided by Pantoja et al. [7].
- Lecithochirium microstomum Chandler, 1935
- Hosts: Prionotus punctatus (Bloch) (Triglidae), Trichiurus lepturus (Linnaeus) (Trichiuridae).
- Infection rates: Pr. punctatus, 1 out of 23; three specimens in total; T. lepturus, one out of one; 10 specimens in total.
- Representative DNA sequences: L. microstomum: OP918119, OP918120, OP918127 (28S); OP918137 (ITS2); OP918021, OP948302, OP918022 (cox1); T. lepturus: OP905634 (cox1).
- Voucher material: CHIOC 39903 a,b; CHIOC 39904 a–g.
Description (Figure 2c,d). (Based on six paragenophores and one hologenophore; measurements of paragenophores in Table 4 and hologenophores in description): Body elongate, dorso-ventrally flattened. Maximum width at ventral sucker level, 679. Tegument smooth. Forebody short, 574. Ecsoma well developed, withdrawn (n = 5) or protruded (n = 2).

Table 4.
Comparative metrical data of Lecithochirium microstomum Chandler, 1935.
Pre-oral lobe distinct, 36 long. Oral sucker muscular, well developed, spherical or subspherical, ventro-subterminal, 194 long, 176 wide. Prepharynx absent. Pharynx muscular, well developed, spherical or subspherical, 112 long, 104 wide. Oesophagus, 80 long (n = 4). “Drüsenmagen” present. Presomatic pit non-aglandular anterior to ventral sucker (Figure 2c,d). Intestinal bifurcation in anterior forebody. Caeca blind, with thick walls and narrow lumen, usually terminates in body or inside ecsoma when extruded (n = 2). Ventral sucker muscular, well developed, subspherical, transversely oval or elongate-oval 564 long, 527 wide, larger than oral sucker (1:2.99), pre-equatorial.
Testes 2, obliquely tandem, contiguous or separated, entire, pre-ovarian, median, in anterior hindbody, separated from ventral sucker; anterior testis elongate-oval, 298 long, 231 wide, posterior testis elongate-oval, 293 long, 224 wide. Distance between ventral sucker and anterior testis 254. Seminal vesicle thin walled, 395 long, 184 wide, tripartite; anterior portion subspherical, 52 long, 48 wide, middle portion transversely oval, 102 long, 145 wide, posterior portion elongate-oval, 241 long, 184 wide (Figure 2d). Seminal vesicle between genital pore and middle level of ventral sucker, dorsal to ventral sucker, connected to pars prostatica by aglandular duct. Pars prostatica short, tubular, invested by prostatic cells, in mid-forebody (Figure 2d). Ejaculatory vesicle conspicuous, spherical, enclosed within sinus-sac. Sinus-sac large, elongate-oval, between pharynx and presomatic pit, with muscular wall, 228 long, 171 wide. Hermaphroditic duct enclosed within sinus-sac, short, straight, opens directly through genital pore. Genital pore median, at level of intestinal bifurcation or slightly posterior to it.
Ovary median (n = 3), dextral (n = 3) or sinistral (n = 1), spherical, subspherical, elongate-oval or transversely oval, entire, 231 long, 316 wide, in posterior half of hindbody, always separated from posterior testis by uterine coils, posterior part dorsal to vitellarium. Distance between posterior testis and ovary 542. Vitellarium in two lateral compact masses, divided into three and four digitiform lobes, 329 long, 516 wide. Juel’s organ and Mehlis’ gland not observed. Uterus coiled, restricted to body or extends to 15% of length of ecsoma (n = 1). Metraterm passes into sinus-sac ventrally, joins male duct just distally to ejaculatory vesicle forming hermaphroditic duct. Eggs numerous, 16–20 × 10–12 (n = 10).
Excretory vesicle not observed; excretory pore terminal.
Remarks: Specimens found in the present study correspond well to the generic diagnosis of Lecithochirium in characters as mentioned above. Following the key to the species-group of Lecithochirium proposed by Bray [68], our specimens belong to the ‘Microstomum-group’. This diagnosis is based on the presence of a non-glandular presomatic pit, a vitellarium of compact masses with distinct and digitiform lobes, terminal genitalia of the ‘musculus’ type, a non-muscular seminal vesicle, and the absence of internal elevations of the ventral sucker.
In comparison to species from the ‘Microstomum-group’, our specimens can be distinguished from L. alectis in the position of the seminal vesicle (antero-dorsal to the ventral sucker vs. entirely anterior to the ventral sucker), from L. mecosaccum, L. antennari, L. chaetodontis, and L. maomao in the position of the testes (obliquely tandem vs. obliquely symmetrical) and from L. priacanthi in the form of a preacetabular pit (poorly developed vs. well developed into typical sucker).
Morphologically, our material closely resembles L. manteri Teixeira de Freitas and Gomes, 1971 and L. microstomum Chandler, 1935, in possessing oblique tandem testes separated from the ventral sucker and a similar ratio of the suckers (1:2.53–3.12 vs. 1:2.81–3.37 vs. 1:2.5–2.8, respectively). However, our material can be distinguished from L. manteri of Teixeira de Freitas and Gomes [69] in possessing a narrower body (641–769 vs. 1040–1310), a smaller pharynx (95–106 × 93–111 vs. 110–120 × 120–130), in partition of seminal vesicle (tripartite vs. bipartite), and a smaller ovary (184–271 × 239–292 vs. 330–420 × 350–430).
Our specimens most closely resemble L. microstomum from the Largehead hairtail, T. lepturus collected in Galveston Bay, Texas, USA particularly in body length (3104–4292 vs. 2750–4800) and by the presence of an oesophagus and tripartite seminal vesicle. However, our specimens differ from the material of Chandler [70] by possessing a narrower body (641–769 vs. 875–1000) and more elongate eggs (18–26 × 10–15 vs. 16–12). The dimensions of our material fall within those of Teixeira de Freitas and Kohn [71], except for the posterior testis width (187–242 vs. 250–450), the seminal vesicle length (188–462 vs. 670–900), and the ovary length (239–292 vs. 330–430). In comparison with the material of Wallet and Kohn [66], our specimens differ as they possess a more elongate oral sucker (170–198 vs. 140–150), a shorter pharynx (95–106 vs. 190–210), a more elongated anterior testis (244–302 vs. 180–230), and a more elongated ovary (184–271 vs. 150–180). Morphology and dimensions of our specimens overlap with specimens collected from fish hosts of the families Carangidae, Engraulidae, Fistularidae, Lutjanidae, and Scombridae in Chamela Bay, Jalisco, Mexico, northeastern Pacific Ocean by León-Règagnon et al. [39]. In comparison with material collected from E. anchoita off the Argentinean and Uruguayan coasts, southwestern Atlantic Ocean by Timi et al. [64], the maxima for body and most internal organs of our specimens are much higher, except for the seminal vesicle width (65–236 vs. 25–74) and the egg size (18–26 × 10–15 vs. 15–19 × 8–11) for which the dimensions overlap.
Lecithochirium microstomum is a parasite of a variety of marine fishes. To date, this species has been reported in fishes belonging to at least 26 families (Anguillidae, Ariidae, Batrachoididae, Bothidae, Carangidae, Centropomidae, Coryphaenidae, Engraulidae, Fistulariidae, Gempylidae, Gerreidae, Labridae, Lutjanidae, Malacanthidae, Paralichthyidae, Percophidae, Phycidae, Pinguipedidae, Pomatomidae, Rachycentridae, Sciaenidae, Scombridae, Serranidae, Sparidae, Synodontidae, and Trichiuridae), with the majority of records coming from the western Atlantic Ocean and eastern Pacific Ocean [9,39,72,73,74]. Razarihelisoa [75] reported this species from Bothus pantherinus (Rüppell) in Nosy Be, Madagascar, southwestern Indian Ocean. Kardousha [76] reported this species from Euthynnus affinis (Cantor) in the Arabian Gulf, northwestern Indian Ocean.
In Brazil, L. microstomum has been reported from fishes of 11 families (Carangidae, Engraulidae, Gempylidae, Gerreidae, Paralichthyidae, Percophidae, Phycidae, Pinguipedidae, Sciaenidae, Scombridae, and Serranidae) [9,67]. Teixeira de Freitas and Kohn [71], Wallet and Kohn [66], Silva et al. [77,78], and Carvalho and Luque [79] reported L. microstomum from the same fish host, T. lepturus, and the same locality as in the present study. Newly collected material of L. microstomum in the present study has allowed us to provide a detailed description of the species together with DNA sequence data.
- Lecithochirium cf. muraenae Manter, 1940
- Host: Gymnothorax vicinus (Castelnau) (Muraenidae).
- Infection rates: one out of two; four specimens in total.
- Representative DNA sequences: OP918128 (28S); OP918138 (ITS2); OP918023, OP948305 (cox1).
- Voucher material: CHIOC 39901 a–d.
Description (Figure 3a,b). (Based on three paragenophores and one hologenophore; measurements of paragenophores in Table 4 and hologenophore in description; Figure 3a): Body small, plump, elongate, pyriform. Maximum width at middle of hindbody, 912. Tegument smooth. Forebody short 303. Ecsoma well developed, withdrawn or protruded.
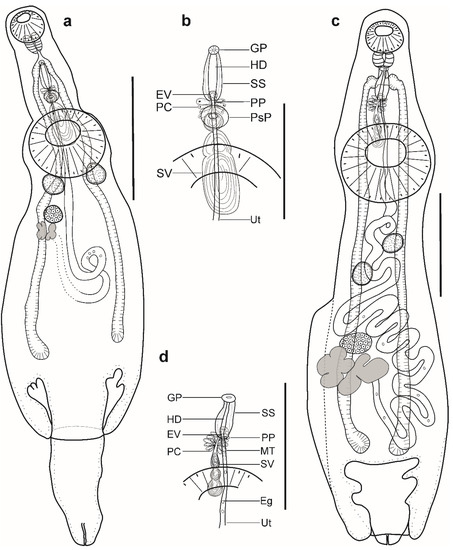
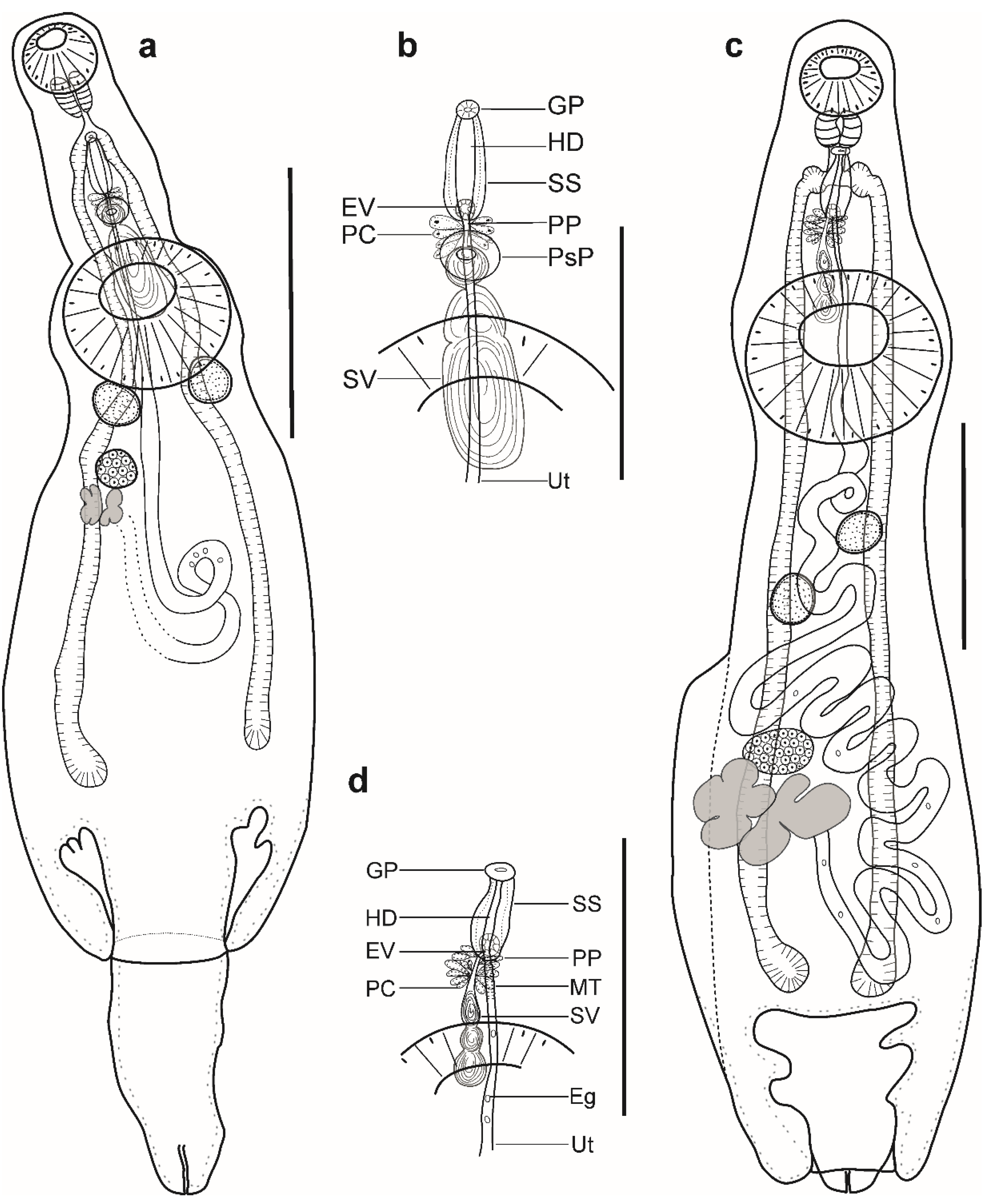
Figure 3.
Hemiurid parasites of marine fishes from Brazil. (a,b) Adult of Lecithochirium cf. muraenae ex Gymnothorax vicinus: (a) complete specimen, ventral view; (b) detail of the terminal genitalia, ventral view. (c,d) Adult of Lecithochirium sp. ex Trichiurus lepturus: (a) complete specimen, ventral view; (b) detail of the terminal genitalia, ventral view. Scale bars (a): 700 µm, (b): 350 µm, (c,d): 500. Abbreviations: Eg, eggs; EV, ejaculatory vesicle; GP, genital pore; HD, hermaphroditic duct; PC, prostatic cells; PP, pars prostatica; SS, sinus-sac; SV, seminal vesicle; Ut, uterus.
Pre-oral lobe short, 13 long. Oral sucker muscular, well developed, subspherical or transversely oval, ventro-subterminal, 160 long, 195 wide. Prepharynx absent. Pharynx muscular, well developed, subspherical or elongate-oval. Oesophagus short, 20 long. Presomatic pit present. Caeca blind, with thick walls and narrow lumen, terminates in body. Ventral sucker muscular, well developed, subspherical or transversely oval, 402 long, 439 wide, larger than oral sucker (1:2.25), pre-equatorial.
Testes 2, symmetrical, separated, entire, pre-ovarian, in anterior hindbody, contiguous with ventral sucker; dextral testis subspherical or transversely oval, 115 long, 161 wide, sinistral testis subspherical or transversely oval, 155 long, 158 wide. Seminal vesicle thin walled, tripartite, anterior and median portions subspherical, posterior portion elongate-oval (Figure 3d). Seminal vesicle between presomatic pit and middle level of ventral sucker, antero-dorsal to ventral sucker, connected to pars prostatica by aglandular duct. Pars prostatica short, tubular, invested by prostatic cells, anterior to ventral sucker (Figure 3b). Ejaculatory vesicle conspicuous, spherical, enclosed within sinus-sac. Sinus-sac elongate-oval, posterior to intestinal bifurcation, with muscular wall. Hermaphroditic duct enclosed within sinus-sac, straight, opens directly through the genital pore. Genital pore median at level of intestinal bifurcation.
Ovary dextral (n = 2) or sinistral (n = 2), entire, transversely oval, 128 long, 172 wide, in anterior half of hindbody, always separated from testes, contiguous with vitellarium. Distance between testes and ovary 63. Vitellarium in two lateral compact masses, divided into three and four short lobes, 205 long, 154 wide. Juel’s organ and Mehlis’ gland not observed. Empty portion of uterus were not observed because of thin uterine wall. Metraterm not differentiated. Eggs small, 10–13 × 9–12 (n = 10).
Excretory vesicle not observed; excretory pore terminal.
Remarks: Specimens collected in this study possess features that fully correspond to the generic diagnosis of the genus Lecithochirium in characters as mentioned above. According to the key to the species-group of Lecithochirium proposed by Bray [68], our specimens belong to the ‘Microstomum-group’ based on the presence of a non-glandular presomatic pit, the absence of internal elevations in the ventral sucker, a non-muscular seminal vesicle, terminal genitalia of the ‘musculus’ type, and a vitellarium represented by compact masses with distinct and digitiform lobes.
Comparing to the species in the ‘Microstomum-group’, our specimens could be distinguished from L. alectis in the position of the seminal vesicle (antero-dorsal to the ventral sucker vs. entirely anterior to the ventral sucker), from L. mecosaccum in possessing a short sinus-sac (116–129 vs. 170–300), and from L. antennari, L. chaetodontis, L. maomao, L. microstomum, and L. priacanthi in the position of the testes (obliquely symmetrical vs. obliquely tandem).
Our specimen is morphologically similar to L. albulae Yamaguti, 1970 described from the bonefish Albula vulpes (Linnaeus) collected in Hawaii, USA, particularly as they possess obliquely symmetrical testes, a tripartite seminal vesicle, a seminal vesicle posterodorsal to the ventral sucker, and similar ratios of suckers (1:2.32–2.47 vs. 1:2.1–2.35). However, they differ in the length of the eggs (10–14 × 9–13 vs. 14–23 × 9–13) and in the form of a preacetabular pit (poorly developed vs. well developed into typical sucker).
Morphologically, our material is most similar to the species of L. muraenae described by Manter [80], from the stomach of the Hourglass moray Muraena clepsydra Gilbert in Cape Elena, Ecuador, southeastern Pacific Ocean, in that they possess a conspicuous and non-glandular presomatic pit, symmetrical testes, a tripartite seminal vesicle, and a vitellarium consisting of two lateral masses divided into three and four short lobes. Nonetheless, specimens in our study differ from the material of Manter [80] by having smaller dimensions for all internal organs (Table 5), in the position of the genital pore (at level of intestinal bifurcation vs. posterior to intestinal bifurcation), and in possessing a straight instead of convoluted hermaphroditic duct. The variation in metrical data, in our opinion, relates to the differences in the fixation method (heat-killed fixation of our material vs. fixation under a cover glass, with the application of slight pressure of the material in Manter [80]). Considering the similarities in morphology and group of hosts (muraenids), we provisionally identify our specimens as L. muraenae.

Table 5.
Comparative metrical data of Lecithochirium spp.
Lecithochirium muraenae is a parasite of muraenid fishes. After the original description, the species was reported in Gymnothorax porphyreus Guichenot (=G. wieneri) in El Callao, Peru, southeastern Pacific Ocean [81]. Our record of L. muraenae infecting G. vicinus off the Brazilian coast potentially represents a new host and geographical record for this species. Additionally, we provide DNA sequence data that may further help to elucidate the taxonomic identity of our specimens.
- Lecithochirium synodi Manter, 1931
- Hosts: Anisotremus virginicus (Linnaeus) (Haemulidae), Pseudopercis numida Miranda Ribeiro (Pinguipedidae).
- Infection rates: A. virginicus, one out of five; four specimens in total; Ps. numida, one out of one; three specimens in total.
- Representative DNA sequences: OP918129, OP918130, OP918132 (28S); OP918139 (ITS2); OP918024, OP918026 (cox1); Ps. numida: OP925860 (cox1).
- Voucher material: CHIOC 39906 a,b; CHIOC 39907 a,b.
Remarks: Specimens found in the present study correspond well to the generic diagnosis of Lecithochirium in characters as mentioned above. Our specimens collected from the haemulid A. virginicus and from the pinguipedid Ps. numida are morphologically similar to those described from A. thazard by Pantoja et al. [7] in Rio de Janeiro, Brazil. The identification of our specimens as L. synodi was confirmed via comparative sequence analyses, which demonstrated that the 28S rDNA sequence of our isolate of Lecithochirium was identical to two isolates of L. synodi reported by Pantoja et al. [7]. Their cox1 sequences differed by 0.23–0.45% (1–2 nt).
Lecithochirium synodi is a parasite of the stomach of a variety of marine fish species. To date, this species has been reported from fishes from at least six families (Haemulidae, Monocanthidae, Paralichthyidae, Pinguipedidae, Scombridae, and Synodontidae), including the present data, with the majority of records coming from the western Atlantic Ocean [7]. Our record of L. synodi infecting A. virginicus and Ps. numida collected off the Brazilian coast represents two new host records for this species. We do not provide the description of the specimens of this species because a detailed morphological description of this species with DNA sequence data was recently provided by Pantoja et al. [7].
- Lecithochirium sp.
- Host: Trichiurus lepturus (Linnaeus) (Trichiuridae).
- Infection rates: one out of one; three specimens in total.
- Representative DNA sequences: not available.
- Voucher material: CHIOC 39905.
Description (Figure 3c,d). (Based on one specimen; measurements in Table 5): Body elongate, dorso-ventrally flattened. Maximum width close to posterior body extremity. Tegument smooth. Forebody short. Ecsoma well developed, withdrawn.
Pre-oral lobe distinct. Oral sucker muscular, well developed, spherical, ventro-subterminal. Prepharynx absent. Pharynx muscular, well developed, subspherical. Oesophagus short. “Drüsenmagen” present. Presomatic pit absent. Intestinal bifurcation in anterior forebody. Caeca blind, with thick walls and narrow lumen, terminates close to posterior extremity of body. Ventral sucker muscular, well developed, subspherical, larger than oral sucker, pre-equatorial.
Testes 2, oblique, separated, entire, pre-ovarian, in anterior hindbody, separated from ventral sucker; dextral testis elongate-oval, sinistral testis subspherical. Post-testicular field almost half of body length. Seminal vesicle thin walled, tripartite; anterior portion pyriform; median and posterior portions subspherical (Figure 3d). Seminal vesicle between intestinal bifurcation and middle level of ventral sucker, antero-dorsal to ventral sucker, connected to pars prostatica by aglandular duct. Pars prostatica short, tubular, invested by prostatic cells, anterior to ventral sucker (Figure 3b). Ejaculatory vesicle conspicuous, spherical, enclosed within sinus-sac. Sinus-sac large, elongate-oval, with muscular wall, at level of intestinal bifurcation. Hermaphroditic duct enclosed within sinus-sac, straight, opens directly through the genital pore. Genital pore just posterior to pharynx.
Ovary dextral, entire, transversely oval, in posterior half of hindbody, always separated from posterior testis by uterine coils, contiguous with vitellarium. Vitellarium in two lateral compact masses, divided into three and four short lobes. Juel’s organ and Mehlis’ gland not observed. Uterus coiled, restricted to body. Metraterm passes into sinus-sac ventrally, joins male duct just distally to ejaculatory vesicle forming hermaphroditic duct. Eggs numerous, small.
Excretory vesicle not observed; excretory pore terminal.
Remarks: The specimen found in the present study correspond well to the generic diagnosis of Lecithochirium in characters as mentioned above. Following the key to the species-group of Lecithochirium proposed by Bray [68], our specimen belongs to the “Musculus-group” based on the absence of a presomatic pit, the presence of a vitellarium comprised of compact masses with distinct and short digitiform lobes, a terminal genitalia of the “musculus” type, a non-muscular seminal vesicle, and the absence of internal elevations in the ventral sucker.
In comparison to species from the “Musculus-group”, our specimen can be distinguished from all other species; from L. brevicirrus (Nicoll, 1915), L. floridense (Manter, 1934), L. medius Acena, 1941, L. microcercus (Manter, 1947) and L. musculus (Looss, 1907) in the position of the testes (not contiguous with the ventral sucker vs. contiguous or just posterior to the ventral sucker or overlapping it), from L. imocavus by having a distinctly wider body (694 vs. 250–400) and from L. monticellii (Linton, 1898) in the position of the testes (contiguous vs. separated by uterine coils).
Our single specimen closely resembles L. monticellii collected from the stomach of T. lepturus in the Atlantic Ocean, Rio de Janeiro, Brazil, by França et al. [15], in that it possesses a genital pore at or slightly posterior to the pharynx, a tripartite seminal vesicle, and testes being separated from the ventral sucker. In comparison with large specimens collected by França et al. [15], our specimen differs by having a wider body (694 vs. 220–520), a longer sinus-sac (179 vs. 50–100), a slightly larger sucker ratio (1:2.46 vs. 1:1.95–2.41), and shorter eggs (11–15 × 9–11 vs. 23–28 × 9–12). In comparison with small specimens collected by França et al. [15], our specimen differs by having a longer body (2607 vs. 1020–1900), a wider body (694 vs. 220–500), a longer seminal vesicle (217 vs. 50–170), a longer sinus-sac (179 vs. 20–100), and shorter eggs (11–15 × 9–11 vs. 15–28 × 10–13) (Table 5). Although our material most resembles the material of França et al. [15], the species identification provided by these authors should be interpreted with caution. Their specimens differ from the type material of L. monticellii described by Linton [82], specifically regarding the position of the testes (separated vs. contiguous in Linton [82]).
Out of the “Musculus-group”, our specimen closely resembles L. trichiuri (‘Keokeo-group’) described from the same host, T. lepturus (=T. haumela) in the China Sea by Gu and Shen [83]; it is similar in body shape, it possesses testes separated from the ventral sucker, and has a similar ratio of the suckers (1:2.46 vs. 1:2.4–2.9). However, our specimen lacks a presomatic pit, whereas L. trichiuri possess a presomatic pit.
Due to the presence of a single specimen in our material and difficulties in assigning this specimen to any known species, we identify it to the genus level as Lecithochirium sp.
3.2. Molecular Results
In this study, 30 novel sequences were generated for 16 isolates. The phylogenetic relationships of the studied species of the Hemiuridae were assessed based on the partial 28S rDNA sequences. Sequences of ITS1-5.8S-ITS2, ITS2, and cox1 were used to calculate the pairwise genetic distances between species and to contribute to a growing DNA sequence library for the Hemiuridae. The phylogenetic tree obtained from the BI analysis based on Alignment 1 (28S rDNA; 1071 nt) is presented in Figure 4. Pairwise genetic distances of this dataset are presented in Table S1. Novel 28S rDNA sequences (n = 14) of seven species were positioned in different clades with the members of the family Hemiuridae.
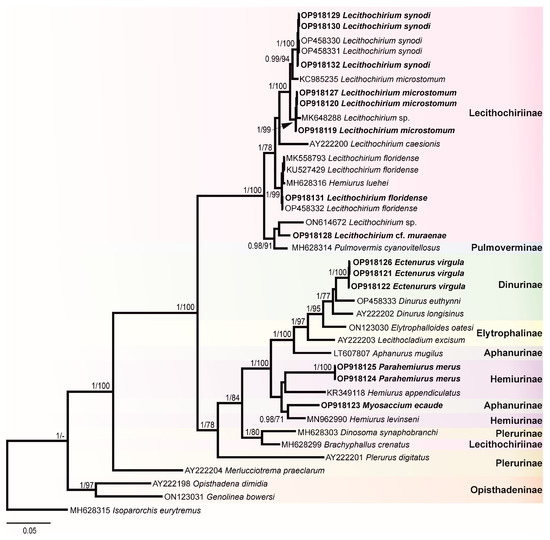
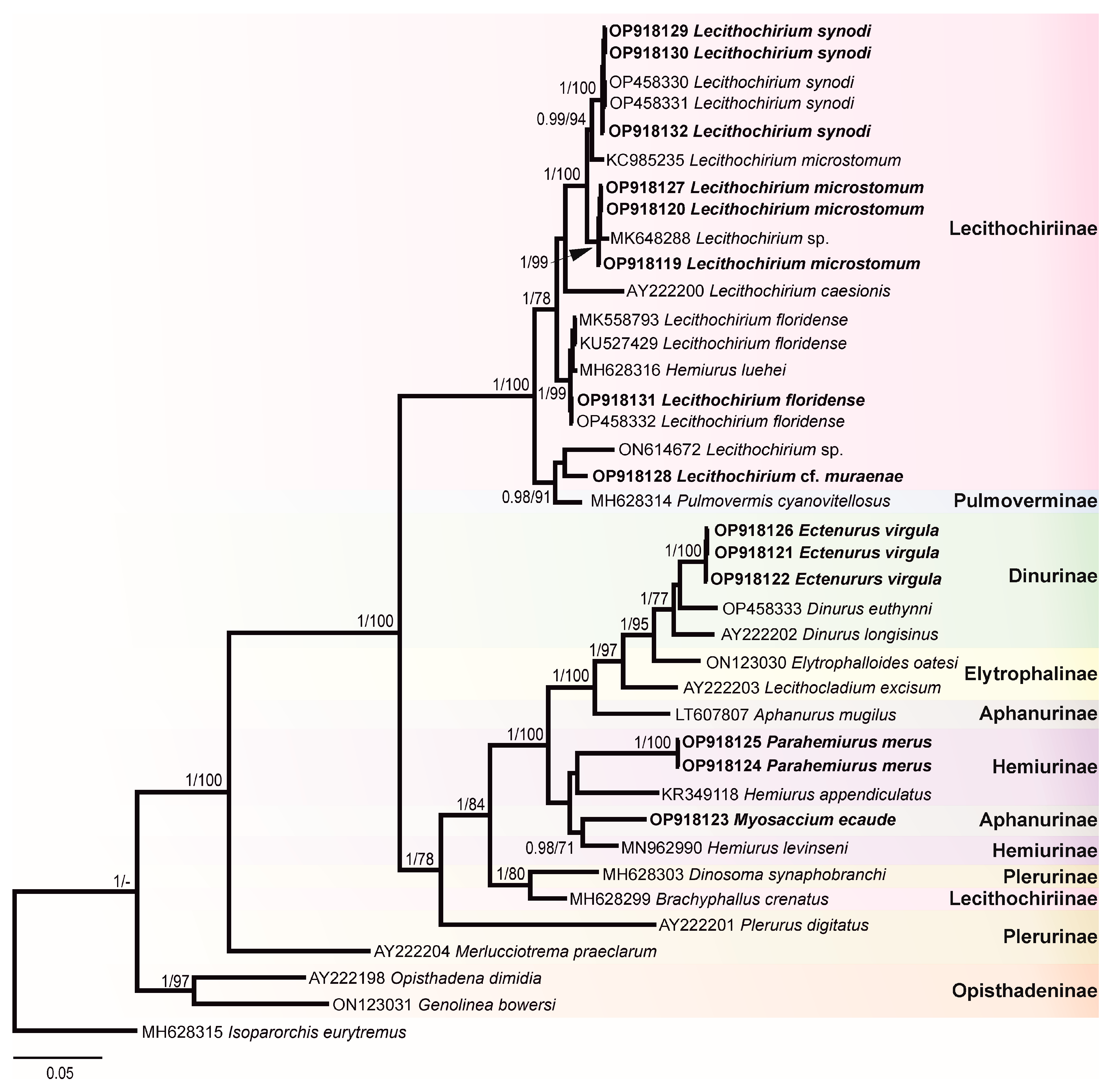
Figure 4.
Phylogenetic tree resulted from Bayesian inference (BI) analysis of the 28S rDNA sequences datasets of the Hemiuridae with nodal support values shown at the node as BI/ML (maximum likelihood). Support values <0.90 (BI) and 70 (ML) are not shown. Sequences generated in the present study are highlighted in bold.
The most surprising result of our molecular analyses is that a sequence of M. ecaude (OP918123), a member of the subfamily Aphanurinae according to classifications based on morphology, clustered with a sequence of Hemiurus levinseni (MN962990) (a member of the Hemiurinae) collected from Cylichna alba (Brown) in White Sea, Russia with strong support. Two identical sequences of P. merus generated in the present study (members of the Hemiurinae) and a sequence of Hemiurus appendiculatus (Rudolphi, 1802) clustered in the same clade, albeit with low support. The sequence divergence between M. ecaude and He. levinseni, He. appendiculatus, and P. merus was 4.84–9.12% (50–79 nt). In comparison with Aphanurus mugilus Tang, 1981 (LT607807), a species of the type genus of the Aphanurinae, our sequence of M. ecaude differed by 8.32% (86 nt). This result suggests that the position of M. ecaude within the Aphanurinae requires revaluation.
Our molecular phylogenetic analyses confirmed the positions of E. virgula within the subfamily Dinurinae and Lecithochirium spp. within the subfamily Lecithochiriinae. The three sequences of E. virgula (OP918121, OP918122, and OP918126) were identical and clustered with sequences of members of the same subfamily Dinurinae, Di. euthynni (OP458333) collected from A. thazard in Rio de Janeiro, Brazil, and Di. longisinus (AY222202) collected from Coryphaena hippurus Linnaeus, Jamaica. The sequence divergence between these three species ranged from 3.21 to 3.80% (33–49 nt).
All sequences generated for species of Lecithochirium in this study clustered in a strongly supported clade. The three novel sequences of L. synodi (GenBank OP918129, OP918130, and OP918132) clustered with sequences of two isolates of the same species (OP458330 and OP458331) found in A. thazard in Rio de Janeiro, Brazil and L. microstomum Chandler, 1935 (KC985235) collected from T. lepturus in the USA. All five sequences of L. synodi were identical. The three novel sequences of L. microstomum (OP918119, OP918120, and OP918127) were identical and clustered with Lecithochirium sp. (MK648288) collected from the same host, T. lepturus in Veracruz, Mexico in the same strongly supported subclade. The sequence divergence between our isolates and the isolate from Mexico was 0.47% (5 nt). The three identical novel sequences of L. microstomum (OP918119, OP918120, and OP918127) differed from L. microstomum (KC985235) by 1.5% (16 nt). The novel sequence of L. floridense (OP918131) from Pe. brasiliensis grouped with sequences of three isolates of the same species (Figure 4) previously reported from S. papillosum in Yucatan Shelf, Mexico (MK558793), in Pterois volitans from Northern Gulf of Mexico, USA (KU527429) and in A. thazard from Rio de Janeiro, Brazil (OP458332) and with the sequence of He. luehei (MH628316). Krupenko et al. [84] reported that the sequence named as He. luehei and published by Sokolov [12] was misidentified. The position of the sequence of He. luehei in this clade was also discussed in Pantoja et al. [7]. The intraspecific divergence between five isolates was 0–0.21% (0–2 nt).
Sequences of Lecithochirium cf. muraenae collected from G. vicinus clustered with the sequence of an unidentified species of Lecithochirium collected from Octopus bimaculatus Verrill, 1883 from Baja California, Mexico, albeit with low support. The third sequence in the same clade was of Pulmovermis cyanovitellosus Coil & Kuntz, 1960 (MH628314) collected from Laticauda semifasciata (Reinwardt) in Ishigaki Island, Japan; the sequence divergence within this clade was 2.81–4.02% (30–43 nt) with sequences of Lecithochirium cf. muraenae and Pu. cyanovitellosus showing the lowest sequence divergence. The interspecific divergence within the clade containing Lecithochirium spp. was 1.21–6.82% (13–73 nt), with Lecithochirium microstomum and L. synodi demonstrating the lowest interspecific divergence, and L. caesionis and Lecithochirium sp. (ON614672) demonstrating the highest interspecific divergence.
Pairwise genetic distances of Alignment 2 containing ITS2 sequences (416 nt) Lecithochirium spp. is presented in Table S2. The ITS2 sequences of L. synodi from A. virginicus (OP918139) and A. thazard (OP458331) were identical. The interspecific divergence within Lecithochirium spp. available for comparison was 1.92–24.33% (8–100 nt), with L. microstomum and L. synodi exhibiting the lowest interspecific divergence, and L. synodi and L. cf. muraenae exhibiting the highest interspecific divergence. The ITS2 sequences of E. virgula (OP918136) and Di. euthynni (OP458333) from A. thazard in Rio de Janeiro, Brazil (both from the subfamily Dinurinae) differed by 2.97% (13 nt).
Pairwise genetic distances of Alignment 3 containing cox1 sequences (443 nt) of Lecithochirium spp. is presented in Table S3. The intraspecific divergence of L. synodi collected from A. virginicus (OP918024), Ps. numida (OP918026), and A. thazard (OP418194) was 0.23–0.45 (1–2 nt). The intraspecific divergence of L. microstomum from T. lepturus (OP918021) and Pr. punctatus (OP918022) was 1.13% (5 nt). The sequences of L. floridense from P. brasiliensis (OP918025) and A. thazard (OP418195) were identical. The interspecific divergence between Lecithochirium spp. available for comparison was 14.9–26% (66–115 nt), with L. cf. muraenae exhibiting the highest interspecific divergence from other Lecithochirium spp.
4. Discussion
Following combined morphological and molecular analyses, we report on eight hemiurid species belonging to four genera, namely: Ectenurus virgula, L. floridense, L. microstomum, Lecithochirium cf. muraenae, L. synodi, Lecithochirium sp., Myosaccium ecaude, and Parahemiurus merus. Our results add new data on the geographical distribution, host associations, and the first DNA sequence data for five hemiurid species.
According to the World Register of Marine Species [85], the genus Ectenurus is currently represented by 28 species globally. In Brazil, only two species have been reported, E. virgula and E. yamagutii, by Nahhas and Powell, 1971 [9] from fishes of three families, Carangidae, Haemulidae, and Phycidae. Ectenurus virgula was previously reported in the carangid O. saurus and in the phycid Urophycis brasiliensis (Kaup) [59,61]. We found this species in taxonomically different hosts, the carangid D. punctatus, the haemulid A. virginicus, and the triglid Pr. punctatus, and confirmed E. virgula to be an euryxenous parasite. The relative position of E. virgula to Di. euthynni and Di. longisinus in our phylogenetic analysis (Figure 4) confirmed its taxonomic position within the subfamily Dinurinae. Notably, the DNA sequence divergence between E. virgula and Di. euthynni was lower than the divergence between congeneric Di. euthynni and Di. longisinus (3.27%, 34 nt vs. 3.80%, 39 nt, respectively). Morphologically, members of both genera are similar in that they have a plicated body surface, a partitioned seminal vesicle, and the presence of a sinus-organ. However, they differ in the length of the pars prostatica.
Lecithochirium is one of the most speciose genera of the family Hemiuridae. The species recognition of the genus, in our view, is often problematic due to the poor descriptions for many species and the lack of critical differentiation analysis at the time of description. Thus, the true species composition of this genus can only be assessed by new, accurate, and precise work, including detailed morphological descriptions together with the incorporation of DNA sequence data. In the present study, the genus Lecithochirium is the richest and is represented by five species. Two of these, L. floridense and L. synodi, were recently reported from A. thazard in Brazil with a morphological description and DNA sequences provided [7]. Therefore, the identification of these species was based on both morphological and comparative sequence analyses. Our findings suggest that the host spectrum for both species is wider. Although L. floridense is reported for the first time in a percophid fish and L. synodi in haemulid and pinguipedid fishes, the euroxenous nature of both species was already observed in previous studies [7,86].
For the three remaining species of Lecithochirium, we provided detailed morphological descriptions, with two of them being genetically characterised, in turn, further helping to avoid ambiguities in species delimitation. Lecithochirium microstomum is an euryxenous parasite widely spread across the tropical and subtropical Atlantic and Pacific oceans [87]. This species is the most reported trematode species in marine fishes form Brazil. So far, fishes from 11 families of 8 orders are known to be the hosts of L. microstomum in the country [9,67]. Our report of L. microstomum in a triglid fish, Pr. punctatus, increased the host spectrum for this species. This species was previously reported five times in the same host, T. lepturus, and in the same locality in Brazil as in the present study [9]. Although numerous records of L. microstomum are available, there were no associated DNA sequence data for this species in Brazil. However, one 28S rDNA sequence of L. microstomum (KC985235) from T. lepturus collected in Gulf of Mexico, Mississippi, USA, by Calhoun et al. [88] is available and our isolate clustered in the same clade with it; the intraspecific sequence divergence was considered high (1.5%; 16 nt) when compared with the intraspecific sequence divergence observed in other Lecithochirium spp. (Figure 4 and Table S1). A morphological description was not provided with the published sequence, and therefore we cannot compare the morphology of the isolates. Based on morphological features and comparisons of the metrical data, our specimens agree with the descriptions of L. microstomum. An unidentified species of Lecithochirium (GenBank number MK648288) collected from T. lepturus in Veracruz, Mexico [89]—even with the lack of a reference morphological voucher for this sequence—is likely conspecific with L. microstomum found in the present study. The difference between the 28S rDNA sequences of our isolates and the isolate from Mexico was low, 0.47% (5 nt).
Molecular data showed that L. synodi and L. microstomum are closely related, although they belong to different morphological groups, i.e., “Synodi-group” and “Microstomum-group”. Morphological similarities are also remarkable, i.e., the absence of internal elevations of the ventral sucker, a tripartite seminal vesicle, the presence of a presomatic pit, obliquely tandem testes, and testes separated from the ventral sucker. A morphological feature that separates L. synodi and L. microstomum between different groups according to the key of Bray [68] is the type of presomatic pit (glandular in the “Synodi-group” and non-glandular in the “Microstomum-group”). Our specimens of L. microstomum possess a non-glandular presomatic pit. However, León-Regàgnon and co-authors [39] examined the type material of L. microstomum and concluded that the presomatic pit is glandular in the material of Chandler [70]. Due to the genus being specious, at the present stage it is not possible to identify which features are more phylogenetically important. Detailed morphological descriptions including molecular data are only available for 5 out of more than 100 nominal species of the genus [7,68,90].
Specimens tentatively identified as L. muraenae in our study were reported for the first time in G. vicinus and in Brazil. Prior to our study, this species was only known from the Pacific Ocean [80,81]. The record of this species in the Atlantic Ocean demonstrates that its geographical distribution is possibly wider than previously reported. Lecithochirium muraenae is a stenoxenous parasite infecting muraenid fishes. DNA sequence data for L. muraenae from muraenids in the Pacific Ocean were not available for comparison.
Interestingly, isolates of Lecithochirium cf. muraenae clustered distantly from other Lecithochirium spp. recorded in this study. It formed a well-supported clade with an unidentified species of Lecithochirium (ON614672) collected from Octopus bimaculatus in Mexico [90] and Pu. cyanovitellosus (the subfamily Pulmoverminae). Although the specimens from octopuses were immature, there are a couple of morphological similarities that we could observe when comparing to specimens of Lecithochirium cf. muraenae, these are: the position of the genital pore at the level of intestinal bifurcation, a blind caeca terminating in the body, symmetrical testes positioned laterally posterior to the ventral sucker, and an ovary closely posterior to dextral or sinistral testis. Morphologically, Lecithochirium cf. muraenae and Pu. cyanovitellosus conspicuously differ in body shape (plump, piriform vs. elongate, slender), the size of the ecsoma (well-developed vs. reduced), the shape and size of the seminal vesicle (short and straight vs. long and convoluted), and the position of the testes in relation to the ventral sucker and to each other (contiguous with the ventral sucker and symmetrical vs. separated from the ventral sucker and tandem). Additionally, these species parasitise different groups of hosts (fish vs. sea-snake) and were found in different sites in the hosts (stomach vs. lung). The published sequence of Pu. cyanovitellosus was not supplemented with either morphological descriptions of the isolate or an electronic voucher, and thus no data is available for morphological comparison of the actual isolates.
Based on their morphology, species of L. muraenae and L. microstomum were placed in the same group, the “Microstomum group” [68]. However, the comparative sequence analysis suggests that these species are rather genetically distant, and thus further revaluation of morphotype groups with DNA sequence data, including data for the type-species, L. rufoviride (Rudolphi, 1819), will aid in the identification of a valid composition of each group.
Currently, and including our data, several species of Lecithochirium are reported from Brazil, namely: L. monticellii (Linton, 1898), L. imovacus (Looss, 1907), L. microstomum Chandler, 1935, L. texanum (Chandler, 1941), L. zeloticum (Travassos, Teixeira de Freitas and Buhrnheim, 1966), L. manteri Teixeira de Freitas and Gomes, 1971, L. perfidum Gomes, Fabio and Rolas, 1972, L. floridense (Manter, 1934) and L. synodi Manter 1931 [7]. The record of L. cf. muraenae in the present study increased the diversity of Lecithochirium to 10 species in marine fish off the Brazilian coast, confirming that this genus is the most diverse in marine fishes in the region. Species of Lecithochirium are parasites of fishes from 14 families in the region [7,9].
The genus Myosaccium accommodates only two species which parasitise marine fishes of the Clupeidae and Carangidae, with their distribution restricted to the Atlantic and Pacific oceans [39]. Due to strong morphological similarity, Overstreet [46] suggested M. opisthonemae to be a synonym of M. ecaude. Although specimens of M. opisthonemae are smaller than those identified as M. ecaude, the main feature used to distinguish between them—the presence or absence of spines and polar filament on eggs—was demonstrated to be erroneous. Molecular genetic characterisation of smaller specimens is needed to assess the taxonomic status of M. opisthonemae. Myosaccium ecaude is a stenoxenous parasite and has previously been reported only in clupeid fishes, whereas M. opisthonemae is an euryxenous parasite and has been reported in clupeid and carangid fishes [9,16].
The relative relation of M. ecaude to He. levinseni in the clade containing the members of the subfamily Hemiurinae was rather surprising (Figure 4) because the genus Myosaccium currently belongs to the subfamily Aphanurinae. We found no convincing morphological characters, potentially justifying the move of Myosaccium into the Hemiurinae. The main feature differentiating Myosaccium and Aphanurus (type genus of the Aphanurinae) is the shape of the vitellarium (two distinct masses vs. one distinct mass, respectively), whereas Hemiurus and Parahemiurus possess vitellarium composed of two distinct masses. The taxonomic value of this character for subfamily classification is still to be confirmed.
The genus Parahemiurus is represented by 22 species [91]. In Brazil, only two species have been reported, the type-species P. merus and P. anchoviae Pereira and Vaz, 1930 [9]. Parahemiurus merus is an euryxenous parasite tending to be most frequently reported in temperate pelagic fishes and is rarely reported in the vast area covered by the tropical Indo-West Pacific region [65]. This species is the second most reported trematode species in marine fishes in Brazil. So far, fishes from 12 families of 9 orders are known to be the hosts of P. merus in Brazil [9]. Despite numerous records of P. merus, there were no DNA sequence data available for this species. Accordingly, and for the first time, we provided DNA sequences for the genus Parahemiurus and confirmed its position within the Hemiurinae based on molecular data.
According to the present and previous phylogenetic analyses of Atopkin et al. [11], Faltýnková et al. [92], and Sokolov et al. [12], it is evident that the subfamily classifications of the Hemiuridae, based entirely on morphological characters, needs to be reconsidered taking into account a wide range information sources, including morphological, molecular, and ecological data. Although the molecular library of the Hemiuridae is slowly growing, the lack of DNA sequences of numerous taxa, the limitation of data from single conservative molecular markers, and the absence of morphological vouchers of the actual isolates used to generate sequences, represents an impediment for the clarification of the taxonomic content of the family. Accordingly, such information is also imperative for understanding the value of the morphological features used to define subfamilies.
5. Conclusions
Based on morphological and molecular genetic analyses, we identified eight hemiurid species belonging to four genera parasitising marine fishes in Brazil. This is the first study to report phylogenetic relationships based on 28S rDNA sequences of members of three hemiurid genera, Ectenurus, Myosaccium, and Parahemiurus. These data contribute to the knowledge of the systematics and evolutionary history of the Hemiuridae which remains incomplete. In phylogenetic analysis, the aphanurines Myosaccium and Aphanurus were rendered paraphyletic. This data casts doubts over the traditional subfamily classification based on the morphology of the members from these genera, which will need to be rearranged in the future when a higher number of sequenced hemiurid taxa will be available. The record of the specimens tentatively identified as Lecithochirium muraenae increases the number of hemiurid species off the Brazilian coast. The first records of digenean trematodes in the fishes Anisotremus virginicus and Decapterus punctatus in the country, indicates the relatively poor coverage of Brazilian marine digenean fauna. Studies on marine fish trematodes using morphological and molecular datasets remain in their infancy in Brazil. Our findings highlight the importance of exploring this region, which is considered as a parasite biodiversity hotspot [93], and the need to describe digenean diversity using integrative data.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12233355/s1, Figure S1: Photomicrographs of the molecular vouchers of fish; Table S1: Pairwise comparisons of genetic distances of the Hemiuridae based on 28S rDNA sequences (957 nt long alignment); Table S2: Nucleotide comparison of the partial ITS2 sequences of Lecithochirium spp. based on 416 nt long alignment. Table S3: Nucleotide comparison of cox1 sequences of Lecithochirium spp. based on 443 nt long alignment.
Author Contributions
Conceptualization, C.P. and O.K.; methodology, C.P. and O.K.; data curation, C.P.; writing—original draft preparation, C.P. and O.K.; writing—review and editing, C.P. and O.K.; project administration, C.P. and O.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Research Council of Lithuania, grant number 09.3.3-LMT-K-712-19-0182.
Institutional Review Board Statement
This study was conducted applying national and international guidelines. All procedures involving animals were conducted in accordance with the Ethics Committee on the Use of Animals (CEUA), Institute of Veterinary, Federal Rural University of Rio de Janeiro, Brazil. Species registration for scientific research purposes was carried out at SisGen (A255046) according to Brazilian legislation on access to the biodiversity.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated in this study are available from the corresponding author upon request.
Acknowledgments
We are grateful to Aline Carvalho Azevedo, Bruno Telles (UFRRJ, Brazil), and Fabiano Paschoal (UFMA, Brazil) for their invaluable help with the collection and dissection of fish.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miloslavich, P.; Klein, E.; Díaz, J.M.; Hernández, C.E.; Bigatti, G.; Campos, L.; Artigas, F.; Castillo, J.; Penchaszadeh, P.; Neill, P.E.; et al. Marine Biodiversity in the Atlantic and Pacific Coasts of South America: Knowledge and Gaps. PLoS ONE 2011, 6, e14631. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2022. Available online: https://www.fishbase.org, version (02/2022) (accessed on 20 October 2022).
- Krasnov, B.R.; Poulin, R. Relationships between Parasite Diversity and Host Diversity. In Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics; Morand, S., Krasnov, B.R., Littlewood, D.T.J., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 27–38. [Google Scholar]
- Kohn, A.; Fernandes, B.M.; Cohen, S.C. South American Trematodes Parasites of Fishes; Fiocruz, Instituto Oswaldo Cruz: Rio de Janeiro, Brazil, 2007; p. 318. [Google Scholar]
- Luque, J.; Pereira, F.; Alves, P.; Oliva, M.; Timi, J. Helminth parasites of South American fishes: Current status and characterization as a model for studies of biodiversity. J. Helminthol. 2017, 91, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.A.; Diaz, P.E.; Cribb, T.H. Knowledge of marine fish trematodes of Atlantic and Eastern Pacific Oceans. Syst. Parasitol. 2016, 93, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, C.; Telles, B.; Paschoal, F.; Luque, J.L.; Kudlai, O. Digenean trematodes infecting the frigate tuna Auxis thazard (Scombriformes, Scombridae) off the Rio de Janeiro coast, Brazil, including molecular data. Parasite 2022, 29, 44. [Google Scholar] [CrossRef]
- Gibson, D.I. Family Hemiuridae Looss, 1899. In Keys to the Trematoda; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CAB International and the Natural History Museum: London, UK, 2002; Volume 1, pp. 305–340. [Google Scholar]
- Eiras, J.C.; Velloso, A.L.; Pereira, J. Parasitos de Peixes Marinhos da América do Sul; FURG: Rio Grande, Brazil, 2016. [Google Scholar]
- Blair, D.; Bray, R.A.; Barker, S.C. Molecules and morphology in phylogenetic studies of the Hemiuroidea (Digenea: Trematoda: Platyhelminthes). Mol. Phylogenetics Evol. 1998, 9, 15–25. [Google Scholar] [CrossRef]
- Atopkin, D.M.; Besprozvannykh, V.V.; Beloded, A.Y.; Ngo, H.D.; Ha, N.V.; Tang, N.V. Phylogenetic relationships of Hemiuridae (Digenea: Hemiuroidea) with new morphometric and molecular data of Aphanurus mugilis Tang, 1981 (Aphanurinae) from mullet fish of Vietnam. Parasitol. Int. 2017, 66, 824–830. [Google Scholar] [CrossRef]
- Sokolov, S.G.; Atopkin, D.M.; Urabe, M.; Gordeev, I.I. Phylogenetic analysis of the superfamily Hemiuroidea (Platyhelminthes, Neodermata: Trematoda) based on partial 28S rDNA sequences. Parasitology 2019, 146, 596–603. [Google Scholar] [CrossRef]
- Louvard, C.; Cutmore, S.C.; Yong, R.Q.Y.; Dang, C.; Cribb, T.H. First elucidation of a didymozoid life cycle: Saccularina magnacetabula n. gen. n. sp. infecting an arcid bivalve. Int. J. Parasitol. 2022, 52, 407–425. [Google Scholar] [CrossRef]
- Almeida, F.D.M.; Barquete, V.; Pereira, J., Jr. Progenetic metacercariae of Parahemiurus merus (Platyhelminthes, Digenea, Hemiurudae) infecting Parasagitta friderici (Chaetognatha) from southern coast Brazil. Atlântica 2009, 31, 35–38. [Google Scholar] [CrossRef]
- França, L.F.; Knoff, M.; Fonseca, M.C.D.; Gomes, D.C.; Ferreira, M.S.; Felizardo, N.N.; São Clemente, S.C.; Mattos, D.P.D. Lecithochirium monticellii digenetic trematode parasites of Trichiurus lepturus (Actinopterygii) from the state of Rio de Janeiro, Brazil, with notes on its taxonomy. An. Da Acad. Bras. De Ciências 2020, 92, e20190161. [Google Scholar] [CrossRef]
- Chaves, L.; Paschoal, P. Community ecology of the metazoan parasites of the Atlantic thread herring, Opisthonema oglinum (Lesueur, 1818) (Actinopterygii: Clupeidae) from the Sepetiba Bay, Rio de Janeiro, Brazil. Braz. J. Biol. 2021, 81, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Pleijel, F.; Jondelius, U.; Norlinder, E.; Nygren, A.; Oxelman, B.; Schander, C.; Sundberg, P.; Thollesson, M. Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Mol. Phylogenetics Evol. 2008, 48, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Faltýnková, A.; Pantoja, C.; Skírnisson, K.; Kudlai, O. Unexpected diversity in northern Europe: Trematodes from salmonid fishes in Iceland with two new species of Crepidostomum Braun, 1900. Parasitol. Res. 2020, 119, 2439–2462. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, S.; Soldánová, M.; Pérez-Del-Olmo, A.; Dangel, D.R.; Sitko, J.; Sures, B.; Kostadinova, A. Molecular prospecting for European Diplostomum (Digenea: Diplostomidae) reveals cryptic diversity. Int. J. Parasitol. 2013, 43, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Blair, D.; McManus, D. A Molecular Phylogeny of the Human Schistosomes. Mol. Phylogenetics Evol. 1995, 4, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Springer, Y.P.; Keeney, D.B.; Poulin, R. Intra- and interclonal phenotypic and genetic variability of the trematode Maritrema novaezealandensis. Biol. J. Linn. Soc. 2011, 103, 106–116. [Google Scholar] [CrossRef]
- Wee, N.Q.-X.; Cribb, T.H.; Bray, R.A.; Cutmore, S.C. Two known and one new species of Proctoeces from Australian teleosts: Variable host-specificity for closely related species identified through multi-locus molecular data. Parasitol. Int. 2017, 66, 16–26. [Google Scholar] [CrossRef]
- Snyder, S.D.; Tkach, V.V. Phylogenetic and biogeographical relationships among some holarctic frog lung flukes (Digenea: Haematoloechidae). J. Parasitol. 2001, 87, 1433–1440. [Google Scholar] [CrossRef]
- Galazzo, D.E.; Dayanandan, S.; Marcogliese, D.J.; McLaughlin, J.D. Molecular systematics of some North American species of Diplostomum (Digenea) based on rDNA-sequence data and comparisons with European congeners. Can. J. Zool. 2002, 80, 2207–2217. [Google Scholar] [CrossRef]
- Tkach, V.V.; Littlewood, D.T.; Olson, P.D.; Kinsella, J.M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; McManus, D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Adlard, R.D.; Bray, R.A. A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). Int. J. Parasitol. 1998, 28, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Cutmore, S.C.; Miller, T.L.; Curran, S.S.; Bennett, M.B.; Cribb, T.H. Phylogenetic relationships of the Gorgoderidae (Platyhelminthes: Trematoda), including the proposal of a new subfamily (Degeneriinae n. subfam.). Parasitol. Res. 2013, 112, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, D.T.; Curini-Galletti, M.; Herniou, E.A. The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Mol. Phylogenetics Evol. 2000, 16, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, D.T.; Rohde, K.; Clough, K.A. Parasite speciation within or between host species?—Phylogenetic evidence from site-specific polystome monogeneans. Int. J. Parasitol. 1997, 27, 1289–1297. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science 1321 Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE); Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2010; pp. 1–8. [Google Scholar]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Menezes, N.A.; Figueiredo, J.L. Manual de Peixes Marinhos do Sudeste do Brasil v Teleostei (4); Museu de Zoologia USP: São Paulo, Brasil, 1985; 105p. [Google Scholar]
- Montgomery, W.R. Studies on Digenetic Trematodes from Marine Fishes of La Jolla, California. Trans. Am. Microsc. Soc. 1957, 76, 13–36. [Google Scholar] [CrossRef]
- León-Règagnon, V.; Pérez-Ponce de León, G.; Lamothe-Argumedo, R. Hemiuriformes de peces marinos de la Bahía de Chamela, México, con la descripción de una nueva especie del género Hysterolecitha (Digenea: Hemiuridae: Lecithasterinae). Ann. Inst. Biol. Zool. Ser. 1997, 68, 1–34. [Google Scholar]
- Pérez-Ponce de León, G.; García Prieto, L.; Rosas Villa, C. Helmintofauna de Opisthonema libertate y Harengula thrissina (Osteichthyes: Clupeidae) de la bahía de Chamela, Jalisco, México. Rev. De Biol. Trop. 2000, 48, 759–763. [Google Scholar]
- Sánchez-Serrano, S.; Cáceres-Martínez, J. Primer registro helmintológico de la sardina monterrey Sardinops sagax en Baja California, México, durante dos estaciones del año. Hidrobiológica 2017, 27, 1–11. [Google Scholar]
- Jacobson, K.; Baldwin, R.; Banks, M.; Emmett, R. Use of parasites to clarify residency and migration patterns of Pacific sardine (Sardinops sagax) in the California Current. Fish. Bull. 2019, 117, 196–210. [Google Scholar] [CrossRef]
- Del-Río-Zaragoza, O.B.; Hernández-Rodríguez, M.; Vivanco-Aranda, M.; Zavala-Hamz, V.A. Blood parameters and parasitic load in Sardinops sagax (Jenyns, 1842) from Todos Santos Bay, Baja California, Mexico. Lat. Am. J. Aquat. Res. 2018, 46, 1110–1115. [Google Scholar] [CrossRef]
- Luque, J.L.; Vinas, R.A.; Paraguassú, A.R.; Alves, D.R. Metazoários Parasitos das sardinhas Sardinella brasiliensis e Harengula clupeola (Osteichthyes, Clupeidae) do litoral do Estado do Rio de Janeiro, Brasil. Rev. Univ. Rural. Ser. Ciências Da Vida 2000, 22, 71–76. [Google Scholar]
- Moreira, J.; Paschoal, F.; Cezar, A.; Luque, J. Community ecology of the metazoan parasites of Brazilian sardinella, Sardinella brasiliensis (Steindachner, 1879) (Actinopterygii: Clupeidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Braz. J. Biol. 2015, 75, 736–741. [Google Scholar] [CrossRef]
- Overstreet, R.M. Digenetic Trematodes of Marine Teleost Fishes from Biscayne Bay, Florida; University of Miami: Coral Gables, FL, USA, 1969. [Google Scholar]
- Siddiqi, A.H.; Cable, R.M. Digenetic trematodes of marine fishes of Puerto Rico. Sci. Surv. Porto Rico Virgin Isl. 1960, 17, 257–369. [Google Scholar]
- Kohn, A.; Buhrnheim, P.F. A new host and new geographic distribution for Myosaccium ecaude Montgomery, 1957 (Trematoda, Hemiuridae). Atas Soc. Biol. 1964, 8, 50–52. [Google Scholar]
- Linton, E. Helminth Fauna of the Dry Tortugas II. Trematodes. In Papers from the Tortugas Laboratory of the Carnegie Institute of Washington; General Books LLC: Memphis, TN, USA, 1910; Volume 4, pp. 11–98. [Google Scholar]
- Manter, H.W. The digenetic trematodes of marine fishes of Tortugas, Florida. Am. Midl. Nat. 1947, 38, 257–416. [Google Scholar] [CrossRef]
- Sparks, A.K. Some digenetic trematodes of marine fishes of the Bahama Islands. Bull. Mar. Sci. Gulf Caribb. 1957, 7, 255–265. [Google Scholar]
- Szidat, L. Versuch Einer Zoogeographie des Sud-Atlantik Mit Hilfe von Leitparasiten der Meeresfische; Parasitologische Schriftenreihe; Fischer: New York, NY, USA, 1961; Volume 13, pp. 1–98. [Google Scholar]
- Nahhas, F.M.; Cable, R.M. Digenetic and aspidogastrid trematodes from marine fishes of Curaçao and Jamaica. Tulane Stud. Zool. 1964, 11, 169–228. [Google Scholar] [CrossRef]
- Rees, G. Some helminth parasites of fishes of Bermuda and an account of the attachment organ of Alcicornis carangis MacCallum,1917 (Digenea: Bucephalidae). Parasitology 1970, 60, 195–221. [Google Scholar] [CrossRef]
- Fischthal, J.H.; Thomas, J.D. Some hemiurid trematodes of marine fishes from Ghana. Helminthol. Soc. Wash. 1971, 38, 181–189. [Google Scholar]
- Fischthal, J.H.; Thomas, J.D. Digenetic trematodes of fish from the Volta River drainage system in Ghana prior to the construction of the Volta Dam at Akosombo in May 1964. J. Helminthol. 1972, 46, 91–105. [Google Scholar] [CrossRef]
- Parukhin, A.M. Parasitic Worms of Food Fishes from the Southern Seas; Akademiya Nauk Ukrainskoi SSR, Ordena Trudovogo Krasnogo Zhameni Institut Biologii Yuzhnykh Morei; Kobalevskogo, A.O., Ed.; Izdatelstvo Naukova Dumka: Kiev, Ukraine, 1976; p. 182. (In Russian) [Google Scholar]
- Braicovich, P.E.; Pantoja, C.; Pereira, A.N.; Luque, J.L.; Timi, J.T. Parasites of the Brazilian flathead Percophis brasiliensis reflect West Atlantic biogeographic regions. Parasitology 2017, 144, 169–178. [Google Scholar] [CrossRef]
- Travassos, L.; Freitas, J.F.T.; Btihrnheim, P.F. Relatório da excursão do Instituto Oswaldo Cruz ao estado do Espírito Santo em novembro de 1964. Bol. Do Mus. De Biol. Mello Leitão 1967, 31, 1–5. [Google Scholar]
- Gibson, D.I.; Bray, R.A. Hemiuridae (Digenea) of fishes from the north-east Atlantic. British Museum (Natural History). Bull. Br. Mus. Zool. Ser. 1986, 51, 1–125. [Google Scholar]
- Pereira, A.N.; Pantoja, C.; Luque, J.L.; Timi, J.T. Parasites of Urophycis brasiliensis (Gadiformes: Phycidae) as indicators of marine ecoregions in coastal areas of the South American Atlantic. Parasitol. Res. 2014, 113, 4281–4292. [Google Scholar] [CrossRef]
- Bray, R.A. Hemiuridae (Digenea) from marine fishes of the southern Indian Ocean: Dinurinae, Elytrophallinae, Glomericirrinae and Plerurinae. Syst. Parasitol. 1990, 17, 183–217. [Google Scholar] [CrossRef]
- Vaz, Z.; Pereira, C. Nouvel hemiuride parasite de Sardinella aurita Cuv. et al., Parahemiurus n.g. Compte Rendu Des Séances De La Société De Biol. 1930, 103, 1315–1317. [Google Scholar]
- Timi, J.T.; Martorelli, S.R.; Sardella, N.H. Digenetic trematodes parasitic on Engraulis anchoita (Pisces: Engraulidae) from Argentina and Uruguay. Folia Parasitol. 1999, 46, 132–138. [Google Scholar]
- Bray, R.A. A review of the genus Parahemiurus Vaz & Pereira, 1930 (Digenea: Hemiuridae). Syst. Parasitol. 1990, 15, 1–21. [Google Scholar]
- Wallet, M.; Kohn, A. Trématodes parasites de poissons marins du littoral de Rio de Janeiro, Brésil. Memórias Do Inst. Oswaldo Cruz 1987, 82, 21–27. [Google Scholar] [CrossRef][Green Version]
- Benicio, L.; Moreira, J.; Paschoal, F. Community ecology of the metazoan parasites of the Atlantic anchoveta, Cetengraulis edentulus (Actinopterygii: Engraulidae) from the Sepetiba Bay, Rio de Janeiro, Brazil. Zoologia 2022, 39, e21034. [Google Scholar] [CrossRef]
- Bray, R.A. Hemiuridae (Digenea) from marine fishes of the southern Indian Ocean: Genus Lecithochirium Lühe, 1901 (Lecithochiriinae). Syst. Parasitol. 1991, 18, 193–219. [Google Scholar] [CrossRef]
- Teixeira de Freitas, J.F.; Gomes, D.C. Sobre uma nova espécie do gênero Lecithochirium Luehe, 1901, (Trematoda, Hemiuroidea). Memórias Do Inst. Oswaldo Cruz 1971, 69, 107–113. [Google Scholar] [CrossRef]
- Chandler, A.C. Parasites of fishes in Galveston Bay. Proc. United States Natl. Mus. 1935, 83, 123–157. [Google Scholar] [CrossRef]
- Teixeira de Freitas, J.F.; Kohn, A. Nova espécie do gênero Glomericirrus Yamaguti, 1937: (Trematoda, Hemiuridae). Memórias Do Inst. Oswaldo Cruz 1965, 63, 229–235. [Google Scholar] [CrossRef]
- Fernandes, B.M.; Arci, A.D.; Cohen, S.C. New data on some species of Monogenea and Digenea parasites of marine fish from the coast of the State of Rio de Janeiro, Brazil. Rev. Bras. De Parasitol. Veterinária 2009, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-F.; Peng, W.-F.; Gao, P.; Fu, M.-J.; Wu, H.-Z.; Lu, M.-K.; Gao, J.-Q.; Xiao, J. Digenean parasites of Chinese marine fishes: A list of species, hosts and geographical distribution. Syst. Parasitol. 2010, 75, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Estrada-García, M.A.; García-Prieto, L.; Garrido-Olvera, L. Description of a new species of Pseudopecoelus (Trematoda: Opecoelidae) with new records of trematodes of marine fishes from the Pacific coast of Mexico. Rev. Mex. Biodivers. 2018, 89, 22–28. [Google Scholar] [CrossRef]
- Razarihelisoa, M. Sur quelques trématodes digènes de poissons de Nossibé (Madagascar). Bull. De La Société Zool. De Fr. 1959, 84, 421–434. [Google Scholar]
- Kardousha, M.M. Redescription of ten species of digenetic trematodes from Marine fishes from the Emirati Coasts of the Arabian Gulf. Arab. Gulf J. Sci. Res. 2003, 21, 217–226. [Google Scholar]
- Silva, L.O.; Luque, J.L.; Alves, D.R. Metazoan parasites of the Atlantic cutlassfish, Trichiurus lepturus (Osteichthyes: Trichiuridae) from coastal zone of the State of Rio de Janeiro, Brazil. Parasitol. Al Dia 2000, 24, 97–101. [Google Scholar]
- Silva, L.O.; Luque, J.L.; Alves, D.R.; Paraguassu, A.R. Ecologia da comunidadeparasitaria do peixe-espada Trichiurus lepturus (Osteichthyes: Trichiuridae) do litoral do estado do Rio de Janeiro, Brasil. Rev. Bras. De Zoociências 2000, 2, 115–133. [Google Scholar]
- Carvalho, A.R.; Luque, J.L. Seasonal variation in metazoan parasites of Trichiurus lepturus (Perciformes: Trichiuridae) of Rio de Janeiro, Brazil. Braz. J. Biol. 2011, 71, 771–782. [Google Scholar] [CrossRef]
- Manter, H.W. Digenetic Trematodes of Fishes from the Galapagos Islands and the Neighboring Pacific; Allan Hancock Pacific Expeditions; The University of Southern California Publications: Los Angeles, CA, USA, 1940; Volume 2, pp. 325–497. [Google Scholar]
- Tantaleán, M.V.; Sarmiento, L.B.; Huiza, A.F. Digeneos (Trematoda) del Peru. Bol. De Lima 1992, 14, 47–84. [Google Scholar]
- Linton, E. Notes on trematode parasites of fishes. Proc. United States Natl. Mus. Wash. 1898, 20, 507–548. [Google Scholar] [CrossRef]
- Gu, C.D.; Shen, J.W. Digenetic trematodes of ribbonfish, Trichiurus haumela (Forskal) and their distribution in the fishing grounds of China Seas. Acta Zool. Sin. 1981, 27, 53–63. [Google Scholar]
- Krupenko, D.Y.; Gonchar, A.G.; Kremnev, G.A.; Uryadova, A.A. On the life cycle of Hemiurus levinseni Odhner, 1905 (Digenea: Hemiuridae). Invertebr. Zoöl. 2020, 17, 205–218. [Google Scholar] [CrossRef]
- WoRMS Editorial Board. World Register of Marine Species. 2022. Available online: https://www.marinespecies.org (accessed on 15 October 2022).
- Bullard, S.A.; Barse, A.M.; Curran, S.S.; Morris, J.A., Jr. First record of a digenean from invasive Lionfish, Pterois cf. volitans, (Scorpaeniformes: Scorpaenidae) in the Northwestern Atlantic Ocean. J. Parasitol. 2011, 97, 833–837. [Google Scholar] [PubMed]
- Pérez-Ponce de León, G.; León-Règagnon, V.; Scott, M. Theletrum lamothei sp. nov. (Digenea), parasite of Echidna nocturna from Cuajiniquil, Guanacaste, and other digenes of marine fishes from Costa Rica. Rev. De Biol. Trop. 1998, 46, 345–354. [Google Scholar]
- Calhoun, D.M.; Curran, S.S.; Pulis, E.E.; Provaznik, J.M.; Franks, J.S. Hirudinella ventricosa (Pallas, 1774) Baird, 1853 represents a species complex based on ribosomal DNA. Syst. Parasitol. 2013, 86, 197–208. [Google Scholar] [CrossRef]
- Pérez-Ponce de León, G.; Hernández-Mena, D.I. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J. Helminthol. 2019, 93, 260–276. [Google Scholar] [CrossRef]
- Chan-Martin, A.D.J.; Castellanos-Martínez, S.; Aguirre-Macedo, M.; Martínez-Aquino, A. Immature trematodes of Lecithochirium sp. (Digenea: Hemiuridae) in the California two-spot octopus (Octopus bimaculatus) from Mexico. Parasitol. Res. 2022, 121, 2651–2660. [Google Scholar] [CrossRef]
- Madhavi, R.; Bray, R.A. Digenetic Trematodes of Indian Marine Fishes; Springer: Heidelberg, Germany, 2018. [Google Scholar]
- Faltýnková, A.; Georgieva, S.; Kostadinova, A.; Bray, R.A. Biodiversity and evolution of digeneans of fishes in the Southern Ocean. In Biodiversity and Evolution of Parasitic Life in the Southern Ocean; Parasitology Research Monographs; Klimpel, S., Kuhn, T., Mehlhorn, H., Eds.; Springer: Cham, Switzerland, 2017; Volume 9, pp. 49–75. [Google Scholar]
- Luque, J.L.; Poulin, R. Metazoan parasite species richness in Neotropical fishes: Hotspots and the geography of biodiversity. Parasitology 2007, 134, 865–878. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).