The Pathology of Fatal Avian Malaria Due to Plasmodium elongatum (GRW6) and Plasmodium matutinum (LINN1) Infection in New Zealand Kiwi (Apteryx spp.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Pathology of Avian Malaria in Kiwi
3.1.1. Gross Lesions
3.1.2. Microscopic Lesions
3.2. Molecular Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, C. Avian malaria in New Zealand dotterel. Kokako 1997, 4, 3. [Google Scholar]
- Alley, M.R.; Fairley, R.A.; Martin, D.G.; Howe, L.; Atkinson, T. An outbreak of avian malaria in captive yellowheads/mohua (Mohoua ochrocephala). N. Z. Vet. J. 2008, 56, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Schoener, E.R.; Alley, M.R.; Howe, L.; Castro, I. Plasmodium relictum identified as a cause of mortality in a blackbird, Turdus merula, from Mokoia Island. Kokako 2009, 16, 39–40. [Google Scholar]
- Baillie, S.M.; Brunton, D.H. Diversity, distribution and biogeographical origins of Plasmodium parasites from the New Zealand bellbird (Anthornis melanura). Parasitology 2011, 138, 1843–1851. [Google Scholar] [CrossRef]
- Ewen, J.G.; Bensch, S.; Blackburn, T.M.; Bonneaud, C.; Brown, R.; Cassey, P.; Clarke, R.H.; Perez-Tris, J. Establishment of exotic parasites: The origins and characteristics of an avian malaria community in an isolated island avifauna. Ecol. Lett. 2012, 15, 1112–1119. [Google Scholar] [CrossRef]
- Howe, L.; Castro, I.C.; Schoener, E.R.; Hunter, S.; Barraclough, R.K.; Alley, M.R. Malaria parasites (Plasmodium spp.) infecting introduced, native and endemic New Zealand birds. Parasitol. Res. 2012, 110, 913–923. [Google Scholar] [CrossRef] [Green Version]
- Banda, M.E.; Howe, L.; Gartrell, B.D.; McInnes, K.; Hunter, S.; French, N.P. A cluster of avian malaria cases in a kiwi management programme. N. Z. Vet. J. 2013, 61, 121–126. [Google Scholar] [CrossRef]
- Hunter, S.A. Avian malaria caused by Plasmodium elongatum in a Fiordland crested penguin. Kokako 2015, 22, 21–22. [Google Scholar]
- Sijbranda, D.C.; Campbell, J.; Gartrell, B.D.; Howe, L. Avian malaria in introduced, native and endemic New Zealand bird species in a mixed ecosystem. N. Z. J. Ecol. 2016, 40, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Alley, M.R.; Hunter, S.A.; Webster, T. An outbreak of avian malaria in yellow-eyed penguins, Megadyptes antipodes. Kokako 2019, 26, 21–22. [Google Scholar]
- Tompkins, D.; Gleeson, D. Relationship between avian malaria distribution and an exotic invasive mosquito in New Zealand. J. R. Soc. N. Z. 2006, 36, 51–62. [Google Scholar] [CrossRef]
- Massey, B.; Gleeson, D.; Slaney, D.; Tompkins, D. PCR detection of Plasmodium and blood meal identification in a native New Zealand mosquito. J. Vector Ecol. 2007, 32, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Schoener, E.; Banda, M.; Howe, L.; Castro, I.; Alley, M. Avian malaria in New Zealand. N. Z. Vet. J. 2014, 62, 189–198. [Google Scholar] [CrossRef]
- Schoener, E.; Tompkins, D.; Parker, K.; Howe, L.; Castro, I. Presence and diversity of mixed avian Plasmodium spp. infections in introduced birds whose distribution overlapped with threatened New Zealand endemic birds. N. Z. Vet. J. 2020, 68, 101–106. [Google Scholar] [CrossRef]
- Argilla, L.; Howe, L.; Gartrell, B.; Alley, M. High prevalence of Leucocytozoon spp. in the endangered yellow-eyed penguin (Megadyptes antipodes) in the sub-Antarctic regions of New Zealand. Parasitology 2013, 140, 672–682. [Google Scholar]
- Fallis, A.; Bisset, S.; Allison, F. Leucocytozoon tawaki n. sp.(Eucoccida: Leucocytozoidae) from the penguin Eudyptes pachyrhynchus, and preliminary observations on its development in Austrosimulium spp.(Diptera: Simuliidae). N. Z. J. Zool. 1976, 3, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, D.; Palinauskas, V.; Iezhova, T.A.; Bernotienė, R.; Ilgūnas, M.; Bukauskaitė, D.; Zehtindjiev, P.; Ilieva, M.; Shapoval, A.P.; Bolshakov, C.V. Plasmodium spp.: An experimental study on vertebrate host susceptibility to avian malaria. Exp. Parasitol. 2015, 148, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G. Immunity, resistance and tolerance in bird–parasite interactions. Parasite Immunol. 2013, 35, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A. Exo-erythrocytic development of avian malaria and related haemosporidian parasites. Malar. J. 2017, 16, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulston, F.; Huff, C.G. Symposium on Exoerythrocytic Forms of Malarial Parasites. IV. The Chemotherapy and Immunology of Pre-Erythrocytic Stages in Avian Malaria. J. Parasitol. 1948, 34, 290–299. [Google Scholar]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, J.P. A review of toxoplasmosis in wild birds. Vet. Parasitol. 2002, 106, 121–153. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E.; Reavill, D.R.; Phalen, D.N. Pathology of Pet and Aviary Birds; Wiley Blackwell: Oxford, UK, 2015. [Google Scholar]
- Casartelli-Alves, L.; Boechat, V.; Couto, R.M.; Ferreira, L.; Nicolau, J.; Neves, L.; Millar, P.; Vicente, R.; Oliveira, R.; Muniz, A. Sensitivity and specificity of serological tests, histopathology and immunohistochemistry for detection of Toxoplasma gondii infection in domestic chickens. Vet. Parasitol. 2014, 204, 346–351. [Google Scholar] [CrossRef]
- Dinhopl, N.; Mostegl, M.M.; Richter, B.; Nedorost, N.; Maderner, A.; Fragner, K.; Weissenbock, H. Application of in-situ hybridization for the detection and identification of avian malaria parasites in paraffin wax-embedded tissues from captive penguins. Avian Pathol. 2011, 40, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Valkiunas, G.; Iezhova, T.A.; Krizanauskiene, A.; Palinauskas, V.; Sehgal, R.N.M.; Bensch, S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J. Parasitol. 2008, 94, 1395–1401. [Google Scholar] [CrossRef]
- Robertson, H.A.; Baird, K.A.; Elliott, G.P.; Hitchmough, R.A.; McArthur, N.J.; Makan, T.D.; Miskelly, C.M.; O’Donnell, C.F.; Sagar, P.M.; Scofield, R.P. New Zealand Threat Classification Series 36: Conservation Status of Birds in Aotearoa New Zealand, 2021; Department of Conservation: Wellington, New Zealand, 2021.
- McLennan, J.A.; Potter, M.A.; Robertson, H.A.; Wake, G.C.; Colbourne, R.; Dew, L.; Joyce, L.; McCann, A.J.; Miles, J.; Miller, P.J.; et al. Role of predation in the decline of kiwi, Apteryx spp., in New Zealand. N. Z. J. Ecol. 1996, 20, 27–35. [Google Scholar]
- Pierce, R.J.; Sporle, W. Causes of Kiwi Mortality in Northland; Department of Conservation: Wellington, New Zealand, 1997; pp. 1–6.
- Gulliver, E.L.; Hunter, S.A.; Vallee, E.; Castillo-Alcala, F. Causes of mortality of kiwi (Apteryx spp.) in New Zealand: A retrospective analysis of postmortem records, 2010–2020. N. Z. Vet. J. 2022; in press. [Google Scholar]
- Jakob-Hoff, R. First record of avian malaria in kiwi. Kokako 2000, 7, 11–12. [Google Scholar]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Aspinall, T.V.; Marlee, D.; Hyde, J.E.; Sims, P.F. Prevalence of Toxoplasma gondii in commercial meat products as monitored by polymerase chain reaction–food for thought? Int. J. Parasitol. 2002, 32, 1193–1199. [Google Scholar] [CrossRef]
- Roe, W.D.; Howe, L.; Baker, E.J.; Burrows, L.; Hunter, S.A. An atypical genotype of Toxoplasma gondii as a cause of mortality in Hector’s dolphins (Cephalorhynchus hectori). Vet. Parasitol. 2013, 192, 67–74. [Google Scholar] [CrossRef]
- Grigg, M.E.; Boothroyd, J.C. Rapid identification of virulent type I strains of the protozoan pathogen Toxoplasma gondii by PCR-restriction fragment length polymorphism analysis at the B1 gene. J. Clin. Microbiol. 2001, 39, 398–400. [Google Scholar] [CrossRef] [Green Version]
- Viscardi, M.; Santoro, M.; Cozzolino, L.; Borriello, G.; Fusco, G. A type II variant of Toxoplasma gondii infects the Eurasian otter (Lutra lutra) in southern Italy. Transbound. Emerg. Dis. 2021, 69, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Germano, J.; Barlow, S.; Castro, I.; Colbourne, R.; Cox, M.; Gillies, C.; Hackwell, K.; Harawira, J.; Impey, M.; Reuben, A.; et al. Threatened Species Recovery Plan 64: Kiwi Recovery Plan 2018–2028; Department of Conservation: Wellington, New Zealand, 2018; pp. 1–60.
- Iurescia, M.; Romiti, F.; Cocumelli, C.; Diaconu, E.L.; Stravino, F.; Onorati, R.; Alba, P.; Friedrich, K.G.; Maggi, F.; Magliano, A. Plasmodium matutinum transmitted by Culex pipiens as a cause of avian malaria in captive african penguins (Spheniscus demersus) in Italy. Front. Vet. Sci. 2021, 8, 621974. [Google Scholar] [CrossRef] [PubMed]
- Vanstreels, R.E.T.; da Silva-Filho, R.P.; Kolesnikovas, C.K.M.; Bhering, R.C.C.; Ruoppolo, V.; Epiphanio, S.; Amaku, M.; Junior, F.C.F.; Braga, É.M.; Catão-Dias, J.L. Epidemiology and pathology of avian malaria in penguins undergoing rehabilitation in Brazil. Vet. Res. 2015, 46, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sijbranda, D.; Hunter, S.; Howe, L.; Lenting, B.; Argilla, L.; Gartrell, B. Cases of mortality in little penguins (Eudyptula minor) in New Zealand associated with avian malaria. N. Z. Vet. J. 2017, 65, 332–337. [Google Scholar] [CrossRef]
- González-Olvera, M.; Hernandez-Colina, A.; Himmel, T.; Eckley, L.; Lopez, J.; Chantrey, J.; Baylis, M.; Jackson, A.P. Molecular and epidemiological surveillance of Plasmodium spp. during a mortality event affecting Humboldt penguins (Spheniscus humboldti) at a zoo in the UK. Int. J. Parasitol. Parasites Wildl. 2022, 19, 26–37. [Google Scholar] [CrossRef]
- Taunde, P.A.; Bianchi, M.V.; Perles, L.; da Silva, F.S.; Guim, T.N.; Stadler, R.A.; André, M.R.; Driemeier, D.; Pavarini, S.P. Pathological and molecular characterization of avian malaria in captive Magellanic penguins (Spheniscus magellanicus) in South America. Parasitol. Res. 2019, 118, 599–606. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Thomas, N.J.; Hunter, D.B. Parasitic Diseases of Wild Birds; John Wiley & Sons: Ames, IA, USA, 2008. [Google Scholar]
- Castro, I.; Howe, L.; Tompkins, D.M.; Barraclough, R.K.; Slaney, D. Presence and seasonal prevalence of Plasmodium spp. in a rare endemic New Zealand passerine (tieke or saddleback, Philesturnus carunculatus). J. Wildl. Dis. 2011, 47, 860–867. [Google Scholar] [CrossRef]
- Colbourne, R.; Bassett, S.; Billing, T.; McCormick, H.; McLennan, J.; Nelson, A.; Robertson, H. The development of Operation Nest Egg as a tool in the conservation management of kiwi. Sci. Conserv. 2005, 259, 24. [Google Scholar]
- Names, G.; Krause, J.S.; Schultz, E.M.; Angelier, F.; Parenteau, C.; Ribout, C.; Hahn, T.P.; Wingfield, J.C. Relationships between avian malaria resilience and corticosterone, testosterone and prolactin in a Hawaiian songbird. Gen. Comp. Endocrinol. 2021, 308, 113784. [Google Scholar] [CrossRef]
- Atkinson, C.; Woods, K.; Dusek, R.J.; Sileo, L.; Iko, W. Wildlife disease and conservation in Hawaii: Pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected Iiwi (Vestiaria coccinea). Parasitology 1995, 111, S59–S69. [Google Scholar] [CrossRef] [PubMed]
- Calero-Riestra, M.; García, J.T. Sex-dependent differences in avian malaria prevalence and consequences of infections on nestling growth and adult condition in the Tawny pipit, Anthus campestris. Malar. J. 2016, 15, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, I.; Hull, R. Serum alterations in avian malaria. J. Protozool. 1960, 7, 171–176. [Google Scholar] [CrossRef]
- Atkinson, C.; Paxton, E. Immunological Markers for Tolerance to Avian Malaria in Hawaiiamakihi: New Tools for Restoring Native Hawaiian Forest Birds? University of Hawaii at Hilo: Hilo, HI, USA, 2013. [Google Scholar]
- Ilgūnas, M.; Palinauskas, V.; Platonova, E.; Iezhova, T.; Valkiūnas, G. The experimental study on susceptibility of common European songbirds to Plasmodium elongatum (lineage pGRW6), a widespread avian malaria parasite. Malar. J. 2019, 18, 290. [Google Scholar] [CrossRef]
- Jarvi, S.I.; Atkinson, C.T.; Fleischer, R.C. Immunogenetics and resistance to avian malaria in Hawaiian honeycreepers (Drepanidinae). Stud. Avian Biol. 2001, 22, 254–263. [Google Scholar]
- Atkinson, C.T.; Dusek, R.J.; Woods, K.L.; Iko, W.M. Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J. Wildl. Dis. 2000, 36, 197–204. [Google Scholar] [CrossRef]
- Graczyk, T.; Cranfield, M.; McCutchan, T.; Bicknese, E. Characteristics of naturally acquired avian malaria infections in naive juvenile African black-footed penguins (Spheniscus demersus). Parasitol. Res. 1994, 80, 634–637. [Google Scholar] [CrossRef]
- Martínez-Girón, R.; Esteban, J.; Ribas, A.; Doganci, L. Protozoa in respiratory pathology: A review. Eur. Respir. J. 2008, 32, 1354–1370. [Google Scholar] [CrossRef]
- Van Riper III, C.; Van Riper, S.G.; Goff, M.L.; Laird, M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 1986, 56, 327–344. [Google Scholar] [CrossRef]
- Buffet, P.A.; Safeukui, I.; Deplaine, G.; Brousse, V.; Prendki, V.; Thellier, M.; Turner, G.D.; Mercereau-Puijalon, O. The pathogenesis of Plasmodium falciparum malaria in humans: Insights from splenic physiology. Blood J. Am. Soc. Hematol. 2011, 117, 381–392. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Bernotienė, R.; Iezhova, T.A. Molecular characterization and distribution of Plasmodium matutinum, a common avian malaria parasite. Parasitology 2017, 144, 1726–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkiunas, G.; Zehtindjiev, P.; Dimitrov, D.; Krizanauskiene, A.; Iezhova, T.A.; Bensch, S. Polymerase chain reaction-based identification of Plasmodium (Huffia) elongatum, with remarks on species identity of haemosporidian lineages deposited in GenBank. Parasitol. Res. 2008, 102, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Cocumelli, C.; Iurescia, M.; Diaconu, E.L.; Galietta, V.; Raso, C.; Buccella, C.; Stravino, F.; Grande, F.; Fiorucci, L.; De Liberato, C. Plasmodium matutinum causing avian malaria in Lovebirds (Agapornis roseicollis) hosted in an Italian zoo. Microorganisms 2021, 9, 1356. [Google Scholar] [CrossRef]
- Meister, S.L.; Richard, O.K.; Hoby, S.; Gurtner, C.; Basso, W.U. Fatal avian malaria in captive Atlantic puffins (Fratercula arctica) in Switzerland. Int. J. Parasitol. Parasites Wildl. 2021, 14, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, A.; Keyser, C.; Ludes, B. Efficiency evaluation of a DNA extraction and purification protocol on archival formalin-fixed and paraffin-embedded tissue. Forensic Sci. Int. 2010, 194, E25–E28. [Google Scholar] [CrossRef] [PubMed]
- Greer, C.E.; Peterson, S.L.; Kiviat, N.B.; Manos, M.M. PCR amplification from paraffin-embedded tissues: Effects of fixative and fixation time. Am. J. Clin. Pathol. 1991, 95, 117–124. [Google Scholar] [CrossRef]
- Freed, L.A.; Cann, R.L. DNA quality and accuracy of avian malaria PCR diagnostics: A review. Condor 2006, 108, 459–473. [Google Scholar] [CrossRef]
- Turner, G. Some aspects of the pathogenesis and comparative pathology of toxoplasmosis. J. South Afr. Vet. Assoc. 1978, 49, 3–8. [Google Scholar]
- Taylor, H.S.; Howe, L.; Bolwell, C.F.; Lenting, B.; Morgan, K.J.; McInnes, K. Toxoplasma gondii exposure prevalence in little spotted kiwi (Apteryx owenii). J. Wildl. Dis. 2022, in press.
- Mirza, V.; Burrows, E.; Gils, S.; Hunter, S.; Gartrell, B.; Howe, L. A retrospective survey into the presence of Plasmodium spp. and Toxoplasma gondii in archived tissue samples from New Zealand raptors: New Zealand falcons (Falco novaeseelandiae), Australasian harriers (Circus approximans) and moreporks (Ninox novaeseelandiae). Parasitol. Res. 2017, 116, 2283–2289. [Google Scholar]

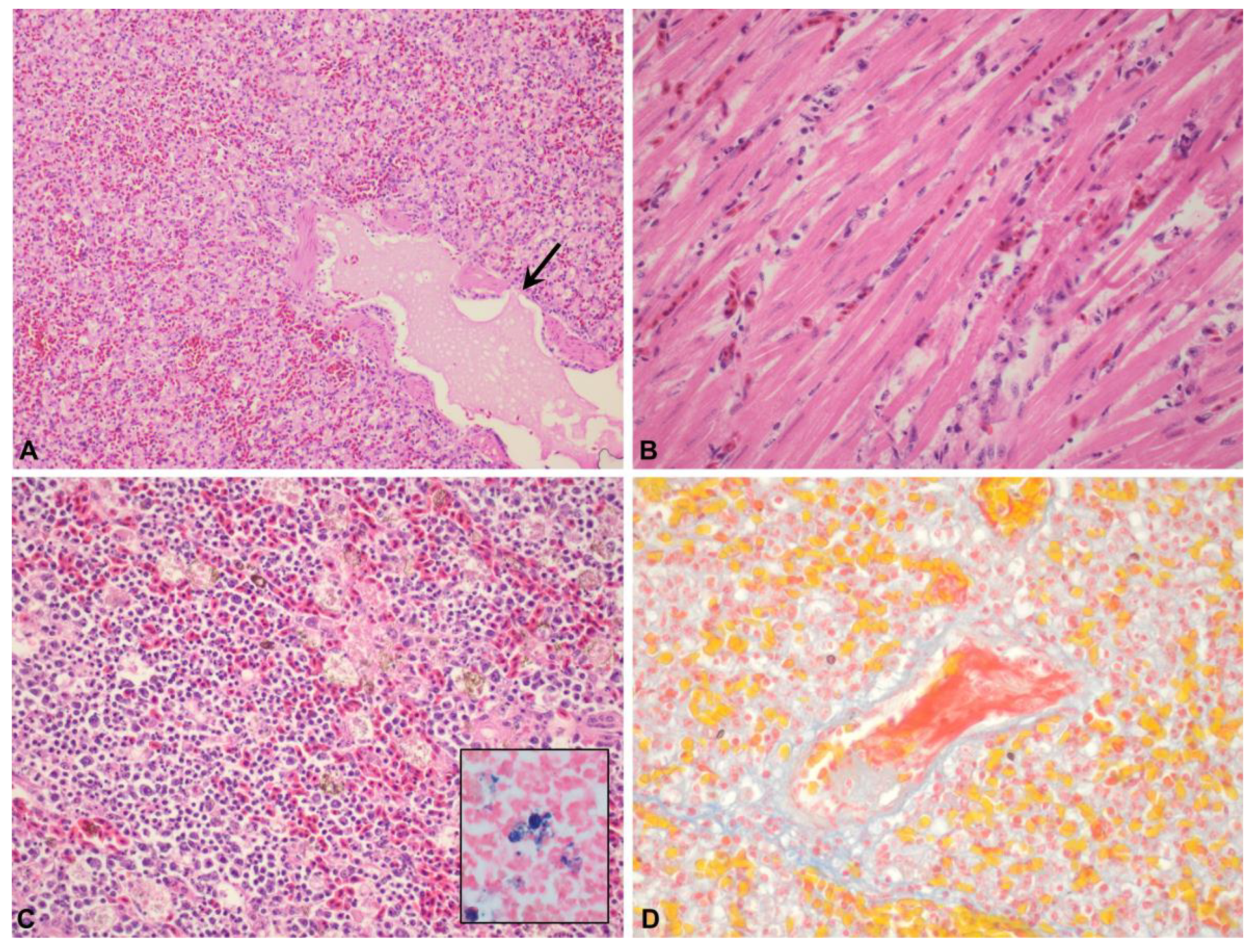
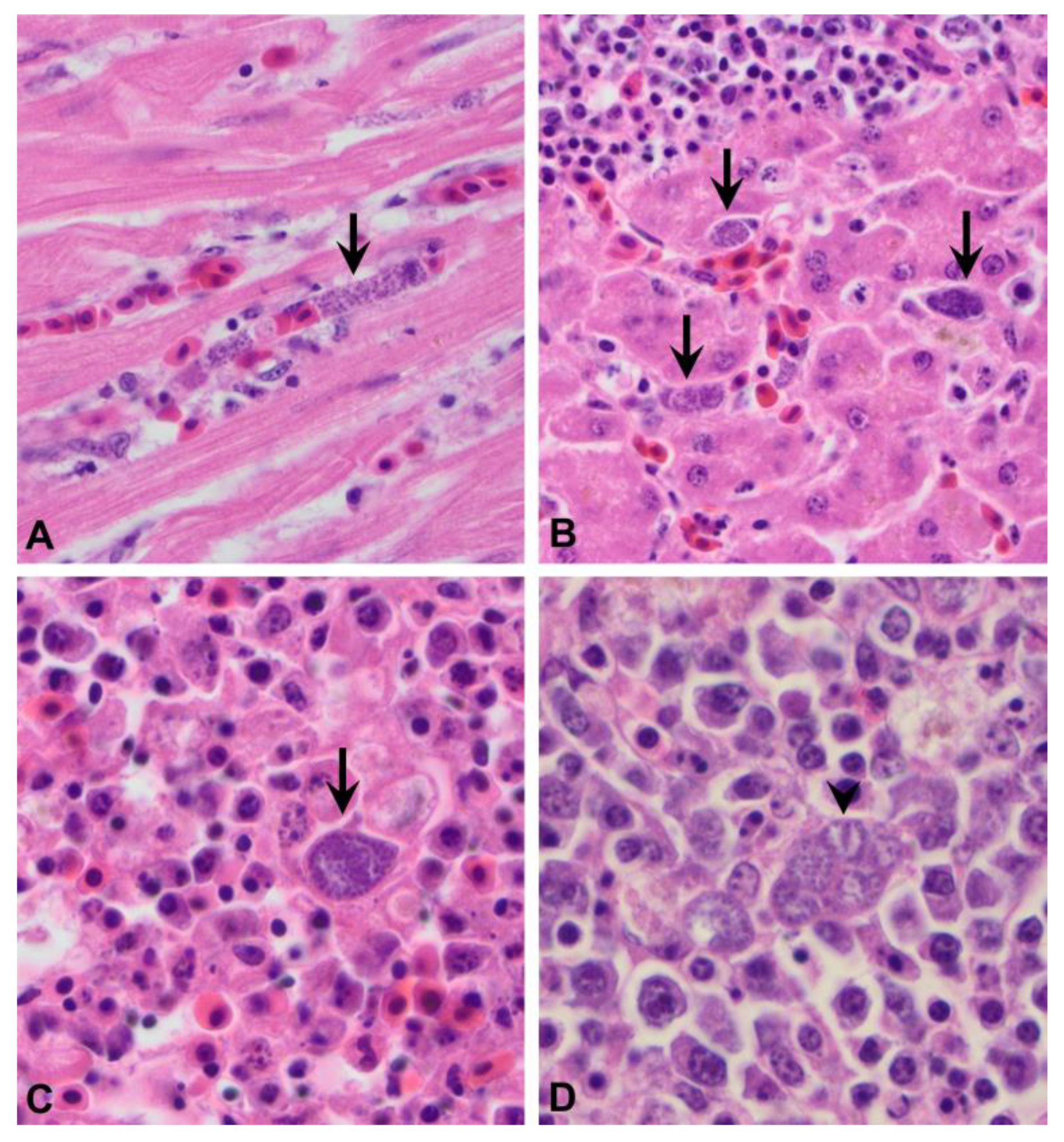
| Case (#) | Species | Age | Sex | Year | Month | Captivity Status | Location | Recorded Cause of Death |
|---|---|---|---|---|---|---|---|---|
| 1 | NIBK | Neonate | M | 2010 | March | Wild | Northland | PM |
| 2 * | GSK | Juvenile | M | 2010 | April | Captive | Canterbury | AM |
| 3 ** | NIBK | Juvenile | F | 2011 | April | Captive | Bay of Plenty | AM |
| 4 | GSK | Juvenile | F | 2012 | April | Créche | West Coast | AM |
| 5 | NIBK | Juvenile | F | 2012 | March | Wild | Auckland | PM |
| 6 | NIBK | Adult | UK | 2013 | February | Captive | Waikato | AM |
| 7 | NIBK | Subadult | M | 2014 | February | Wild | Northland | PM |
| 8 | NIBK | Juvenile | F | 2014 | March | Créche | Hawke’s Bay | AM Toxoplasmosis |
| 9 | NIBK | Subadult | M | 2014 | March | Wild | Waikato | AM |
| 10 | NIBK | Juvenile | M | 2015 | April | Créche | Hawke’s Bay | AM |
| 11 | NIBK | Juvenile | F | 2016 | March | Sanctuary | Hawke’s Bay | MF |
| 12 | NIBK | Juvenile | M | 2017 | February | Créche | Hawke’s Bay | AM |
| 13 | NIBK | Juvenile | UK | 2017 | February | Créche | Hawke’s Bay | MF |
| 14 | NIBK | Juvenile | F | 2017 | March | Créche | Hawke’s Bay | MF |
| 15 | NIBK | Juvenile | F | 2018 | February | Wild | Waikato | AM |
| 16 | LSK | Adult | F | 2018 | April | Sanctuary | Auckland | AM |
| 17 | LSK | Subadult | F | 2018 | April | Sanctuary | Auckland | AM |
| 18 | LSK | Subadult | M | 2018 | April | Sanctuary | Auckland | AM |
| 19 | LSK | Adult | F | 2018 | April | Sanctuary | Auckland | AM |
| 20 | LSK | Adult | F | 2018 | May | Sanctuary | Auckland | AM |
| 21 | NIBK | Juvenile | M | 2019 | March | Wild | Hawke’s Bay | AM |
| 22 | NIBK | Juvenile | F | 2020 | March | Captive | Hawke’s Bay | AM |
| 23 | NIBK | Juvenile | F | 2020 | March | Wild | Bay of Plenty | AM |
| Case (#) | Previous Test Results | Tissues Tested | Plasmodium spp. PCR | Plasmodium spp. Sequencing | Toxoplasma gondii PCR |
|---|---|---|---|---|---|
| 1 | N/A | Liver, heart | Negative | Positive | |
| 2 | Positive–Plasmodium spp. (LINN1) on fresh liver * | Heart, spleen, lung, thymus | Positive | P. matutinum (LINN1) 1 | Negative |
| 3 | Positive–Plasmodium spp. on fresh spleen ** | Liver, spleen, lung, adrenal gland | Negative | Negative | |
| 4 | Positive–Plasmodium spp. (AFTUR5/LINN1) on fresh liver | Spleen | Negative | Negative | |
| 5 | N/A | None available | N/D | N/D | |
| 6 | Positive–P. elongatum on spleen | None available | N/D | N/D | |
| 7 | N/A | Liver, lung, spleen | Negative | Negative | |
| 8 | Positive–P. elongatum and T. gondii on fresh lung and spleen | Liver, lung | Negative | Weak Positive | |
| 9 | N/A | Liver | Negative | Negative | |
| 10 | Positive–P. elongatum on formalin-fixed heart | Liver, heart | Positive | P. elongatum (GRW6) 2 | Negative |
| 11 | N/A | Spleen, lung, bursa | Negative | Positive | |
| 12 | N/A | Heart, spleen, liver | Negative | Positive | |
| 13 | N/A | Lung, liver, heart, spleen | Negative | Negative | |
| 14 | N/A | Lung, spleen, liver, kidney | Negative | Negative | |
| 15 | N/A | Spleen, liver, lung, adrenal gland | Positive | P. elongatum (GRW6) 2 | Negative |
| 16 | N/A | Liver, spleen, lung | Negative | Negative | |
| 17 | N/A | Lung, heart | Positive | P. matutinum (LINN1) 3 | Negative |
| 18 | N/A | Lung, spleen, liver | Positive | P. matutinum (LINN1) 3 | Negative |
| 19 | N/A | Spleen, liver, kidney, heart | Positive | P. matutinum (LINN1) 3 | Negative |
| 20 | N/A | Lung, liver, spleen, kidney | Positive | P. matutinum (LINN1) 3 | Negative |
| 21 | N/A | Spleen, liver, lung | Negative | Positive | |
| 22 | N/A | Spleen, liver, kidney, lung | Positive | P. elongatum (GRW6) 2 | Negative |
| 23 | N/A | Liver, spleen, lung | Positive | P. elongatum (GRW6) 2 | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulliver, E.; Hunter, S.; Howe, L.; Castillo-Alcala, F. The Pathology of Fatal Avian Malaria Due to Plasmodium elongatum (GRW6) and Plasmodium matutinum (LINN1) Infection in New Zealand Kiwi (Apteryx spp.). Animals 2022, 12, 3376. https://doi.org/10.3390/ani12233376
Gulliver E, Hunter S, Howe L, Castillo-Alcala F. The Pathology of Fatal Avian Malaria Due to Plasmodium elongatum (GRW6) and Plasmodium matutinum (LINN1) Infection in New Zealand Kiwi (Apteryx spp.). Animals. 2022; 12(23):3376. https://doi.org/10.3390/ani12233376
Chicago/Turabian StyleGulliver, Emma, Stuart Hunter, Laryssa Howe, and Fernanda Castillo-Alcala. 2022. "The Pathology of Fatal Avian Malaria Due to Plasmodium elongatum (GRW6) and Plasmodium matutinum (LINN1) Infection in New Zealand Kiwi (Apteryx spp.)" Animals 12, no. 23: 3376. https://doi.org/10.3390/ani12233376





