Brown Seaweed (Padina australis) Extract can Promote Performance, Innate Immune Responses, Digestive Enzyme Activities, Intestinal Gene Expression and Resistance against Aeromonas hydrophila in Common Carp (Cyprinus carpio)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. P. australis Extract Preparation
2.2. Characterization of P. australis Extract
2.3. Diet Preparation
2.4. Experimental Procedure
2.5. Growth Performance
2.6. Sample Collection
2.7. Digestive Enzyme Activities
2.8. Immune Assays
2.9. Real-Time PCR Analysis
2.10. Challenge Test
2.11. Statistical Analysis
3. Results
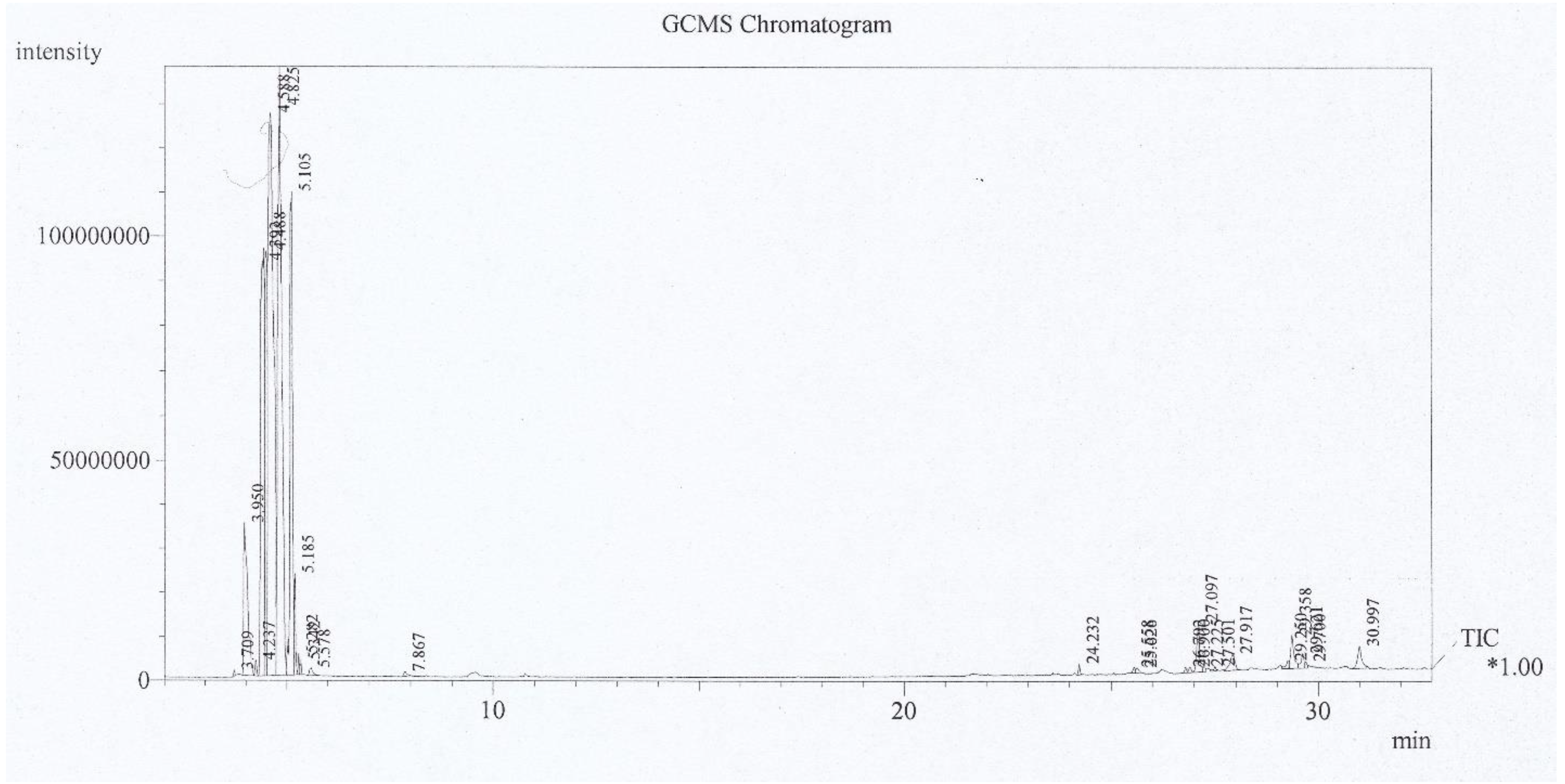
3.1. Analysis of P. australis Extract
3.2. Growth Performance and Carcass Composition
3.3. Digestive Enzymes
3.4. Innate Immune Response
3.5. Intestinal-Integrity-Related Gene Expression
3.6. Intestinal-Immunity-Related Gene Expression
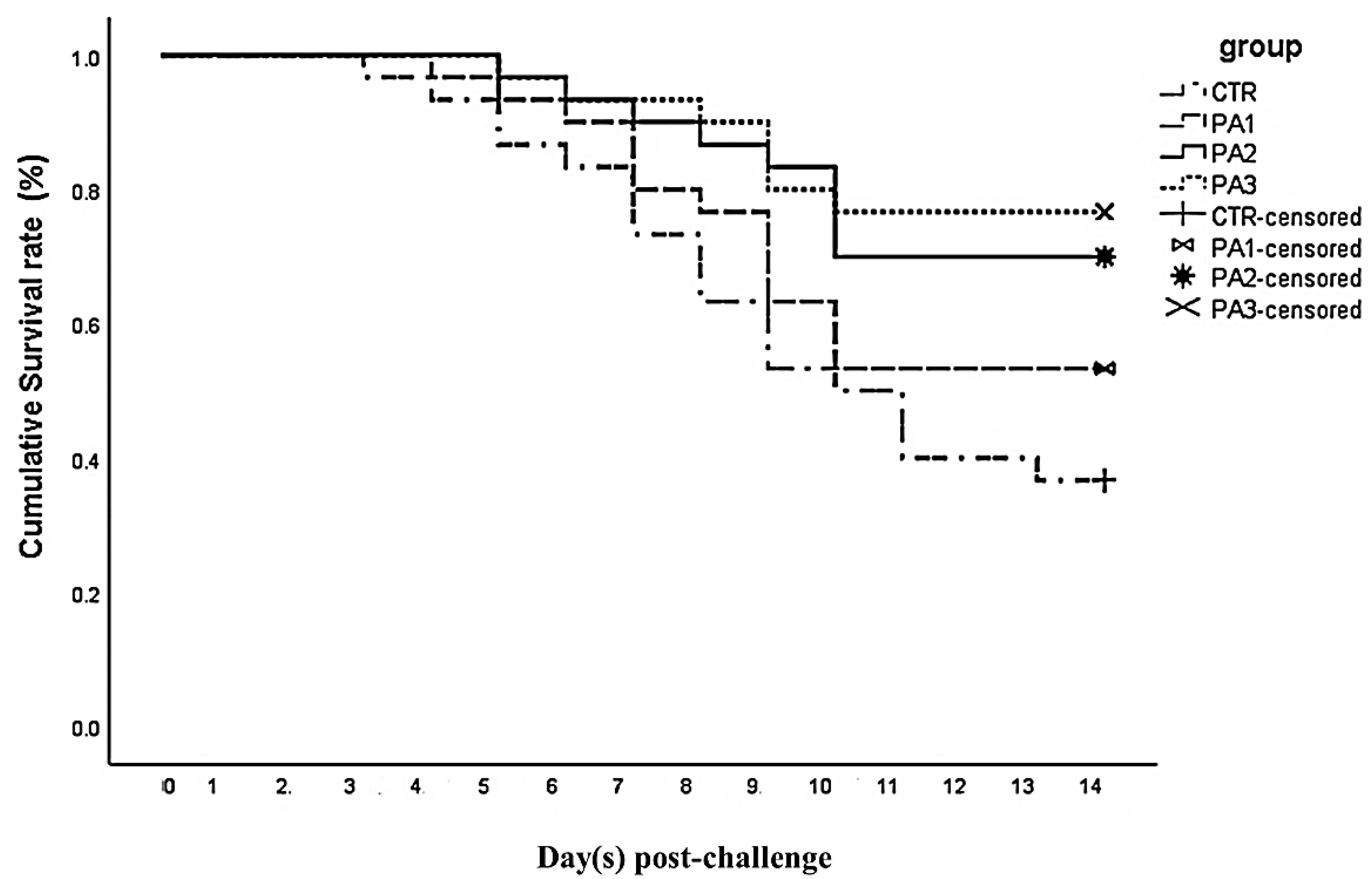
3.7. Disease Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Sustainability in action; FAO: Rome, Italy, 2020. [Google Scholar]
- Khalafalla, M.M.; Zayed, N.F.A.; Amer, A.A.; Soliman, A.A.; Zaineldin, A.I.; Gewaily, M.S.; Hassan, A.M.; Van Doan, H.; Tapingkae, W.; Dawood, M.A.O. Dietary Lactobacillus acidophilus ATCC 4356 Relieves the Impacts of Aflatoxin B1 Toxicity on the Growth Performance, Hepatorenal Functions, and Antioxidative Capacity of Thinlip Grey Mullet (Liza ramada) (Risso 1826). Probiotics Antimicrob. Proteins 2022, 14, 189–203. [Google Scholar] [CrossRef]
- Gharib, A.A.; Abdel-Hamid, E.A.; Mousa, M.A.; Naiel, M.A. Improving water quality, growth performance, and modulating some stress physiological biomarkers in Cyprinus carpio using raw date nuclei as a zinc adsorbent agent. Appl. Water Sci. 2022, 12, 159. [Google Scholar] [CrossRef]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review. Aquac. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Abdelnour, S.A.; Alotaibi, M.A.; AlGabbani, Q.; Naiel, M.A.; Shokrollahi, B.; Noreldin, A.E.; Jahejo, A.R.; Shah, M.A.; Alagawany, M.; et al. MicroRNAs mediated environmental stress responses and toxicity signs in teleost fish species. Aquaculture 2022, 546, 737310. [Google Scholar] [CrossRef]
- El-Sherbeny, E.M.E.; Khoris, E.A.; Kassem, S. Assessment the efficacy of some various treatment methods, in vitro and In Vivo, against Aeromonas hydrophila infection in fish with regard to side effects and residues. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 253, 109246. [Google Scholar] [CrossRef] [PubMed]
- Mehrinakhi, Z.; Ahmadifar, E.; Sheikhzadeh, N.; Moghadam, M.S.; Dawood, M.A. Extract of grape seed enhances the growth performance, humoral and mucosal immunity, and resistance of common carp (Cyprinus carpio) against Aeromonas hydrophila. Ann. Anim. Sci. 2021, 21, 217–232. [Google Scholar] [CrossRef]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: Risks, current concern, and future thinking. Environ. Sci. Pollut. Res. 2022, 29, 11054–11075. [Google Scholar] [CrossRef]
- Choi, W.; Moniruzzaman, M.; Bae, J.; Hamidoghli, A.; Lee, S.; Choi, Y.-H.; Min, T.; Bai, S.C. Evaluation of Dietary Probiotic Bacteria and Processed Yeast (GroPro-Aqua) as the Alternative of Antibiotics in Juvenile Olive Flounder Paralichthys olivaceus. Antibiotics 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Alizadeh-Salteh, S.; Khani Oushani, A.; Firouzamandi, M.; Mardani, K. Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol. Biochem. 2020, 46, 777–786. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Ramezanzadeh, S.; Abedian Kenari, A.; Esmaeili, M. Immunohematological parameters of rainbow trout (Oncorhynchus mykiss) fed supplemented diet with different forms of barberry root (Berberis vulgaris). Comp. Clin. Pathol. 2020, 29, 177–187. [Google Scholar] [CrossRef]
- Purcell-Meyerink, D.; Packer, M.A.; Wheeler, T.T.; Hayes, M. Aquaculture Production of the Brown Seaweeds Laminaria digitata and Macrocystis pyrifera: Applications in Food and Pharmaceuticals. Molecules 2021, 26, 1306. [Google Scholar] [CrossRef]
- Duarte, C.M.; Bruhn, A.; Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2021, 5, 185–193. [Google Scholar] [CrossRef]
- Negm, S.S.; Ismael, N.E.; Ahmed, A.I.; Asely, A.M.E.; Naiel, M.A. The efficiency of dietary Sargassum aquifolium on the performance, innate immune responses, antioxidant activity, and intestinal microbiota of Nile Tilapia (Oreochromis niloticus) raised at high stocking density. J. Appl. Phycol. 2021, 33, 4067–4082. [Google Scholar] [CrossRef]
- Saeed, M.; Arain, M.A.; Ali Fazlani, S.; Marghazani, I.B.; Umar, M.; Soomro, J.; Bhutto, Z.A.; Soomro, F.; Noreldin, A.E.; Abd El-Hack, M.E.; et al. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquac. Nutr. 2021, 27, 633–654. [Google Scholar] [CrossRef]
- Deepitha, R.P.; Xavier, K.A.M.; Layana, P.; Nayak, B.B.; Balange, A.K. Quality improvement of pangasius fillets using aqueous seaweed (Padina tetrastromatica) extract. LWT 2021, 137, 110418. [Google Scholar] [CrossRef]
- Zeilab Sendijani, R.; Abedian Kenari, A.; Smiley, A.H.; Esmaeili, M. The effect of extract from dill Anethum graveolens on the growth performance, body composition, immune system, and antioxidant system of rainbow trout. N. Am. J. Aquac. 2020, 82, 119–131. [Google Scholar] [CrossRef]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnik, M.; Johnston, C.; Evans, B.; Paul, N.A. Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.-S.; Jena, M. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Tran, V.T.T.; Bui, L.M.; Takebe, S.; Suzuki, S.; Nakajima, N.; Kitamura, S.; Thanh, T.T.T. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 2016, 147, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.H.; Pham, H.N.T.; Nguyen, T.H. Optimization of ultrasound-assisted extraction conditions for phenolics, antioxidant, and tyrosinase inhibitory activities of Vietnamese brown seaweed (Padina australis). J. Food Process. Preserv. 2021, 45, e15386. [Google Scholar] [CrossRef]
- Salosso, Y.; Aisiah, S.; Toruan, L.N.L.; Pasaribu, W. Nutrient content, active compound and antibacterial activity of Padina australis against Aeromonas hydropilla. Pharm. J. 2020, 12, 771–776. [Google Scholar] [CrossRef]
- Akbary, P.; Aminikhoei, Z. Effects of Padina australis (Hauck) polysaccharide extract on growth, antioxidant and non-specific immune parameters of the western white leg shrimp, Litopenaeus vannamei (Boone). Iran. J. Aquat. Anim. Health 2018, 4, 73–85. [Google Scholar]
- Shahraki, N. Effect of Padina Astraulis Hauck Extract on Growth, Carcass Chemical Composition, Fatty Acids and Some of Liver Param-eters in Grey Mullet (Mugil cephalus Linnaeus, 1758) Larvae. Master’s Thesis, Chabahar Maritime University, Chah Bahar, Iran, 2016. (In Persian). [Google Scholar]
- Akbary, P.; Shahraki, N. Effect of dietary supplementation of Padina astraulis (Hauk) extract on biochemical response and digestive enzyme activities of grey mullet, Mugil cephalus (Linnaeus). Iran. J. Fish. Sci. 2020, 19, 2118–2127. [Google Scholar]
- Bita, S.; Akbary, P.; Soltanpur, F. Effect of Sargassum ilicifolium alcoholic extract on growth, feed, body composition and digestive enzymatic activities in Mugil cephalus. Aquat. Physiol. Biotechnol. 2019, 6, 61–78. [Google Scholar]
- Win, N.-N.; Wai, M.-K.; Geraldino, P.J.L.; Liao, L.M.; Aye, C.-T.P.; Mar, N.N.; Hanyuda, T.; Kawai, H.; Tokeshi, M. Taxonomy and species diversity of Padina (Dictyotales, Phaeophyceae) from the Indo-Pacific with the description of two new species. Eur. J. Phycol. 2022, 57, 1–17. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT-Food Sci. Technol. 2011, 44, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Babaei Mahani Nejad, S.; Yousefzadi, M.; Soleimani, S. Phlorotannins extracted from macroalgae as a new antioxidant source. Aquat. Physiol. Biotechnol. 2020, 8, 69–94. [Google Scholar]
- Cuilel, I. Methodology for the Analysis of Vegetables and Drugs; Chemical Industry Division, NNIDO Romania: Bucharest, Romania, 1994; pp. 24–67. [Google Scholar]
- AOAC (Association of Official Agricultural Chemists). Official methods of analysis of AOAC International, volume 1. In Agriculture Chemicals, Contaminants, Drugs, 16th ed.; AOAC International: Arlington, Virginia, 1995. [Google Scholar]
- Bernfeld, P. Amylase a and b. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 149–158. [Google Scholar]
- Bülow, L.; Mosbach, K. The expression in E. coli of a polymeric gene coding for an esterase mimic catalyzing the hydrolysis of p-nitrophenyl esters. FEBS Lett. 1987, 210, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers, N.E.; Bayne, C.J. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 1997, 21, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Andani, H.R.R.; Tukmechi, A.; Meshkini, S.; Sheikhzadeh, N. Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss). J. Appl. Ichthyol. 2012, 28, 728–734. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet. Immunol. Immunopathol. 1994, 41, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 1, 102269. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- He, M.; Liu, G.; Liu, Y.; Yang, K.; Qi, X.; Huang, A.; Liu, T.; Wang, G.; Wang, E. Effects of geniposide as immunostimulant on the innate immune response and disease resistance in crucian carp. Aquaculture 2020, 529, 735713. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; El-Borady, O.M.; Mahmoud, A.E.D. Comparative study between Phragmites australis root and rhizome extracts for mediating gold nanoparticles synthesis and their medical and environmental applications. Adv. Powder Technol. 2021, 32, 2268–2279. [Google Scholar] [CrossRef]
- Santoso, J.; Yoshie, Y.; Suzuki, T. Polyphenolic compounds from seaweeds: Distribution and their antioxidative effect. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 2004; pp. 169–177. [Google Scholar]
- Akbary, P.; Aminikhoei, Z. Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J. Appl. Phycol. 2018, 30, 1345–1353. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Sheikhzadeh, N.; Roshanaei, K.; Dargahi, N.; Faggio, C. Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture 2019, 507, 341–348. [Google Scholar] [CrossRef]
- Venkatramalingam, K.; Christopher, J.G.; Citarasu, T. Zingiber officinalis an herbal appetizer in the tiger shrimp Penaeus monodon (Fabricius) larviculture. Aquac. Nutr. 2007, 13, 439–443. [Google Scholar] [CrossRef]
- Naiel, M.A.; Khames, M.K.; Abdel-Razek, N.; Gharib, A.A.; El-Tarabily, K.A. The dietary administration of miswak leaf powder promotes performance, antioxidant, immune activity, and resistance against infectious diseases on Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2021, 20, 100707. [Google Scholar] [CrossRef]
- Junopia, A.; Natsir, H.; Dali, S. Effectiveness of brown algae (Padina australis) extract as antioxidant agent. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; p. 012012. [Google Scholar]
- Esmaeili, M.; Abedian Kenari, A.; Rombenso, A. Effects of fish meal replacement with meat and bone meal using garlic (Allium sativum) powder on growth, feeding, digestive enzymes and apparent digestibility of nutrients and fatty acids in juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Aquac. Nutr. 2017, 23, 1225–1234. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Chen, L.; Feng, L.; Jiang, W.-D.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Intestinal immune function, antioxidant status and tight junction proteins mRNA expression in young grass carp (Ctenopharyngodon idella) fed riboflavin deficient diet. Fish Shellfish. Immunol. 2015, 47, 470–484. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Jiang, W.-D.; Liu, Y.; Wu, P.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q. Vitamin A deficiency suppresses fish immune function with differences in different intestinal segments: The role of transcriptional factor NF-κB and p38 mitogen-activated protein kinase signalling pathways. Br. J. Nutr. 2017, 117, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-C.; Zhu, Y.-R.; Zhao, Z.-H.; Jiang, P.; Yin, F.-Q. Effects of Dietary Supplementation of Algae-Derived Polysaccharides on Morphology, Tight Junctions, Antioxidant Capacity and Immune Response of Duodenum in Broilers under Heat Stress. Animals 2021, 11, 2279. [Google Scholar] [CrossRef]
- Lian, P.; Braber, S.; Garssen, J.; Wichers, H.J.; Folkerts, G.; Fink-Gremmels, J.; Varasteh, S. Beyond Heat Stress: Intestinal Integrity Disruption and Mechanism-Based Intervention Strategies. Nutrients 2020, 12, 734. [Google Scholar] [CrossRef] [Green Version]
- Naiel, M.A.; Abd El-hameed, S.A.; Arisha, A.H.; Negm, S.S. Gum Arabic-enriched diet modulates growth, antioxidant defenses, innate immune response, intestinal microbiota and immune related genes expression in tilapia fish. Aquaculture 2022, 556, 738249. [Google Scholar] [CrossRef]
- Yang, G.; Bibi, S.; Du, M.; Suzuki, T.; Zhu, M.-J. Regulation of the intestinal tight junction by natural polyphenols: A mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3830–3839. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Sheikhzadeh, N.; Hamidian, G.; Mardani, K.; Oushani, A.K.; Firouzamandi, M.; Esteban, M.Á.; Shohreh, P. Changes in rainbow trout (Oncorhynchus mykiss) growth and mucosal immune parameters after dietary administration of grape (Vitis vinifera) seed extract. Fish Physiol. Biochem. 2021, 47, 547–563. [Google Scholar] [CrossRef]
- Naiel, M.A.; Alagawany, M.; Patra, A.K.; El-Kholy, A.I.; Amer, M.S.; Abd El-Hack, M.E. Beneficial impacts and health benefits of macroalgae phenolic molecules on fish production. Aquaculture 2021, 534, 736186. [Google Scholar] [CrossRef]
- Naiel, M.A.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The risk assessment of high-fat diet in farmed fish and its mitigation approaches: A review. J. Anim. Physiol. Anim. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Frosali, S.; Pagliari, D.; Gambassi, G.; Landolfi, R.; Pandolfi, F.; Cianci, R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. J. Immunol. Res. 2015, 2015, 489821. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Cao, L.-P.; Du, J.-L.; He, Q.; Gu, Z.-Y.; Jeney, G.; Xu, P.; Yin, G.-J. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish Shellfish. Immunol. 2020, 104, 391–401. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | % | Proximate Analysis | |
|---|---|---|---|
| Fish meal (61.6%) Soybean meal (44.2%) Wheat flour Cotton seed meal Rice bran Corn flour Cellulose Zeolite Soy lecithin Vitamin premix 1 Mineral premix 2 | 30 17 8 20 4 8 2 1 4 3 3 | Crude protein (%) Crude lipid (%) Dry matter (%) Ash (%) Gross energy (kcal/kg) | 33.21 10.30 87.46 8.08 4167.21 |
| Function | Target Gene | Accession Number | Annealing Temperature (°C) | Efficacy (%) | Product Size (bp) | Primer Sequences |
|---|---|---|---|---|---|---|
| Nrf-2 | JX462955 | 60 | 95 | 158 | TTCCCGCTGGTTTACCTTAC CGTTTCTTCTGCTTGTCTTT | |
| TLR-2 | HQ731681 | 60 | 95 | 94 | GTGCTCCTGTGAGTTTGTATCT TGGTGTGTCGCACACATAATAG | |
| MyD-88 | HQ380208 | 58 | 94 | 107 | GCCCAGGAACTCACTCTAAAC GGGTCTGGTGTAATCACAGATG | |
| Immune-related genes | IL-1β | KC008576.1 | 60 | 93 | 189 | CTCTACCTTGCTTGTACCCAG AGCTGTGCTAATAAACCATCCAG |
| Lysozyme-C | AB027305 | 60 | 96 | 359 | GTGTCTGATGTGGCTGTGCT TTCCCCAGGTATCCCATGAT | |
| C3 | AB016211 | 60 | 98 | 155 | CAATGCCCGAGTGTCCTA TCGTTCACAGGTGTAGCC | |
| Occludin | KF975606.1 | 55.5 | 93 | 145 | ATCGGTTCAGTACAATCAGG GACAATGAAGCCCATAACAA | |
| Cldn-3 | JQ767157 | 56.6 | 94 | 114 | GCACCAACTGTATCGAGGATG GGTTTGCCACCCAAGCCACCGGAATGA | |
| Tight junction-related genes | Cldn-7 | JQ767155 | 56 | 94 | 104 | CTTCTATAACCCCTTCACACCAG ACATGCCTCCACCCATTATG |
| ZO-1 | KY290394 | 65 | 93 | 107 | AGGAAGTTCTCCCTCGTACTC CCTCTGTTGTGGTTGAGTGTAG | |
| Housekeeping gene | β-actin | M24113.1 | 60 | 93 | 110 | TCACCACCACAGCCGAGAG CAGGGAGGAGGAGGAAGCAG |
| Retention Time (min) | Compound Name | Yield (%) |
|---|---|---|
| 23.611 | n-Eicosane | 0.72 |
| 23.748 | Limonen dioxide | 0.92 |
| 24.142 | 7-oxabicyclo [4.1.0] heptane, 1-methyl-4-(2-methyloxiranyl) | 1.13 |
| 24.292 | Myristic acid | 6.25 |
| 25.085 | n-Heptadecane | 0.61 |
| 25.575 | Hexahydrofarnesyl acetone | 0.82 |
| 25.644 | Myristaldehyde | 2.56 |
| 26.849 | 9-Hexadecenoic acid | 3.69 |
| 26.961 | Oleic acid | 4.2 |
| 27.199 | Palmitic acid | 36 |
| 27.364 | Arachidic acid | 3.1 |
| 27.525 | Ethyl palmitate | 5.43 |
| 27.701 | Behenic acid | 2.05 |
| 27.812 | Stearic acid | 1.43 |
| 29.168 | Phytol | 0.62 |
| 29.406 | 9-Hexadecenoic acid | 15.79 |
| 29.651 | n-Octadecenoic acid | 3.08 |
| 29.723 | Ethyl 9-octadeccnoet | 1.85 |
| 31.012 | 1-Hentetracontanol | 9.03 |
| 32.084 | n-Octadecyl isocyanate | 0.72 |
| Parameters | Experimental Groups | p Value | |||||
|---|---|---|---|---|---|---|---|
| CTR | PA1 | PA2 | PA3 | Combined | Linear | Quadratic | |
| IW (g) | 14.75 ± 0.03 | 14.78 ± 0.04 | 14.78 ± 0.04 | 14.78 ± 0.03 | 0.314 | 0.339 | 0.289 |
| IL (cm) | 5.23 ± 0.02 | 5.23 ± 0.03 | 5.25 ± 0.04 | 5.24 ± 0.03 | 0.284 | 0.285 | 0.283 |
| FW (g) | 31.19 ± 0.08 c | 31.97 ± 0.59 c | 35.32 ± 0.75 b | 39.34 ± 0.92 a | 0.021 | 0.019 | 0.077 |
| FL (cm) | 12.78 ± 0.03 b | 12.87 ± 0.17 b | 13.31 ± 0.16 a | 13.70 ± 0.19 a | 0.004 | 0.002 | 0.086 |
| WGR (%) | 111.51 ± 2.76 c | 116.50 ± 3.97 c | 138.97 ± 2.97 b | 166.18 ± 2.18 a | 0.015 | 0.068 | 0.014 |
| CF | 1.49 ± 0.01 b | 1.50 ± 0.01 b | 1.50 ± 0.01 b | 1.53 ± 0.02 a | 0.034 | 0.033 | 0.071 |
| SGR (%/d)2 FCR (g/g) SR (%) | 1.34 ± 0.01 a 1.86 ± 0.02 a 100 | 1.38 ± 0.13 a 1.81 ± 0.03 a 100 | 1.56 ± 0.19 b 1.74 ± 0.03 b 100 | 1.75 ± 0.11 b 1.66 ± 0.02 c 100 | 0.014 | 0.287 | 0.012 |
| 0.002 | 0.084 | 0.002 | |||||
| 0.055 | 0.061 | 0.059 | |||||
| Parameters | Experimental Groups | p Value | |||||
|---|---|---|---|---|---|---|---|
| CTR | PA1 | PA2 | PA3 | Combined | Linear | Quadratic | |
| Crude protein | 18.28 ± 0.02 | 18.25 ± 0.02 | 18.30 ± 0.03 | 18.30 ± 0.03 | 0.587 | 0.594 | 0.601 |
| Crude lipid | 3.34 ± 0.02 | 3.39 ± 0.03 | 3.36 ± 0.02 | 3.34 ± 0.02 | 0.354 | 0.349 | 0.299 |
| Ash | 0.92 ± 0.01 | 0.91 ± 0.01 | 0.92 ± 0.01 | 0.92 ± 0.01 | 0.076 | 0.078 | 0.081 |
| Moisture | 76.20 ± 0.04 | 76.26 ± 0.03 | 76.29 ± 0.03 | 76.27 ± 0.03 | 0.094 | 0.096 | 0.091 |
| Parameters | Experimental Groups | p Value | |||||
|---|---|---|---|---|---|---|---|
| CTR | PA1 | PA2 | PA3 | Combined | Linear | Quadratic | |
| Amylase | 10.26 ± 0.09 | 10.29 ± 0.08 | 10.31 ± 0.12 | 10.38 ± 0.14 | 0.241 | 0.257 | 0.231 |
| Lipase | 1.16 ± 0.12 c | 2.02 ± 0.09 b | 2.15 ± 0.28 a | 2.04 ± 0.10 b | 0.016 | 0.0001 | 0.087 |
| Trypsin | 0.223 ± 0.09 c | 0.224 ± 0.08 c | 0.246 ± 0.01 b | 0.270 ± 0.22 a | 0.030 | 0.011 | 0.068 |
| Chymotrypsin | 0.076 ± 0.21 b | 0.082 ± 0.17 b | 0.095 ± 0.12 a | 0.091 ± 0.15 a | 0.011 | 0.009 | 0.099 |
| Parameters | Experimental Groups | p Value | |||||
|---|---|---|---|---|---|---|---|
| CTR | PA1 | PA2 | PA3 | Combined | Linear | Quadratic | |
| TIg (%) | 16.09 ± 0.19 c | 16.07 ± 0.13 c | 17.15 ±0.21 b | 17.43 ± 0.17 a | 0.005 | 0.036 | 0.058 |
| LYZ (μg/mL) | 28.91 ± 0.88 c | 30.17 ± 0.92 c | 39.33 ± 0.69 b | 44.90 ± 1.46 a | 0.017 | 0.042 | 0.112 |
| ACH50 (Unit/mL) | 115.3 ± 2.36 c | 122.8 ± 3.41 c | 130.3 ± 3.97 b | 144.2 ± 2.53 a | 0.002 | 0.031 | 0.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikhzadeh, N.; Ahmadifar, E.; Soltani, M.; Tayefi-Nasrabadi, H.; Mousavi, S.; Naiel, M.A.E. Brown Seaweed (Padina australis) Extract can Promote Performance, Innate Immune Responses, Digestive Enzyme Activities, Intestinal Gene Expression and Resistance against Aeromonas hydrophila in Common Carp (Cyprinus carpio). Animals 2022, 12, 3389. https://doi.org/10.3390/ani12233389
Sheikhzadeh N, Ahmadifar E, Soltani M, Tayefi-Nasrabadi H, Mousavi S, Naiel MAE. Brown Seaweed (Padina australis) Extract can Promote Performance, Innate Immune Responses, Digestive Enzyme Activities, Intestinal Gene Expression and Resistance against Aeromonas hydrophila in Common Carp (Cyprinus carpio). Animals. 2022; 12(23):3389. https://doi.org/10.3390/ani12233389
Chicago/Turabian StyleSheikhzadeh, Najmeh, Ehsan Ahmadifar, Mehdi Soltani, Hossein Tayefi-Nasrabadi, Shalaleh Mousavi, and Mohammed A. E. Naiel. 2022. "Brown Seaweed (Padina australis) Extract can Promote Performance, Innate Immune Responses, Digestive Enzyme Activities, Intestinal Gene Expression and Resistance against Aeromonas hydrophila in Common Carp (Cyprinus carpio)" Animals 12, no. 23: 3389. https://doi.org/10.3390/ani12233389







