Economic Improvement of Artisanal Fishing by Studying the Survival of Discarded Plectorhinchus mediterraneus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Geographical Location of the Fisheries, Vessels, and Fishing Gears Characteristics
2.3. Survival Rates
2.4. Physiological Recovery
2.5. Serum and Mucus Measurements
2.6. Growth Parameters and Historical Data
2.7. Statistics
3. Results
3.1. Survival Rates
3.2. Physiological Recovery after Capture
3.3. Growth Rates and Selling Prices on the Market
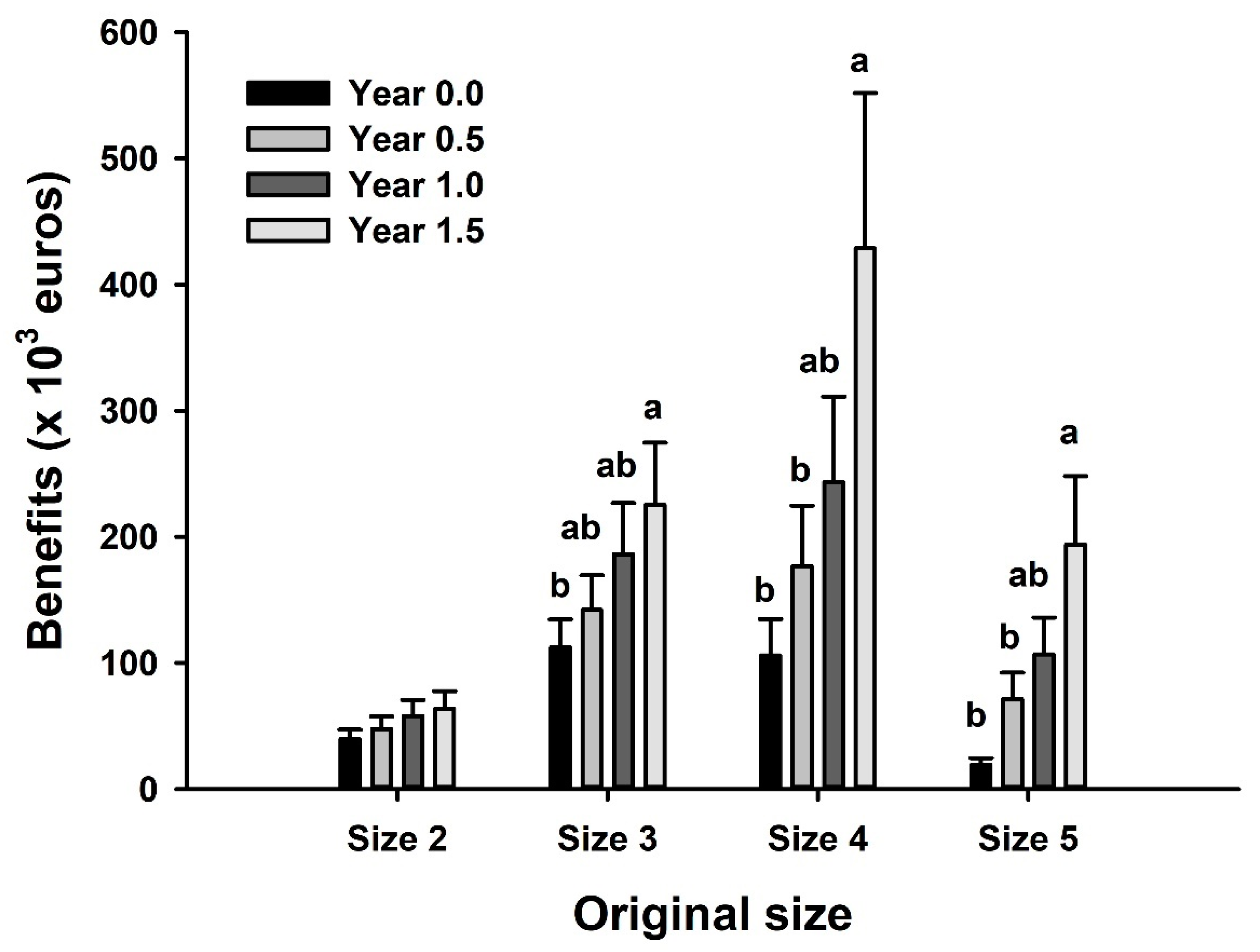
3.4. Economic Benefits in the Case of Discards
4. Discussion
4.1. Survival of Discards
4.2. Physiological Recovery after Capture
4.3. Economic Benefits Due to the Recapture of Discards
4.4. Future Strategies to Improve Artisanal Fisheries
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, H.; Basurto, X. Defining small-scale fisheries and examining the role of science in shaping perceptions of who and what counts: A systematic review. Front. Mar. Sci. 2019, 6, 236. [Google Scholar] [CrossRef]
- Crona, B.; Pomeroy, R.S.; Purcell, S.W. Editorial: Small-scale and artisanal fisheries. Front. Mar. Sci. 2020, 30, 455. [Google Scholar] [CrossRef]
- Purcell, S.W.; Fraser, N.J.; Tagica, S.; Lalavanua, W.; Ceccarelli, D.M. Discriminating catch composition and fishing modes in an artisanal multispecies fishery. Front. Mar. Sci. 2018, 5, 243. [Google Scholar] [CrossRef]
- FAO. Voluntary Guidelines for Securing Sustainable Small-Scale Fisheries in the Context of Food Security and Poverty Eradication; FAO: Rome, Italy, 2015; p. 34. [Google Scholar]
- Batista, V.S.; Fabré, N.N.; Malhado, A.C.M.; Ladle, R.J. Tropical artisanal coastal fisheries: Challenges and future directions. Rev. Fish. Sci. Aquac. 2014, 22, 1–15. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guenette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Pauly, D.; Alder, J.; Bennett, E.; Christensen, V.; Tyedmers, P.; Watson, R. The future for fisheries. Science 2003, 302, 1359–1361. [Google Scholar] [CrossRef]
- Aguión, A.; Ojea, E.; Garcia-Florez, L.; Cruz, T.; Garmendia, J.M.; Davoult, D.; Queiroga, H.; Rivera, A.; Acuna-Fernandez, J.L.; Macho, G. Establishing a governance threshold in small-scale fisheries to achieve sustainability. Ambio 2022, 51, 652–665. [Google Scholar] [CrossRef]
- Allison, E.H.; Ellis, F. The livelihoods approach and management of small-scale fisheries. Mar. Policy 2001, 25, 377–388. [Google Scholar] [CrossRef]
- EU. Regulation (EU) No 508/2014 of the European Parliament and of the Council, of 15 May 2014; EU: Maastricht, The Netherlands, 2014. [Google Scholar]
- STECF. The 2021 Annual Economic Report on the EU Fishing Fleet (STECF 21–08); Publications Office of the European Union: Luxembourg, 2021; p. 540. [Google Scholar]
- Guyader, O.; Berthou, P.; Koutsikopoulos, C.; Alban, F.; Demanèche, S.; Gaspar, M.B.; Eschbaum, R.; Fahy, E.; Tully, O.; Reynal, L.; et al. Small scale fisheries in Europe: A comparative analysis based on a selection of case studies. Fish. Res. 2013, 140, 1–13. [Google Scholar] [CrossRef]
- INE. Instituto Nacional de Estatística—Estatísticas da Pesca: 2019; INE: Lisboa, Portugal, 2020. [Google Scholar]
- FranceAgriMer. Available online: https://www.franceagrimer.fr/Accompagner/Plan-de-relance-Peche-et-Aquaculture (accessed on 25 September 2022).
- Di Cintio, A.; Scianna, C.; Prato, G. Analysis of small-scale fisheries value chain: An interview-based approach in Italian marine protected areas. Fish. Res. 2022, 252, 106358. [Google Scholar] [CrossRef]
- MAPA. La Flota Española: Situación a 31 de Diciembre de 2021; MAPA: Madrid, Spain, 2021; p. 14. [Google Scholar]
- Fernández-González, R.; Pérez-Pérez, M.; Hervés-Estévez, J.; Garza-Gil, M.D. Socio-economic impact of Covid-19 on the fishing sector: A case study of a region highly dependent on fishing in Spain. Ocean Coast. Manag. 2022, 221, 106131. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Abdallah, P.R.; Gasalla, M.A. Harvesting costs and revenues: Implication of the performance of open-access industrial fishing fleets off Rio Grande, Brazil. Mar. Policy 2018, 93, 104–112. [Google Scholar] [CrossRef]
- Carvalho, N.; Guillen, J. Economic impact of eliminating the fuel tax exemption in the EU fishing fleet. Sustainability 2021, 13, 2719. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Fernández-Castro, M.; Jerez-Cepa, I.; Barragán-Méndez, C.; Pérez, M.; Pérez, E.; Gil, J.; Canoura, J.; Farias, C.; Mancera, J.M.; et al. Survival and physiological recovery after capture by hookline: The case study of the blackspot seabream (Pagellus bogaraveo). Fishes 2021, 6, 16. [Google Scholar] [CrossRef]
- Marcalo, A.; Guerreiro, P.M.; Bentes, L.; Rangel, M.; Monteiro, P.; Oliveira, F.; Afonso, C.M.L.; Pousao-Ferreira, P.; Benoit, H.P.; Breen, M.; et al. Effects of different slipping methods on the mortality of sardine, Sardina pilchardus, after purse-seine capture off the Portuguese Southern coast (Algarve). PLoS ONE 2018, 13, e0195433. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, S.S.; Ampe, B.; Van Bogaert, N.; Vanden Berghe, C.; Vanelslander, B. Flatfish survivors have tales to tell: Cold seawater and reduced deployment duration contribute to the survival of European plaice (Pleuronectes platessa) discarded by Belgian beam trawlers. Fish. Res. 2021, 240, 105966. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; Ruiz-Jarabo, I.; Fuentes, J.; Mancera, J.M.; Sobrino, I. Survival rates and physiological recovery responses in the lesser-spotted catshark (Scyliorhinus canicula) after bottom-trawling. Comp. Biochem. Physiol. A 2019, 233, 1–9. [Google Scholar] [CrossRef]
- Ellis, J.R.; Burt, G.J.; Grilli, G.; McCully Phillips, S.R.; Catchpole, T.L.; Maxwell, D.L. At-vessel mortality of skates (Rajidae) taken in coastal fisheries and evidence of longer-term survival. J. Fish Biol. 2018, 92, 1702–1719. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; Sobrino, I.; Marin-Rincon, A.; Fernandez-Boo, S.; Costas, B.; Mancera, J.M.; Ruiz-Jarabo, I. Acute-stress biomarkers in three Octopodidae species after bottom trawling. Front. Physiol. 2019, 10, 784. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; González-Duarte, M.M.; Sobrino, I.; Vila, Y.; Mancera, J.M.; Ruiz-Jarabo, I. Physiological recovery after bottom trawling as a method to manage discards: The case study of Nephrops norvegicus and Squilla mantis. Mar. Policy 2020, 116, 103895. [Google Scholar] [CrossRef]
- Fox, C.J.; Albalat, A.; Valentinsson, D.; Nilsson, H.C.; Armstrong, F.; Randall, P.; Catchpole, T. Survival rates for Nephrops norvegicus discarded from Northern European trawl fisheries. ICES J. Mar. Sci. 2020, 77, 1698–1710. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Amanajas, R.D.; Baldisserotto, B.; Mancera, J.M.; Val, A.L. Tambaqui (Colossoma macropomum) acclimated to different tropical waters from the Amazon basin shows specific acute-stress responses. Comp. Biochem. Physiol. A 2020, 245, 110706. [Google Scholar] [CrossRef]
- Cook, K.V.; Hinch, S.G.; Drenner, S.M.; Raby, G.D.; Patterson, D.A.; Cooke, S.J. Dermal injuries caused by purse seine capture result in lasting physiological disturbances in coho salmon. Comp. Biochem. Physiol. A 2019, 227, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Methling, C.; Skov, P.V.; Madsen, N. Reflex impairment, physiological stress, and discard mortality of European plaice Pleuronectes platessa in an otter trawl fishery. ICES J. Mar. Sci. 2017, 496, 207–218. [Google Scholar] [CrossRef]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. C 1998, 120, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jarabo, I.; Barragán-Méndez, C.; Jerez-Cepa, I.; Fernandez-Castro, M.; Sobrino, I.; Mancera, J.M.; Aerts, J. Plasma 1alpha-hydroxycorticosterone as biomarker for acute stress in catsharks (Scyliorhinus canicula). Front. Physiol. 2019, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Paullada-Salmerón, J.A.; Jerez-Cepa, I.; Gonçalves Neto, J.B.; Bystriansky, J.S.; Mancera, J.M. Acute stress in lesser-spotted catshark (Scyliorhinus canicula Linnaeus, 1758) promotes amino acid catabolism and osmoregulatory imbalances. Animals 2022, 12, 1192. [Google Scholar] [CrossRef]
- Cabrera-Busto, J.; Mancera, J.M.; Ruiz-Jarabo, I. Cortisol and dexamethasone mediate glucocorticoid actions in the lesser spotted catshark (Scyliorhinus canicula). Biology 2022, 11, 20. [Google Scholar] [CrossRef]
- Tenningen, M.; Vold, A.; Olsen, R.E. The response of herring to high crowding densities in purse-seines: Survival and stress reaction. ICES J. Mar. Sci. 2012, 69, 1523–1531. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Gregório, S.F.; Fuentes, J. Regulation of stanniocalcin secretion by calcium and PTHrP in gilthead seabream (Sparus aurata). Biology 2022, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Skrzynska, A.K.; Maiorano, E.; Bastaroli, M.; Naderi, F.; Miguez, J.M.; Martinez-Rodriguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Impact of air exposure on vasotocinergic and isotocinergic systems in gilthead sea bream (Sparus aurata): New insights on fish stress response. Front. Physiol. 2018, 9, 15. [Google Scholar] [CrossRef]
- McCormick, S.D.; Taylor, M.L.; Regish, A.M. Cortisol is an osmoregulatory and glucose-regulating hormone in Atlantic sturgeon, a basal ray-finned fish. J. Exp. Biol. 2020, 223, jeb220251. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An important tool to assess the welfare of aquatic animals. Biology 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, T.; Uhlmann, S.; Breen, M.; Adão, C.; Arregi, L.; Benoît, H.; Campos, A.; Castro, M.; Ferter, K.; Karlsen, J.; et al. Working Group on Methods for Estimating Discard Survival (Wgmeds; Outputs from 2019 Meeting); International Council for the Exploration of the Sea: Copenhagen, Denmark, 2020; p. 81. [Google Scholar]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Gonçalves, O.; Pâscoa, I.; Martín del Río, M.P.; Mancera, J.M. Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup, 1858) to osmotic challenge: Implications for osmoregulation, energy metabolism and growth. Aquaculture 2009, 287, 419–426. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Barton, B.A.; McLeay, D.J. Stress and acclimation. In Methods of Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 451–489. [Google Scholar]
- FAO. Rapport du Groupe de Travail sur les Merlus et les Crevettes d’Eaux Profondes dans la Zone nord du COPACE; FAO: Rome, Italy, 1990. [Google Scholar]
- Fernández-Alacid, L.; Sanahuja, I.; Ordóñez-Grande, B.; Sánchez-Nuño, S.; Viscor, G.; Gisbert, E.; Herrera, M.; Ibarz, A. Skin mucus metabolites in response to physiological challenges: A valuable non-invasive method to study teleost marine species. Sci. Total Environ. 2018, 644, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Ouréns, R.; Dione, L.; Samba, I.; Sánchez-Carnero, N.; Freire, J. Length–weight relationships for 13 fish species from a coastal artisanal fishery at Cape Verde peninsula (Senegal). J. Appl. Ichthyol. 2014, 31, 1177–1179. [Google Scholar] [CrossRef]
- Meissa, B.; Gascuel, D. Overfishing of marine resources: Some lessons from the assessment of demersal stocks off Mauritania. ICES J. Mar. Sci. 2015, 72, 414–427. [Google Scholar] [CrossRef]
- Wegner, N.C.; Portner, E.J.; Nguyen, D.T.; Bellquist, L.; Nosal, A.P.; Pribyl, A.L.; Stierhoff, K.L.; Fischer, P.; Franke, K.; Vetter, R.D.; et al. Post-release survival and prolonged sublethal effects of capture and barotrauma on deep-dwelling rockfishes (genus Sebastes): Implications for fish management and conservation. ICES J. Mar. Sci. 2021, 78, 3230–3244. [Google Scholar] [CrossRef]
- Humborstad, O.B.; Breen, M.; Davis, M.W.; Lokkeborg, S.; Mangor-Jensen, A.; Midling, K.O.; Olsen, R.E. Survival and recovery of longline- and pot-caught cod (Gadus morhua) for use in capture-based aquaculture (CBA). Fish. Res. 2016, 174, 103–108. [Google Scholar] [CrossRef]
- van der Reijden, K.J.; Molenaar, P.; Chen, C.; Uhlmann, S.S.; Goudswaard, P.C.; van Marlen, B. Survival of undersized plaice (Pleuronectes platessa), sole (Solea solea), and dab (Limanda limanda) in North Sea pulse-trawl fisheries. ICES J. Mar. Sci. 2017, 74, 1672–1680. [Google Scholar] [CrossRef]
- Cook, K.V.; Hinch, S.G.; Watson, M.S.; Patterson, D.A.; Reid, A.J.; Cooke, S.J. Experimental capture and handling of chum salmon reveal thresholds in injury, impairment, and physiology: Best practices to improve bycatch survival in a purse seine fishery. Fish. Res. 2018, 206, 96–108. [Google Scholar] [CrossRef]
- Martins, C.L.; Walker, T.I.; Reina, R.D. Stress-related physiological changes and post-release survival of elephant fish (Callorhinchus milii) after longlining, gillnetting, angling and handling in a controlled setting. Fish. Res. 2018, 204, 116–124. [Google Scholar] [CrossRef]
- Frick, L.H.; Reina, R.D.; Walker, T.I. Stress related physiological changes and post-release survival of Port Jackson sharks (Heterodontus portusjacksoni) and gummy sharks (Mustelus antarcticus) following gill-net and longline capture in captivity. J. Exp. Mar. Biol. Ecol. 2010, 385, 29–37. [Google Scholar] [CrossRef]
- Costas, B.; Conceicao, L.; Aragao, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.; Afonso, A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76. [Google Scholar] [CrossRef]
- Tracey, S.R.; Hartmann, K.; Leef, M.; McAllister, J. Capture-induced physiological stress and postrelease mortality for Southern bluefin tuna (Thunnus maccoyii) from a recreational fishery. Can. J. Fish. Aquat. Sci. 2016, 73, 1547–1556. [Google Scholar] [CrossRef]
- Uhlmann, S.S.; Broadhurst, M.K.; Millar, R.B. Effects of modified handling on the physiological stress of trawled-and-discarded yellowfin bream (Acanthopagrus australis). PLoS ONE 2015, 10, e0131109. [Google Scholar] [CrossRef]
- Olsen, R.E.; Oppedal, F.; Tenningen, M.; Vold, A. Physiological response and mortality caused by scale loss in Atlantic herring. Fish. Res. 2012, 129, 21–27. [Google Scholar] [CrossRef]
- Iki, A.; Anderson, W.G.; Deck, C.A.; Ogihara, M.H.; Ikeba, K.; Kataoka, H.; Hyodo, S. Measurement of 1alpha hydroxycorticosterone in the Japanese banded houndshark, Triakis scyllium, following exposure to a series of stressors. Gen. Comp. Endocr. 2020, 292, 113440. [Google Scholar] [CrossRef]
- Santos, P.; Peixoto, D.; Ferreira, I.; Passos, R.; Pires, P.; Simoes, M.; Pousao-Ferreira, P.; Baptista, T.; Costas, B. Short-term immune responses of gilthead seabream (Sparus aurata) juveniles against Photobacterium damselae subsp. piscicida. Int. J. Mol. Sci. 2022, 23, 1561. [Google Scholar] [CrossRef]
- Machado, M.; Azeredo, R.; Diaz-Rosales, P.; Afonso, A.; Peres, H.; Oliva-Teles, A.; Costas, B. Dietary tryptophan and methionine as modulators of European seabass (Dicentrarchus labrax) immune status and inflammatory response. Fish Shellfish Immunol. 2015, 42, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pita, C.; Villasante, S.; Pascual-Fernandez, J.J. Managing small-scale fisheries under data poor scenarios: Lessons from around the world. Mar. Policy 2019, 101, 154–157. [Google Scholar] [CrossRef]
- Koeck, B.; Zavorka, L.; Aldven, D.; Naslund, J.; Arlinghaus, R.; Thornqvist, P.O.; Winberg, S.; Bjornsson, B.; Johnsson, J.I. Angling selects against active and stress-resilient phenotypes in rainbow trout. Can. J. Fish. Aquat. Sci. 2019, 76, 320–333. [Google Scholar] [CrossRef]
- Pascual-Fernandez, J.J.; Pita, C.; Josupeit, H.; Said, A.; Garcia Rodrigues, J. Markets, distribution and value chains in small-scale fisheries: A special focus on Europe. In Transdisciplinary for Small-Scale Fisheries Governance; Chuenpagdee, R., Jentoft, S., Eds.; Springer: Cham, Switzerland, 2019; Volume 21, pp. 141–162. [Google Scholar]



| Parameter | Fishing Gear | 0 h | 24 h | 48 h |
|---|---|---|---|---|
| pH | Gillnet | 7.26 ± 0.03 b | 7.63 ± 0.01 a | 7.59 ± 0.01 a |
| Hookline | 7.35 ± 0.06 b | 7.55 ± 0.02 a | 7.60 ± 0.02 a | |
| Phosphate (mM) | Gillnet | 7.2 ± 0.5 a | 3.6 ± 0.2 bc | 2.8 ± 0.2 c |
| Hookline | 4.8 ± 0.6 b | 3.2 ± 0.2 c | 2.8 ± 0.1 c | |
| Glucose (mM) | Gillnet | 7.9 ± 0.9 a | 3.4 ± 0.4 bc | 2.9 ± 0.2 c |
| Hookline | 5.0 ± 0.5 b | 3.9 ± 0.4 bc | 2.9 ± 0.1 c | |
| Total proteins (g dL−1) | Gillnet | 4.2 ± 0.4 a | 2.4 ± 0.1 b | 2.7 ± 0.1 b |
| Hookline | 3.6 ± 0.2 a | 2.5 ± 0.1 b | 2.8 ± 0.1 b | |
| Na+ (mM) | Gillnet | 130 ± 6 ab | 140 ± 9 a | 114 ± 4 b |
| Hookline | 154 ± 4 a | 140 ± 8 a | 129 ± 3 ab | |
| Cl− (mM) | Gillnet | 182 ± 5 | 194 ± 2 | 191 ± 3 |
| Hookline | 189 ± 4 | 190 ± 2 | 188 ± 1 | |
| Ca2+ (mM) | Gillnet | 3.6 ± 0.1 a | 2.3 ± 0.1 b | 2.2 ± 0.0 bc |
| Hookline | 2.5 ± 0.1 b | 2.2 ± 0.1 bc | 2.0 ± 0.1 c | |
| Mg2+ (mM) | Gillnet | 3.5 ± 0.4 a | 2.1 ± 0.2 b | 2.0 ± 0.2 b |
| Hookline | 1.7 ± 0.5 b | 1.1 ± 0.2 b | 1.3 ± 0.2 b | |
| K+ (mM) | Gillnet | 7.4 ± 0.4 a | 4.7 ± 0.3 b | 4.1 ± 0.3 bc |
| Hookline | 6.6 ± 0.9 a | 3.6 ± 0.5 c | 4.1 ± 0.3 bc | |
| Peroxidase activity (U mL−1) | Gillnet | 97 ± 16 ab | 68 ± 12 b | 109 ± 9 a |
| Hookline | 107 ± 18 ab | 88 ± 13 ab | 91 ± 9 ab | |
| Lysozyme activity (µg mL−1) | Gillnet | 8.9 ± 0.9 ab | 7.0 ± 0.7 b | 10.7 ± 1.1 ab |
| Hookline | 9.7 ± 1.0 ab | 9.3 ± 1.6 ab | 12.7 ± 1.5 a |
| Parameter | Fishing Gear | 0 h | 24 h | 48 h |
|---|---|---|---|---|
| Glucose (mM) | Gillnet | 0.46 ± 0.06 b | 0.63 ± 0.06 a | 0.65 ± 0.04 a |
| Hookline | 0.72 ± 0.06 a | 0.37 ± 0.04 b | 0.45 ± 0.04 b | |
| Lactate (mM) | Gillnet | 0.14 ± 0.02 c | 0.62 ± 0.07 a | 0.45 ± 0.04 ab |
| Hookline | 0.15 ± 0.04 c | 0.43 ± 0.08 abc | 0.30 ± 0.03 bc | |
| Total proteins (g dL−1) | Gillnet | 0.86 ± 0.17 b | 0.66 ± 0.08 b | 0.91 ± 0.07 b |
| Hookline | 1.60 ± 0.36 a | 0.45 ± 0.10 b | 0.77 ± 0.07 b | |
| Peroxidase activity (U mL−1) | Gillnet | 56 ± 10 bc | 87 ± 7 ab | 104 ± 8 a |
| Hookline | 87 ± 12 ab | 57 ± 13 bc | 38 ± 4 c | |
| Lysozyme activity (µg mL−1) | Gillnet | 0.47 ± 0.15 ab | 0.66 ± 0.11 ab | 0.56 ± 0.11 ab |
| Hookline | 1.17 ± 0.41 a | 0.18 ± 0.09 b | 0.55 ± 0.09 ab |
| Year | Size | Total Captures (kg) | Total Sales | ||
|---|---|---|---|---|---|
| Hookline | Gillnet | (Euros) | (Euros kg−1) | ||
| 2016 | 1 | 8605 | 11,767 | 128,263 | 6.29 |
| 2 | 3739 | 10,024 | 67,235 | 4.89 | |
| 3 | 6344 | 17,500 | 87,448 | 3.67 | |
| 4 | 4544 | 10,254 | 33,639 | 2.27 | |
| 5 | 1101 | 2975 | 4723 | 1.16 | |
| 2017 | 1 | 7199 | 13,036 | 129,490 | 6.40 |
| 2 | 2280 | 10,208 | 54,757 | 4.38 | |
| 3 | 4077 | 12,136 | 55,819 | 3.44 | |
| 4 | 2051 | 9503 | 23,968 | 2.07 | |
| 5 | 328 | 4742 | 6187 | 1.22 | |
| 2018 | 1 | 1118 | 7726 | 63,405 | 7.14 |
| 2 | 928 | 7873 | 54,120 | 6.15 | |
| 3 | 3246 | 17,341 | 107,481 | 5.21 | |
| 4 | 2692 | 33,610 | 147,958 | 4.06 | |
| 5 | 760 | 20,405 | 33,509 | 1.57 | |
| 2019 | 1 | 1435 | 3918 | 37,465 | 6.96 |
| 2 | 1363 | 2534 | 27,570 | 7.03 | |
| 3 | 5705 | 14,915 | 119,640 | 5.80 | |
| 4 | 3352 | 46,384 | 181,418 | 3.64 | |
| 5 | 955 | 23,478 | 38,577 | 1.58 | |
| 2020 | 1 | 1289 | 1578 | 24,369 | 8.50 |
| 2 | 2034 | 1915 | 29,997 | 7.60 | |
| 3 | 11,141 | 14,116 | 151,761 | 6.01 | |
| 4 | 5284 | 30,454 | 124,285 | 3.47 | |
| 5 | 1363 | 12,537 | 21,663 | 1.56 | |
| 2021 | 1 | 2033 | 3071 | 41,696 | 8.17 |
| 2 | 1997 | 1867 | 32,410 | 8.39 | |
| 3 | 19,978 | 10,690 | 216,156 | 7.05 | |
| 4 | 9365 | 36,494 | 201,368 | 4.39 | |
| 5 | 3024 | 13,670 | 25,371 | 1.52 | |
| 2022 (to May 28) | 1 | 640 | 1874 | 21,234 | 8.44 |
| 2 | 474 | 601 | 10,361 | 9.64 | |
| 3 | 3121 | 2524 | 48,635 | 8.61 | |
| 4 | 1390 | 4353 | 28,913 | 5.03 | |
| 5 | 324 | 2069 | 4911 | 2.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Jarabo, I.; Partida, B.; Page, M.; Madera, D.; Saiz, N.; Alonso-Gómez, A.; Herrera-Castillo, L.; Isorna, E.; Alonso-Gómez, Á.L.; Valenciano, A.I.; et al. Economic Improvement of Artisanal Fishing by Studying the Survival of Discarded Plectorhinchus mediterraneus. Animals 2022, 12, 3423. https://doi.org/10.3390/ani12233423
Ruiz-Jarabo I, Partida B, Page M, Madera D, Saiz N, Alonso-Gómez A, Herrera-Castillo L, Isorna E, Alonso-Gómez ÁL, Valenciano AI, et al. Economic Improvement of Artisanal Fishing by Studying the Survival of Discarded Plectorhinchus mediterraneus. Animals. 2022; 12(23):3423. https://doi.org/10.3390/ani12233423
Chicago/Turabian StyleRuiz-Jarabo, Ignacio, Blanca Partida, María Page, Diego Madera, Nuria Saiz, Aitana Alonso-Gómez, Lisbeth Herrera-Castillo, Esther Isorna, Ángel L. Alonso-Gómez, Ana I. Valenciano, and et al. 2022. "Economic Improvement of Artisanal Fishing by Studying the Survival of Discarded Plectorhinchus mediterraneus" Animals 12, no. 23: 3423. https://doi.org/10.3390/ani12233423
APA StyleRuiz-Jarabo, I., Partida, B., Page, M., Madera, D., Saiz, N., Alonso-Gómez, A., Herrera-Castillo, L., Isorna, E., Alonso-Gómez, Á. L., Valenciano, A. I., de Pedro, N., Saez, J., & Delgado, M. J. (2022). Economic Improvement of Artisanal Fishing by Studying the Survival of Discarded Plectorhinchus mediterraneus. Animals, 12(23), 3423. https://doi.org/10.3390/ani12233423







