Greater Horseshoe Bats Recognize the Sex and Individual Identity of Conspecifics from Their Echolocation Calls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Husbandry of Bats
2.2. Sound Recording and Editing
2.2.1. Sound Recording
2.2.2. Playback Stimuli for Sex Recognition
2.2.3. Playback Stimuli for Individual Recognition
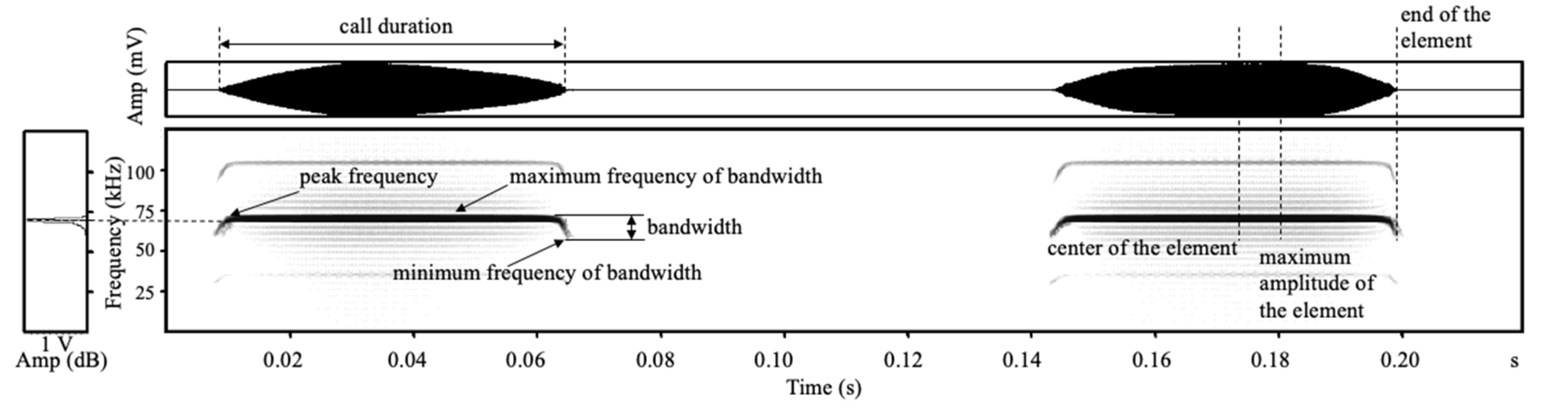
2.3. Sound Analysis and Playback
2.4. Behavioral Observations and Response Measures
- (1)
- Nodding. This behavior was characterized by a movement of the head to the chin. The number was counted as 1 each time the subject bat raised its head and then lowered its head. If the bat only raised its head, the number was counted as 0.5.
- (2)
- Ear movement: movement of the ears around to detect the caller. This was often accompanied by echolocation. The number was counted as 1 when the bat moved its left or right ear.
- (3)
- Body movement: rotation of the body toward the microphone to aid in detection. The number was counted as 1 when the bat turned to the left or right.
- (4)
- Echolocation calls. The number of echolocation pulses emitted by the bat was counted.
2.5. Statistical Analysis
2.6. Ethical Note
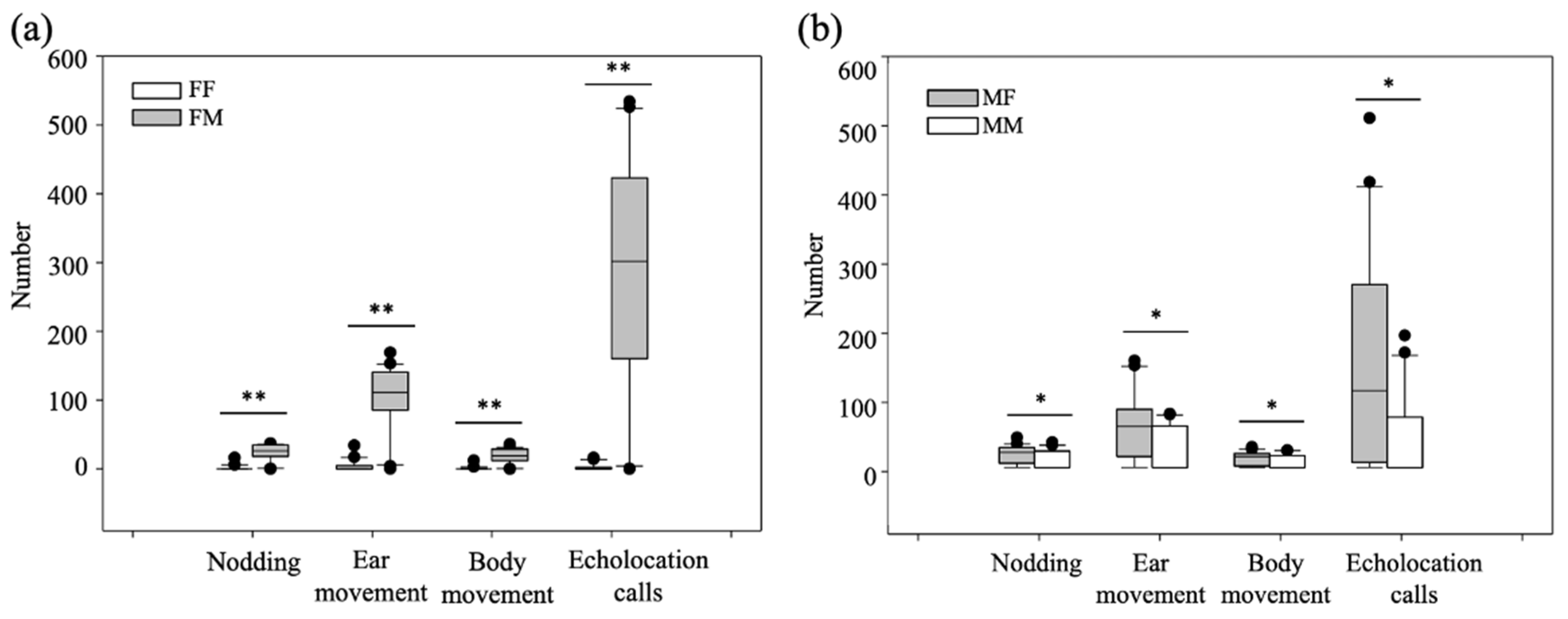
3. Results
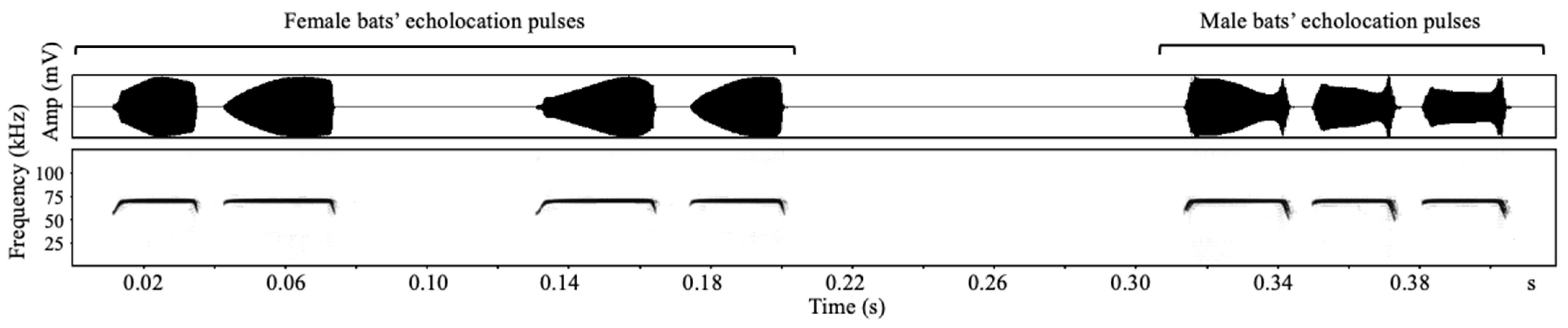
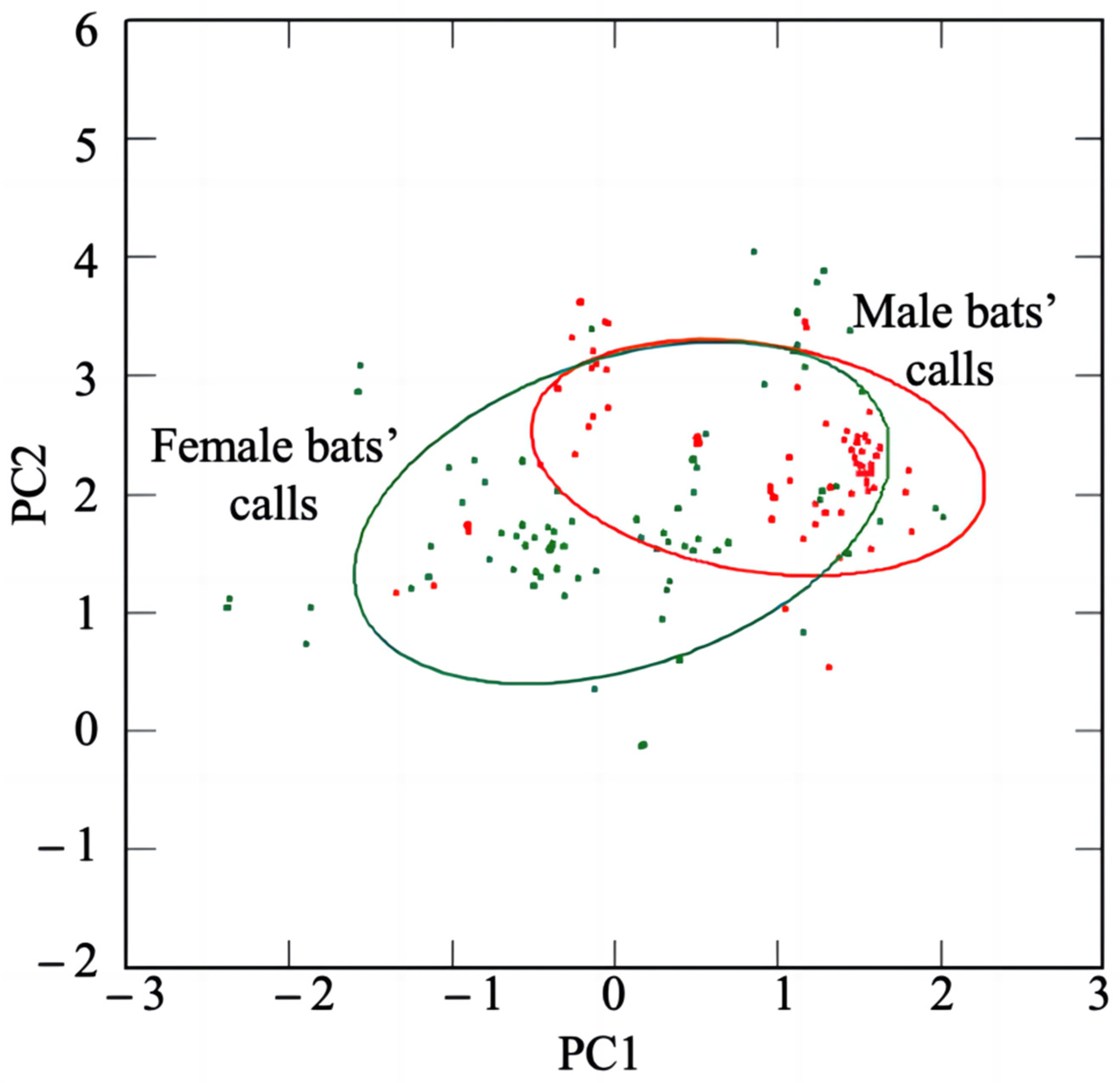
3.1. Sexual Dimorphism in Echolocation Calls and Sex Recognition
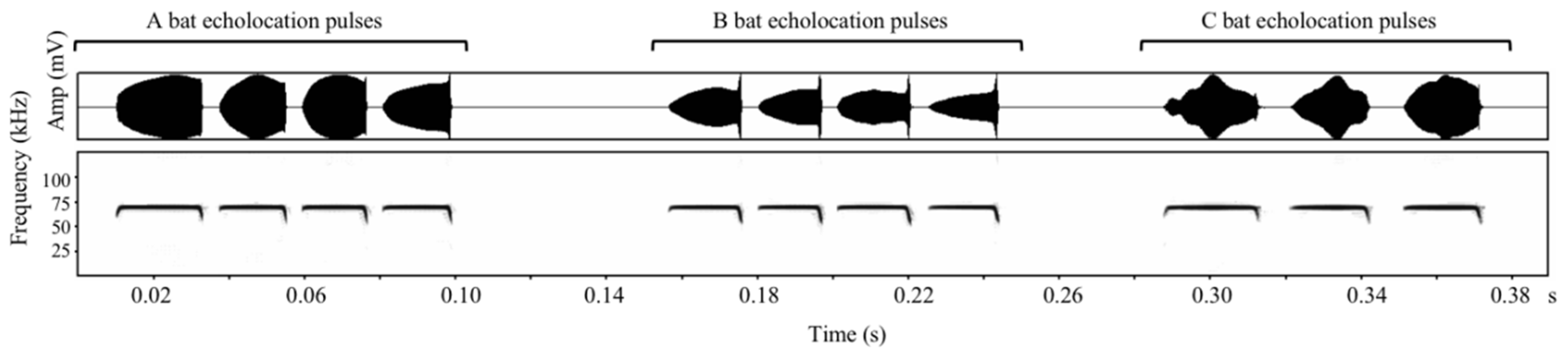
3.2. Individual Signature in Echolocation Calls and Individual Identity Recognition
4. Discussion
4.1. Sexual Dimorphism of and Sex Identification from Acoustic Signals
4.2. Individual Signature and Individual Recognition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherman, P.; Reeve, H.; Pfennig, D. Recognition Systems. In Behavioural Ecology, 4th ed.; Krebs, J., Davies, N., Eds.; Blackwell Science: Oxford, UK, 1997; pp. 69–96. [Google Scholar]
- Bee, M. Individual Recognition in Animal Species. In Encyclopedia of Language & Linguistics, 2nd ed.; Elsevier: Oxford, UK, 2006; pp. 617–626. [Google Scholar]
- Qvarnström, A.; Haavie, J.; Saether, S.A.; Eriksson, D.; Pärt, T. Song similarity predicts hybridization in flycatchers. J. Evol. Biol. 2006, 19, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, E.A.; Dale, J. Individual recognition: It is good to be different. Trends Ecol. Evol. 2007, 22, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Mönkkönen, M.; Forsman, J.T.; Helle, P. Mixed-Species Foraging Aggregations and Heterospecific Attraction in Boreal Bird Communities. Oikos 1996, 77, 127–136. [Google Scholar] [CrossRef]
- Suzuki, T.N. Referential calls coordinate multi-species mobbing in a forest bird community. J. Ethol. 2016, 34, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrath, R.D.; Haff, T.M.; Fallow, P.M.; Radford, A.N. Eavesdropping on heterospecific alarm calls: From mechanisms to consequences. Biol. Rev. 2015, 90, 560–586. [Google Scholar] [CrossRef]
- Insley, S.J.; Paredes, R.; Jones, I.L. Sex differences in razorbill Alca torda parent-offspring vocal recognition. J. Exp. Biol. 2003, 206 Pt 1, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Chuang, M.-F.; Kam, Y.-C.; Bee, M.A. Territorial olive frogs display lower aggression towards neighbours than strangers based on individual vocal signatures. Anim. Behav. 2017, 123, 217–228. [Google Scholar] [CrossRef]
- Clark, C.J.; Jaworski, J.W. Introduction to the symposium: Bio-inspiration of quiet flight of owls and other flying animals: Recent advances and unanswered questions. Integr. Comp. Biol. 2020, 60, 1025–1035. [Google Scholar] [CrossRef]
- Sauvé, C.C.; Beauplet, G.; Hammill, M.O.; Charrier, I. Mother-pup vocal recognition in harbour seals: Influence of maternal behaviour, pup voice and habitat sound properties. Anim. Behav. 2015, 105, 109–120. [Google Scholar] [CrossRef]
- Ravignani, A.; Kello, C.T.; de Reus, K.; Kotz, S.A.; Dalla Bella, S.; Mendez-Arostegui, M.; Rapado-Tamarit, B.; Rubio-Garcia, A.; de Boer, B. Ontogeny of vocal rhythms in harbor seal pups: An exploratory study. Curr. Zool. 2019, 65, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, G.; Ancillotto, L.; Russo, D. Social communication in bats. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1938–1954. [Google Scholar] [CrossRef] [PubMed]
- Kazial, K.A.; Kenny, T.L.; Burnett, S.C. Little brown bats (Myotis lucifugus) recognize individual identity of conspecifics using sonar calls. Ethology 2008, 114, 469–478. [Google Scholar] [CrossRef]
- Ancillotto, L.; Russo, D. Selective aggressiveness in European free-tailed bats (Tadarida teniotis): Influence of familiarity, age and sex. Naturwissenschaften 2014, 101, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorado-Correa, A.M.; Goerlitz, H.R.; Siemers, B.M. Interspecific acoustic recognition in two European bat communities. Front. Physiol. 2013, 4, 192. [Google Scholar] [CrossRef] [Green Version]
- Kao, M.T.; Liu, J.N.; Cheng, H.C.; Nakazawa, T. Social signatures in echolocation calls of a leaf-roosting bat, Kerivoula furva. Bioacoustics 2020, 29, 461–480. [Google Scholar] [CrossRef]
- Russo, D.; Ancillotto, L.; Jones, G. Bats are still not birds in the digital era: Echolocation call variation and why it matters for bat species identification. Can. J. Zool. 2017, 96, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Schuchmann, M.; Siemers, B.M. Behavioral evidence for community-wide species discrimination from echolocation calls in bats. Am. Nat. 2010, 176, 72–82. [Google Scholar] [CrossRef]
- Pearl, D.L.; Fenton, M.B. Can echolocation calls provide information about group identity in the little brown bat (Myotis lucifugus). Can. J. Zool. 1996, 74, 2184–2192. [Google Scholar] [CrossRef]
- Kastein, H.B.; Winter, R.; Vinoth Kumar, A.K.; Kandula, S.; Schmidt, S. Perception of individuality in bat vocal communication: Discrimination between, or recognition of, interaction partners? Anim. Cogn. 2013, 16, 945–959. [Google Scholar] [CrossRef]
- Masters, W.M.; Raver, K.A.S.; Kazial, K.A. Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age and family affiliation. Anim. Behav. 1995, 50, 1243–1260. [Google Scholar] [CrossRef]
- Jones, G.; Kokurewicz, T. Sex and age variation in echolocation calls and flight morphology of Daubenton’s Bats Myotis daubentonii. Mammalia 1994, 58, 41–50. [Google Scholar] [CrossRef]
- Kazial, K.A.; Burnett, S.C.; Masters, W.M. Individual and group variation in echolocation calls of big brown bats, Eptesicus fuscus (Chiroptera: Vespertilionidae). J. Mammal. 2001, 82, 339–351. [Google Scholar] [CrossRef]
- Schuchmann, M.; Puechmaille, S.J.; Siemers, B.M. Horseshoe bats recognise the sex of conspecifics from their echolocation calls. Acta. Chiropt. 2012, 14, 161–166. [Google Scholar] [CrossRef]
- Siemers, B.M.; Kerth, G. Do echolocation calls of wild colony-living Bechstein’s bats (Myotis bechsteinii) provide individual-specific signatures? Behav. Ecol. Sociobiol. 2006, 59, 443–454. [Google Scholar] [CrossRef]
- Finger, N.M.; Bastian, A.; Jacobs, D.S. To seek or speak? Dual function of an acoustic signal limits its versatility in communication. Anim. Behav. 2017, 127, 135–152. [Google Scholar] [CrossRef]
- Dechmann, D.; Voigt-Heucke, S.; Giuggioli, L.; Safi, K.; Voigt, C.; Wikelski, M. Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. R. Soc. B 2009, 276, 2721–2728. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.K.; Allen, K.M.; Moss, C.F. Communication with self, friends and foes in active-sensing animals. J. Exp. Biol. 2021, 224, jeb242637. [Google Scholar] [CrossRef]
- Simmons, N.B.; Cirranello, A.L. Bat Species of the World: A Taxonomic and Geographic Database. Available online: https://batnames.org. (accessed on 19 July 2022).
- Simmons, J.A.; Grinnell, A.D. The performance of echolocation: Acoustic images perceived by echolocating bats. In Animal Sonar: Processes and Performance; Nachtigall, P.E., Moore, P.W.B., Eds.; Springer US: Boston, MA, USA, 1988; pp. 353–385. [Google Scholar]
- Liu, Y.; Feng, J.; Jiang, Y.; Wu, L.; Sun, K. Vocalization development of greater horseshoe bat, Rhinolophus ferrumequinum (Rhinolophidae, Chiroptera). Folia Zool. 2007, 56, 126–136. [Google Scholar]
- Möhres, F. Communicative characters of sonar signals in bats. In Animal Sonar Systems: Biology and Bionics; Busnel, R.G., Ed.; NATO Advanced Study Institute: Frascati, Italy, 1967; Volume 2, pp. 939–945. [Google Scholar]
- Lin, A.; Liu, H.; Chang, Y.; Lu, G.; Feng, J. Behavioural response of the greater horseshoe bat to geographical variation in echolocation calls. Behav. Ecol. Sociobiol. 2016, 70, 1765–1776. [Google Scholar] [CrossRef]
- Racey, P. Reproductive assessment of bats. In Ecological and Behavioral Methods for the Study of Bats; Kunz, T.H., Parsons, S., Eds.; The John Hopkins University Press: Baltimore, MD, USA, 2009; pp. 249–264. [Google Scholar]
- Jin, L.; Yang, S.; Kimball, R.T.; Xie, L.; Yue, X.; Luo, B.; Sun, K.; Feng, J. Do pups recognize maternal calls in pomona leaf-nosed bats, Hipposideros pomona? Anim. Behav. 2015, 100, 200–207. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, X.; Hui, W.; Feng, J. Size and quality information in acoustic signals of Rhinolophus ferrumequinum in distress situations. Physiol. Behav. 2017, 173, 252–257. [Google Scholar] [CrossRef]
- Sun, C.; Jiang, T.; Kanwal, J.S.; Guo, X.; Luo, B.; Lin, A.; Feng, J. Great Himalayan leaf-nosed bats modify vocalizations to communicate threat escalation during agonistic interactions. Behav. Process. 2018, 157, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Balcombe, J.P.; Mccracken, G.F. Vocal recognition in Mexican free-tailed bats: Do pups recognize mothers? Anim. Behav. 1992, 43, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, D.L.; McCracken, G.F. Individual variation in the isolation calls of Mexican free-tailed bat pups (Tadarida brasiliensis mexicana). Anim. Behav. 1986, 34, 1078–1086. [Google Scholar] [CrossRef]
- Gillam, E.H.; Chaverri, G. Strong individual signatures and weaker group signatures in contact calls of Spix’s disc-winged bat, Thyroptera tricolor. Anim. Behav. 2012, 83, 269–276. [Google Scholar] [CrossRef]
- Li, Y.; Jing, W.; Metzner, W.; Luo, B.; Jiang, T.; Yang, S.; Shi, L.; Huang, X.; Yue, X.; Feng, J. Behavioral responses to echolocation calls from sympatric heterospecific bats: Implications for interspecific competition. Behav. Ecol. Sociobiol. 2014, 68, 657–667. [Google Scholar] [CrossRef]
- Mundry, R.; Sommer, C. Discriminant function analysis with nonindependent data: Consequences and an alternative. Anim. Behav. 2007, 74, 965–976. [Google Scholar] [CrossRef]
- Siemers, B.; Beedholm, K.; Dietz, C.; Dietz, I.; Ivanova, T. Is species identity, sex, age or individual quality conveyed by echolocation call frequency in European horseshoe bats? Acta Chiropt. 2009, 7, 259–274. [Google Scholar] [CrossRef]
- Yoshino, H.; Matsumura, S.; Kinjo, K.; Tamura, H.; Ota, H.; Izawa, M. Geographical variation in echolocation call and body size of the Okinawan least horseshoe bat, Rhinolophus pumilus (Mammalia: Rhinolophidae), on Okinawa-jima Island, Ryukyu Archipelago, Japan. Zool. Sci. 2006, 23, 661–667. [Google Scholar] [CrossRef]
- Chen, S.-F.; Jones, G.; Rossiter, S.J. Determinants of echolocation call frequency variation in the Formosan lesser horseshoe bat (Rhinolophus monoceros). Proc. R. Soc. B Biol. Sci. 2009, 276, 3901–3909. [Google Scholar] [CrossRef] [Green Version]
- Russo, D.; Jones, G.; Mucedda, M. Influence of age, sex and body size on echolocation calls of Mediterranean and Mehely’s horseshoe bats, Rhinolophus euryale and R.mehelyi (Chiroptera: Rhinotophidae). Mammalia 2001, 65, 429–436. [Google Scholar] [CrossRef]
- Russo, D.; Mucedda, M.; Bello, M.; Biscardi, S.; Pidinchedda, E.; Jones, G. Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): A case of character displacement? J. Biogeogr. 2007, 34, 2129–2138. [Google Scholar] [CrossRef]
- Curé, C.; Mathevon, N.; Aubin, T. Mate vocal recognition in the Scopoli’s shearwater Calonectris diomedea: Do females and males share the same acoustic code? Behav. Process. 2016, 128, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Volodin, I.A.; Volodina, E.V.; Klenova, A.V.; Matrosova, V.A. Gender identification using acoustic analysis in birds without external sexual dimorphism. Avian Res. 2015, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.-S.; Yuan, H.-W.; Yao, C.-T.; Hsieh, C.-F. Male and female Steere’s liocichlas respond differently to solo and stereo duet playback. Anim. Behav. 2012, 83, 487–493. [Google Scholar] [CrossRef]
- Kazial, K.A.; Masters, W.M. Female big brown bats, Eptesicus fuscus, recognize sex from a caller’s echolocation signals. Anim. Behav. 2004, 67, 855–863. [Google Scholar] [CrossRef]
- Grilliot, M.E.; Burnett, S.C.; Mendonça, M.T. Sexual Dimorphism in Big Brown Bat (Eptesicus fuscus) Ultrasonic Vocalizations Is Context Dependent. J. Mammal. 2009, 90, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Knörnschild, M.; Jung, K.; Nagy, M.; Metz, M.; Kalko, E. Bat echolocation calls facilitate social communication. Proc. Biol. Sci. 2012, 279, 4827–4835. [Google Scholar] [CrossRef] [Green Version]
- Puechmaille, S.J.; Borissov, I.M.; Zsebok, S.; Allegrini, B.; Hizem, M.; Kuenzel, S.; Schuchmann, M.; Teeling, E.C.; Siemers, B.M. Female mate choice can drive the evolution of high frequency echolocation in bats: A case study with Rhinolophus mehelyi. PLoS ONE 2014, 9, e103452. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.; Siemers, B.M. The communicative potential of bat echolocation pulses. J. Comp. Physiol. 2011, 197, 447–457. [Google Scholar] [CrossRef]
- Yovel, Y.; Melcon, M.L.; Franz, M.O.; Denzinger, A.; Schnitzler, H.U. The voice of bats: How greater mouse-eared bats recognize individuals based on their echolocation calls. PLoS Comput. Biol. 2009, 5, e1000400. [Google Scholar] [CrossRef] [PubMed]
- Esser, K.H.; Schmidt, U. Mother-infant communication in the lesser spear-nosed bat Phyllostomus discolor (Chiroptera, Phyllostomidae)-evidence for acoustic learning. Ethology 1989, 82, 156–168. [Google Scholar] [CrossRef]
- Voigt-Heucke, S.L.; Taborsky, M.; Dechmann, D.K. A dual function of echolocation: Bats use echolocation calls to identify familiar and unfamiliar individuals. Anim. Behav. 2010, 80, 59–67. [Google Scholar] [CrossRef]
- Müller, C.A.; Manser, M.B. Mutual recognition of pups and providers in the cooperatively breeding banded mongoose. Anim. Behav. 2008, 75, 1683–1692. [Google Scholar] [CrossRef]
- Mumm, C.A.S.; Urrutia, M.C.; Knörnschild, M. Vocal individuality in cohesion calls of giant otters, Pteronura brasiliensis. Anim. Behav. 2014, 88, 243–252. [Google Scholar] [CrossRef]
- Matrosova, V.A.; Blumstein, D.T.; Volodin, I.A.; Volodina, E.V. The potential to encode sex, age, and individual identity in the alarm calls of three species of Marmotinae. Naturwissenschaften 2011, 98, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, S.L.; Connor, R.C.; Montgomery, S.H. Social and vocal complexity in bottlenose dolphins. Trends Neurosci. 2022, 45, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Cerchio, S.; Weir, C.R. Mid-frequency song and low-frequency calls of sei whales in the Falkland Islands. R. Soc. Open Sci. 2022, 9, 220738. [Google Scholar] [CrossRef]


| Parameters | Female (Mean ± SD) | Male (Mean ± SD) | p-Value |
|---|---|---|---|
| Call duration | 0.029 ± 0.010 | 0.033 ± 0.010 | 0.005 ** |
| Peak frequency (end) | 63.91 ± 3.28 | 59.32 ± 4.02 | 0.000 ** |
| Minimum frequency of bandwidth (end) | 58.77 ± 3.44 | 55.04 ± 5.39 | 0.000 ** |
| Maximum frequency of bandwidth (end) | 71.07 ± 1.33 | 69.03 ± 7.45 | 0.017 * |
| Bandwidth (end) | 12.28 ± 3.66 | 13.94 ± 7.63 | 0.081 |
| Peak frequency (center) | 69.08 ± 0.42 | 68.90 ± 0.49 | 0.017 * |
| Minimum frequency of bandwidth (center) | 67.60 ± 0.46 | 67.11 ± 0.37 | 0.000 ** |
| Maximum frequency of bandwidth (center) | 72.67 ± 0.50 | 72.02 ± 0.62 | 0.000 ** |
| Bandwidth (center) | 4.97 ± 0.42 | 4.83 ± 0.45 | 0.043 * |
| Peak frequency (maximum) | 68.77 ± 1.14 | 67.87 ± 2.97 | 0.012 * |
| Minimum frequency of bandwidth (maximum) | 67.01 ± 1.94 | 65.82 ± 3.21 | 0.005 ** |
| Maximum frequency of bandwidth (maximum) | 72.53 ± 0.63 | 71.27 ± 2.81 | 0.000 ** |
| Bandwidth (maximum) | 5.44 ± 1.60 | 5.38 ± 0.84 | 0.781 |
| Parameters | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| Call duration | 0.012 | 0.036 | 0.02 | 0.01 |
| Peak frequency (end) | 54.6 | 66.4 | 59.82 | 2.08 |
| Minimum frequency of bandwidth (end) | 50.7 | 61.5 | 55.06 | 1.69 |
| Maximum frequency of bandwidth (end) | 62.5 | 71.2 | 67.23 | 2.06 |
| Bandwidth (end) | 8.0 | 18.5 | 12.11 | 2.22 |
| Peak frequency (center) | 67.3 | 69.3 | 68.52 | 0.61 |
| Minimum frequency of bandwidth (center) | 65.4 | 67.3 | 66.90 | 0.63 |
| Maximum frequency of bandwidth (center) | 70.3 | 72.2 | 71.70 | 0.67 |
| Bandwidth (center) | 4.1 | 5.1 | 4.73 | 0.23 |
| Peak frequency (maximum) | 60.5 | 69.3 | 67.70 | 2.10 |
| Minimum frequency of bandwidth (maximum) | 55.6 | 67.3 | 65.68 | 3.18 |
| Maximum frequency of bandwidth (maximum) | 65.6 | 72.2 | 71.28 | 1.36 |
| Bandwidth (maximum) | 4.1 | 12.6 | 5.53 | 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Lin, A.; Sun, K.; Jin, L.; Feng, J. Greater Horseshoe Bats Recognize the Sex and Individual Identity of Conspecifics from Their Echolocation Calls. Animals 2022, 12, 3490. https://doi.org/10.3390/ani12243490
Tan X, Lin A, Sun K, Jin L, Feng J. Greater Horseshoe Bats Recognize the Sex and Individual Identity of Conspecifics from Their Echolocation Calls. Animals. 2022; 12(24):3490. https://doi.org/10.3390/ani12243490
Chicago/Turabian StyleTan, Xiao, Aiqing Lin, Keping Sun, Longru Jin, and Jiang Feng. 2022. "Greater Horseshoe Bats Recognize the Sex and Individual Identity of Conspecifics from Their Echolocation Calls" Animals 12, no. 24: 3490. https://doi.org/10.3390/ani12243490
APA StyleTan, X., Lin, A., Sun, K., Jin, L., & Feng, J. (2022). Greater Horseshoe Bats Recognize the Sex and Individual Identity of Conspecifics from Their Echolocation Calls. Animals, 12(24), 3490. https://doi.org/10.3390/ani12243490





