Chicken LEAP2 Level Substantially Changes with Feed Intake and May Be Regulated by CDX4 in Small Intestine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. ELISA for Serum Ghrelin and LEAP2
2.3. RNA Isolation and Quantitative Real-Time PCR
2.4. Vector Construction
2.5. Cell Culture and Dual Luciferase Assay
2.6. Bioinformatics and Statistical Analysis
3. Results
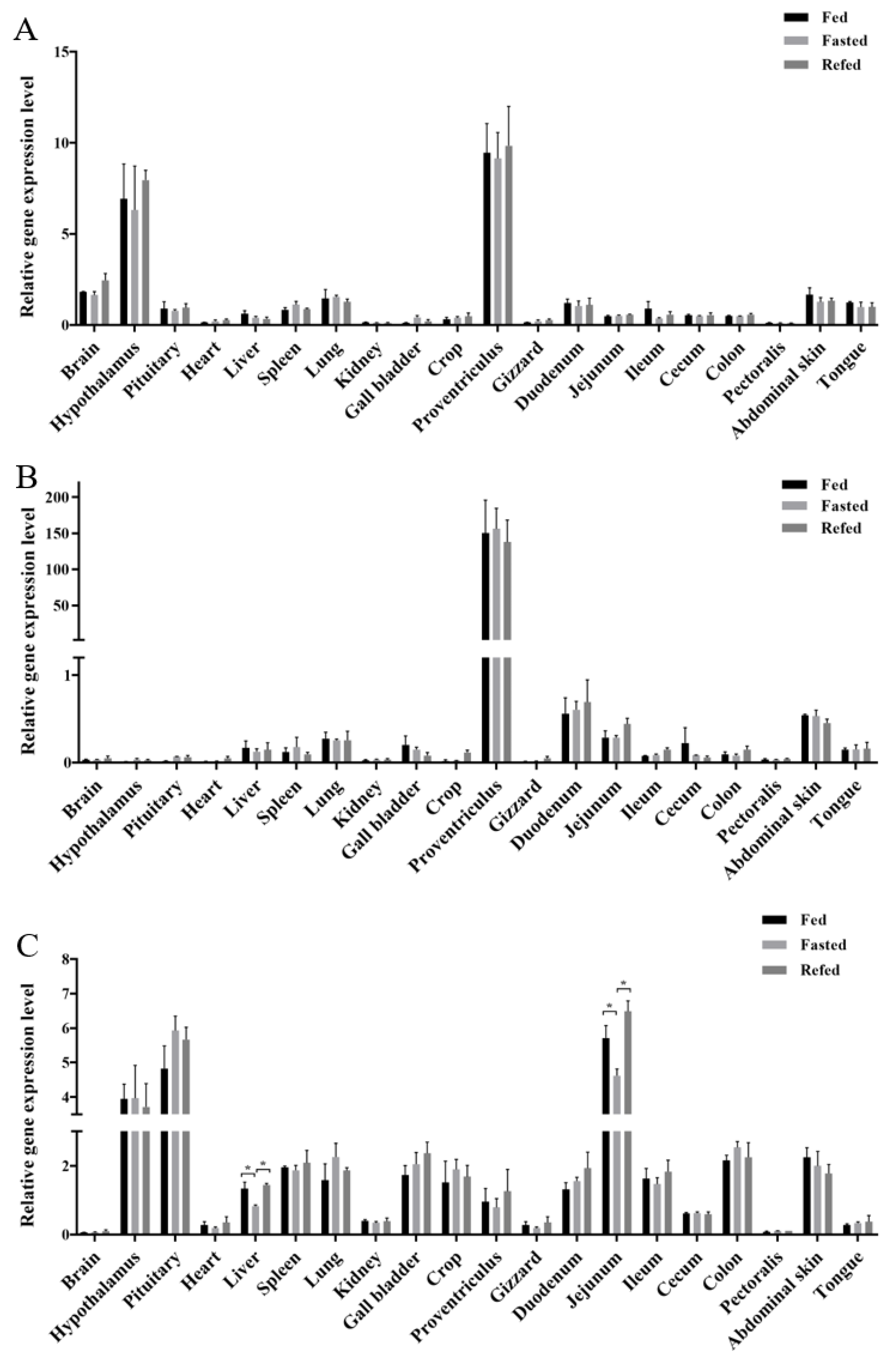
3.1. Tissue Expression Profiles of GOAT, Ghrelin, and GHSR in Chicks
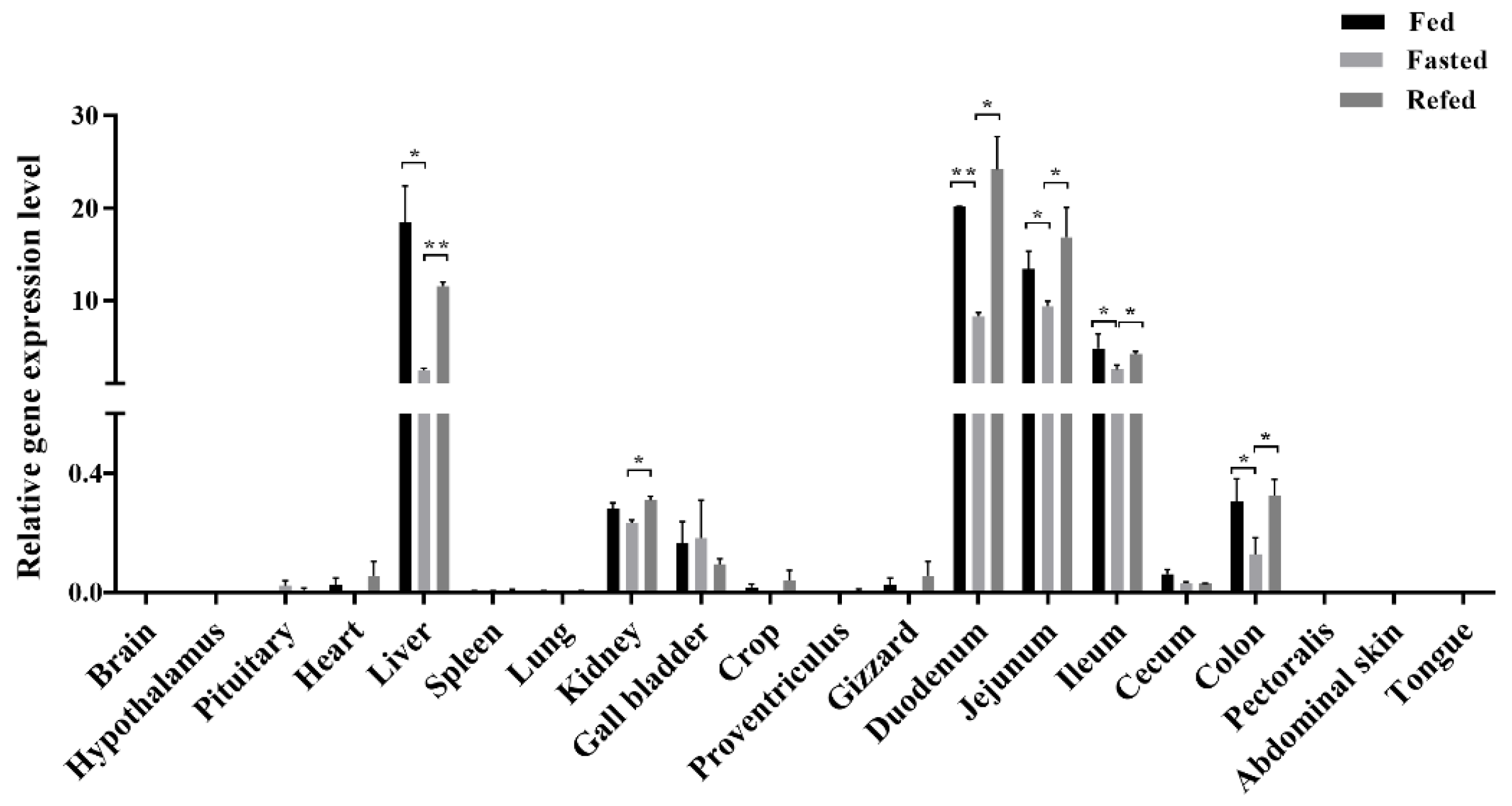
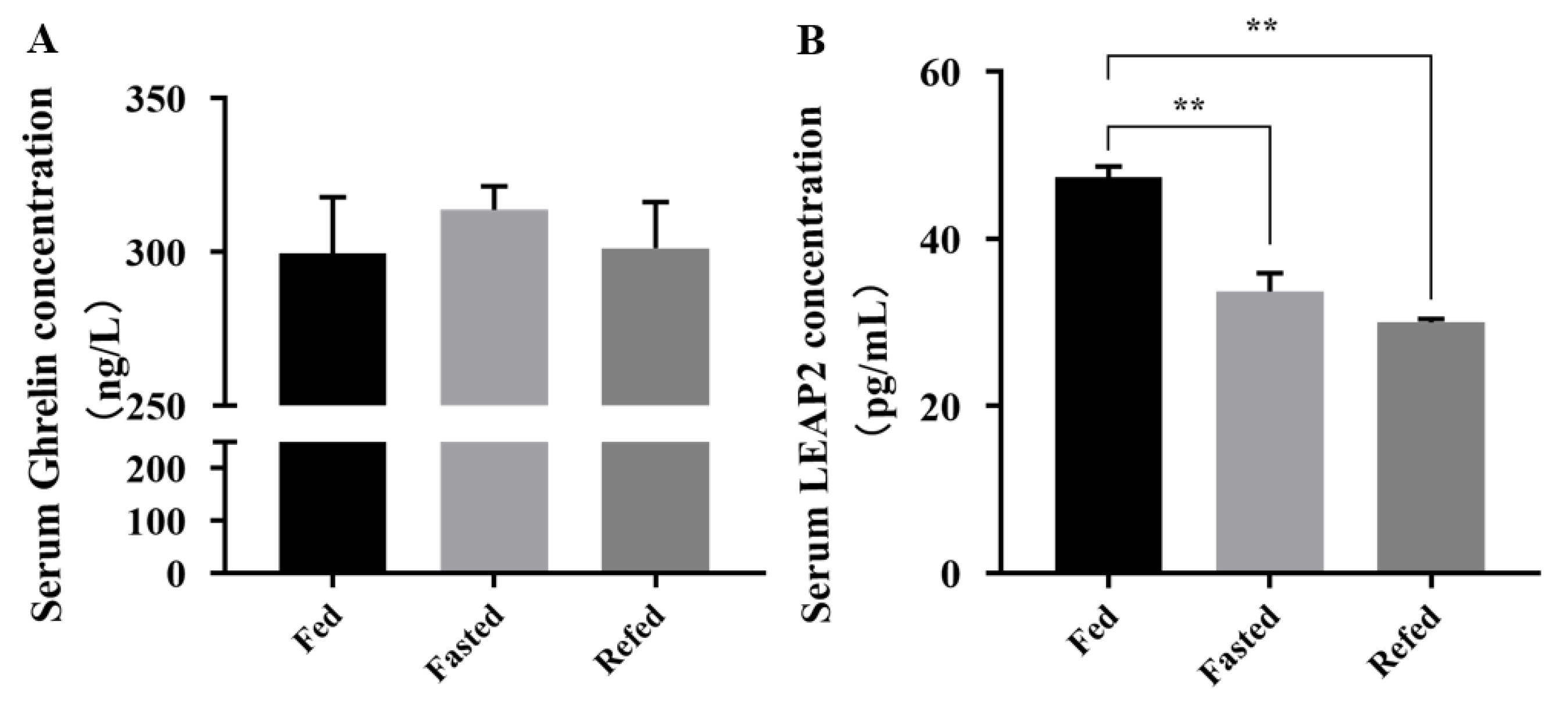
3.2. LEAP2 Changes with Feed Intake in the RNA Expression Level and Serum Content
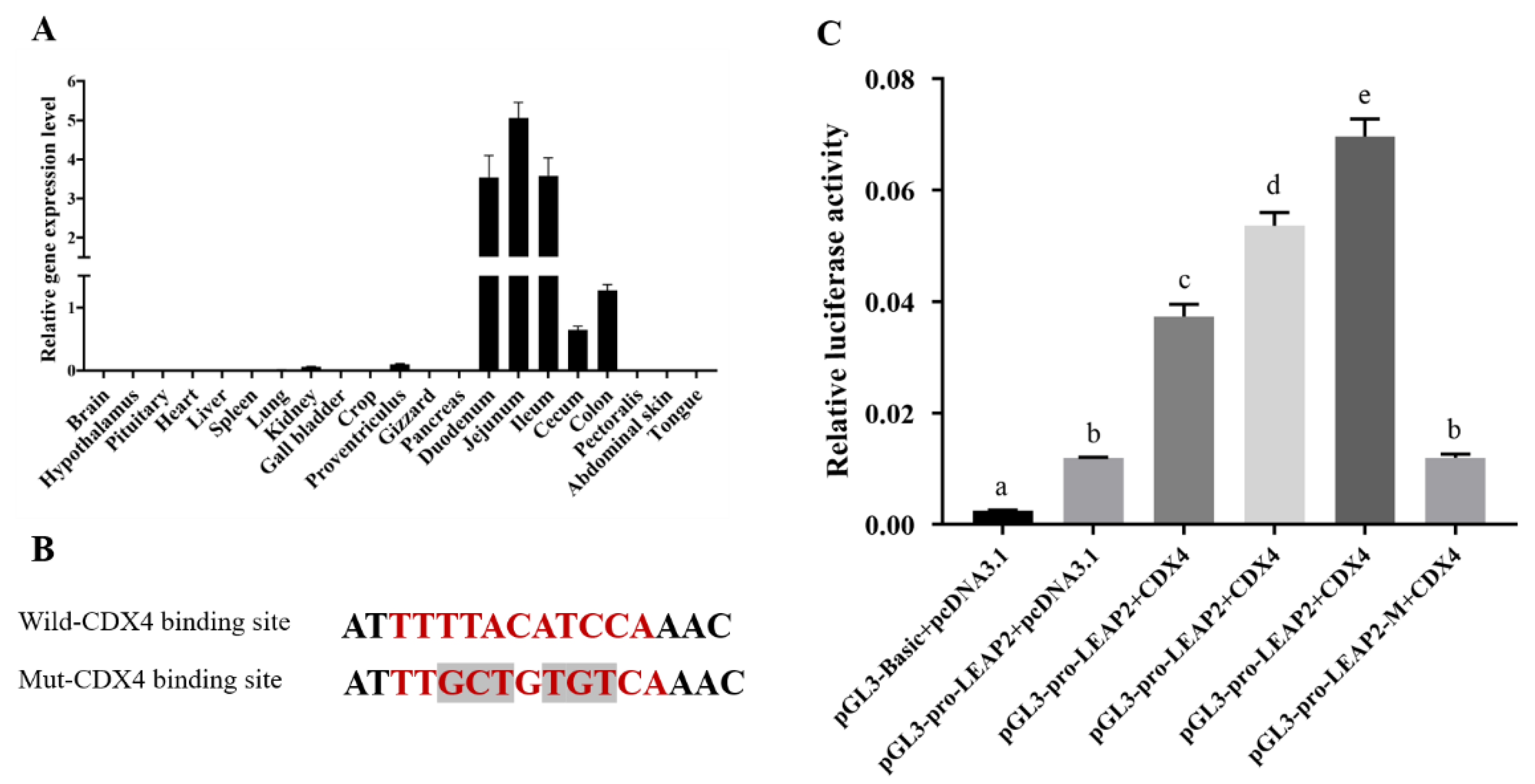
3.3. CDX4 Is a Potential Transcriptional Factor of LEAP2 Highly Expressed in the Small Intestine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, J.A.; Solenberg, P.J.; Perkins, D.R.; Willency, J.A.; Knierman, M.D.; Jin, Z.; Witcher, D.R.; Luo, S.; Onyia, J.E.; Hale, J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA 2008, 105, 6320–6325. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Kirchner, H.; Heppner, K.; Pfluger, P.T.; Tschöp, M.H.; Nogueiras, R. GOAT: The master switch for the ghrelin system? Eur. J. Endocrinol. 2010, 163, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Mulholland, M.; Zhang, W. Ghrelin O-acyltransferase (GOAT) and energy metabolism. Sci. China Life Sci. 2016, 59, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Toshinai, K.; Mondal, M.S.; Nakazato, M.; Date, Y.; Murakami, N.; Kojima, M.; Kangawa, K.; Matsukura, S. Upregulation of Ghrelin Expression in the Stomach upon Fasting, Insulin-Induced Hypoglycemia, and Leptin Administration. Biochem. Bioph. Res. Co. 2001, 281, 1220–1225. [Google Scholar] [CrossRef]
- Saito, E.S.; Kaiya, H.; Takagi, T.; Yamasaki, I.; Denbow, D.M.; Kangawa, K.; Furuse, M. Chicken ghrelin and growth hormone-releasing peptide-2 inhibit food intake of neonatal chicks. Eur. J. Pharmacol. 2002, 453, 75–79. [Google Scholar] [CrossRef]
- Vizcarra, J.A.; Wright, H.; Vizcarra, A. The effect of passive immunization against ghrelin on feed and water intake in turkeys1. Poultry Sci. 2012, 91, 2305–2309. [Google Scholar] [CrossRef]
- Saito, E.; Kaiya, H.; Tachibana, T.; Tomonaga, S.; Denbow, D.M.; Kangawa, K.; Furuse, M. Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul. Pept. 2005, 125, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kaiya, H.; Saito, E.; Tachibana, T.; Furuse, M.; Kangawa, K. Changes in ghrelin levels of plasma and proventriculus and ghrelin mRNA of proventriculus in fasted and refed layer chicks. Domest. Anim. Endocrin. 2007, 32, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Shousha, S.; Nakahara, K.; Kojima, M.; Miyazato, M.; Hosoda, H.; Kangawa, K.; Murakami, N. Different effects of peripheral and central ghrelin on regulation of food intake in the Japanese quail. Gen. Comp. Endocr. 2005, 141, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Krause, A. Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Sci. 2003, 12, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Ball, S.L.; Siou, G.P.; Wilson, J.A.; Howard, A.; Hirst, B.H.; Hall, J. Expression and immunolocalisation of antimicrobial peptides within human palatine tonsils. J. Laryngol. Otol. 2007, 121, 973–978. [Google Scholar] [CrossRef]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Andrews, Z.B. The next big LEAP2 understanding ghrelin function. J. Clin. Investig. 2019, 129, 3542–3544. [Google Scholar] [CrossRef]
- Mani, B.K.; Puzziferri, N.; He, Z.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Chhina, N.; Gaylinn, B.; Thorner, M.O.; Thomas, E.L.; et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Investig. 2019, 129, 3909–3923. [Google Scholar] [CrossRef]
- Howard, A.; Townes, C.; Milona, P.; Nile, C.J.; Michailidis, G.; Hall, J. Expression and functional analyses of liver expressed antimicrobial peptide-2 (LEAP-2) variant forms in human tissues. Cell. Immunol. 2010, 261, 128–133. [Google Scholar] [CrossRef]
- Islam, M.N.; Mita, Y.; Maruyama, K.; Tanida, R.; Zhang, W.; Sakoda, H.; Nakazato, M. Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol. 2020, 244, 13–23. [Google Scholar] [CrossRef]
- Li, H.; Shou, L.; Shao, X.; Liu, Y.; Xu, Z.; Guo, Z. Identifying key residues and key interactions for the binding of LEAP2 to receptor GHSR1a. Biochem. J. 2020, 477, 3199–3217. [Google Scholar] [CrossRef] [PubMed]
- Townes, C.L.; Michailidis, G.; Hall, J. The interaction of the antimicrobial peptide cLEAP-2 and the bacterial membrane. Biochem. Bioph. Res. Co. 2009, 387, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Dwyer, D.M.; Miska, K.B.; Fetterer, R.H.; Jenkins, M.C.; Wong, E.A. Expression of host defense peptides in the intestine of Eimeria-challenged chickens. Poult. Sci. 2017, 96, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Jahejo, A.R.; Jia, F.; Li, X.; Ning, G.; Zhang, D.; Ma, H.; Hao, W.; Gao, W.; Zhao, Y.; et al. Transcripts of antibacterial peptides in chicken erythrocytes infected with Marek’s disease virus. BMC Vet. Res. 2018, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Monson, M.; Kaiser, M.; Lamont, S.J. Induction of Chicken Host Defense Peptides within Disease-Resistant and -Susceptible Lines. Genes 2020, 11, 1195. [Google Scholar] [CrossRef]
- Lynn, D.J.; Higgs, R.; Gaines, S.; Tierney, J.; James, T.; Lloyd, A.T.; Fares, M.A.; Mulcahy, G.; Farrelly, C.O. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics 2004, 56, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Michailidis, G. Expression of chicken LEAP-2 in the reproductive organs and embryos and in response to Salmonella enterica infection. Vet. Res. Commun. 2010, 34, 459–471. [Google Scholar] [CrossRef]
- Kaiya, H.; van der Geyten, S.; Kojima, M.; Hosoda, H.; Kitajima, Y.; Matsumoto, M.; Geelissen, S.; Darras, V.M.; Kangawa, K. Chicken Ghrelin: Purification, cDNA Cloning, and Biological Activity. Endocrinology 2002, 143, 3454–3463. [Google Scholar] [CrossRef] [Green Version]
- Geelissen, S.M.; Beck, I.M.; Darras, V.M.; Kuhn, E.R.; Van der Geyten, S. Distribution and regulation of chicken growth hormone secretagogue receptor isoforms. Gen. Comp. Endocrinol. 2003, 134, 167–174. [Google Scholar] [CrossRef]
- Song, X.; Jiao, H.; Zhao, J.; Wang, X.; Lin, H. Ghrelin serves as a signal of energy utilization and is involved in maintaining energy homeostasis in broilers. Gen. Comp. Endocr. 2019, 272, 76–82. [Google Scholar] [CrossRef]
- Fang, M.; Nie, Q.; Luo, C.; Zhang, D.; Zhang, X. An 8bp indel in exon 1 of Ghrelin gene associated with chicken growth. Domest. Anim. Endocrin. 2007, 32, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Nie, Q.; Luo, C.; Zhang, D.; Zhang, X. Associations of GHSR gene polymorphisms with chicken growth and carcass traits. Mol. Biol. Rep. 2010, 37, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, T.; Hiraga, T.; Teraoka, H.; Yaosaka, N.; Kaiya, H. Correlation of ghrelin concentration and ghrelin, ghrelin-O-acetyltransferase (GOAT) and growth hormone secretagogue receptor 1a mRNAs expression in the proventriculus and brain of the growing chicken. Peptides 2015, 63, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, S.; Shamsara, M.; Khateri, S.; Soroush, M.R.; Ghorbanmehr, N.; Tavallaei, M.; Nourani, M.R.; Mowla, S.J. Identification of Reliable Reference Genes for Quantification of MicroRNAs in Serum Samples of Sulfur Mustard-Exposed Veterans. Cell J. 2015, 17, 494–501. [Google Scholar] [CrossRef]
- Hauger, R.L.; Grigoriadis, D.E.; Dallman, M.F.; Plotsky, P.M.; Vale, W.W.; Dautzenberg, F.M. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol. Rev. 2003, 55, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huszar, D.; Lynch, C.A.; Fairchild-Huntress, V.; Dunmore, J.H.; Fang, Q.; Berkemeier, L.R.; Gu, W.; Kesterson, R.A.; Boston, B.A.; Cone, R.D.; et al. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell 1997, 88, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shipp, S.L.; Yi, J.; Dridi, S.; Gilbert, E.R.; Cline, M.A. The central anorexigenic mechanism of adrenocorticotropic hormone involves the caudal hypothalamus in chicks. Neuropeptides 2015, 53, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Sugimoto, I.; Ogino, M.; Sakirul Islam Khan, M.; Masuda, K.; Ukena, K.; Wang, Y. Central administration of chicken growth hormone-releasing hormone decreases food intake in chicks. Physiol. Behav. 2015, 139, 195–201. [Google Scholar] [CrossRef]
- Levine, A.S.; Morley, J.E. Neuropeptide Y: A potent inducer of consummatory behavior in rats. Peptides 1984, 5, 1025–1029. [Google Scholar] [CrossRef]
- Chen, G.; Yang, F.; Wu, T.; Jiang, J.; Zhou, W. The Stimulatory Effect of Cerebral Intraventricular Injection of cNPY on Precocial Feeding Behavior in Neonatal Chicks (Gallus domesticus). PLoS ONE 2016, 11, e153342. [Google Scholar] [CrossRef] [PubMed]
- Seroussi, E.; Cinnamon, Y.; Yosefi, S.; Genin, O.; Smith, J.G.; Rafati, N.; Bornelöv, S.; Andersson, L.; Friedman-Einat, M. Identification of the Long-Sought Leptin in Chicken and Duck: Expression Pattern of the Highly GC-Rich Avian leptin Fits an Autocrine/Paracrine Rather Than Endocrine Function. Endocrinology 2016, 157, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkašová, H.; Hron, T.; Pačes, J.; Pajer, P.; Elleder, D. Identification of a GC-rich leptin gene in chicken. Agri. Gene 2016, 1, 88–92. [Google Scholar] [CrossRef]
- Seroussi, E.; Pitel, F.; Leroux, S.; Morisson, M.; Bornelöv, S.; Miyara, S.; Yosefi, S.; Cogburn, L.A.; Burt, D.W.; Anderson, L.; et al. Mapping of leptin and its syntenic genes to chicken chromosome 1p. BMC Genet. 2017, 18, 77. [Google Scholar] [CrossRef] [Green Version]
- Richards, M.P.; Poch, S.M.; Mcmurtry, J.P. Characterization of turkey and chicken ghrelin genes, and regulation of ghrelin and ghrelin receptor mRNA levels in broiler chickens. Gen. Comp. Endocr. 2006, 145, 298–310. [Google Scholar] [CrossRef]
- Fujino, K.; Inui, A.; Asakawa, A.; Kihara, N.; Fujimura, M.; Fujimiya, M. Ghrelin Induces Fasted Motor Activity of the Gastrointestinal Tract in Conscious Fed Rats. J. Physiol. 2003, 550, 227–240. [Google Scholar] [CrossRef]
- Kitazawa, T.; Kaiya, H. Regulation of Gastrointestinal Motility by Motilin and Ghrelin in Vertebrates. Front. Endocrinol. 2019, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Shankar, K.; Metzger, N.P.; Singh, O.; Mani, B.K.; Osborne-Lawrence, S.; Varshney, S.; Gupta, D.; Ogden, S.B.; Takemi, S.; Richard, C.P.; et al. LEAP2 deletion in mice enhances ghrelin’s actions as an orexigen and growth hormone secretagogue. Mol. Metab. 2021, 53, 101327. [Google Scholar] [CrossRef]
- Li, H.; Shou, L.; Shao, X.; Li, N.; Liu, Y.; Xu, Z.; Guo, Z. LEAP2 has antagonized the ghrelin receptor GHSR1a since its emergence in ancient fish. Amino Acids 2021, 53, 939–949. [Google Scholar] [CrossRef]
- Xiao, Y.; Hughes, A.L.; Ando, J.; Matsuda, Y.; Cheng, J.F.; Skinner-Noble, D.; Zhang, G. A genome-wide screen identifies a single beta-defensin gene cluster in the chicken: Implications for the origin and evolution of mammalian defensins. BMC Genom. 2004, 5, 56. [Google Scholar] [CrossRef]
- Suh, E.; Chen, L.; Taylor, J.; Traber, P.G. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol. Cell. Biol. 1994, 14, 7340–7351. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Gu, X.; Golden, H.M.; Suh, E.; Rhoads, D.B.; Reinecker, H. Cloning of the Human Claudin-2 5′-Flanking Region Revealed a TATA-less Promoter with Conserved Binding Sites in Mouse and Human for Caudal-related Homeodomain Proteins and Hepatocyte Nuclear Factor-1α. J. Biol. Chem. 2002, 277, 21361–21370. [Google Scholar] [CrossRef] [PubMed]
| Primer Names | Primer Sequence (5′→3′) | GenBank Number | Size (bp) | Anneal Temperature (°C) |
|---|---|---|---|---|
| Ghrelin-F Ghrelin-R | TTTGAAGCACTGCCTAAAGAA GTCATCTTCTCCCTCTGTTTCAT | CGNC: 6373 | 229 | 58 |
| GOAT-F GOAT-R | GACCTGCTCATCCTTCTCCCT TGAGAAGCAGCGTGGCATAA | CGNC: 8705 | 181 | 58 |
| GHSR-F GHSR-R | CATCATCAGGGACAAGAACAAC AAGGCAACCAGCAGAGTATGA | CGNC: 6983 | 83 | 58 |
| LEAP2-F LEAP2-R | TTCTGAGACTGAAGCGGATGA AGGCCGTTCTAAGGAAGCAG | CGNC: 5360 | 132 | 58 |
| CDX4-F CDX4-R | CTCACCCACCAACCAAAGG CAGGGAACTTGTTATTTCATTAGG | CGNC: 49264 | 352 | 58 |
| HPRT-F HPRT-R | CCCAAACATTATGCAGACGA TGTCCTGTCCATGATGAGC | CGNC: 4576 | 66 | 58 |
| GAPDH-F GAPDH-R | CTCTGTTGTTGACCTGACCT CAACCTGGTCCTCTGTGTAT | CGNC: 49077 | 125 | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Chen, Z.; Zhuang, W.; Zhang, J.; He, J.; Xie, Y.; Chen, J. Chicken LEAP2 Level Substantially Changes with Feed Intake and May Be Regulated by CDX4 in Small Intestine. Animals 2022, 12, 3496. https://doi.org/10.3390/ani12243496
Zheng X, Chen Z, Zhuang W, Zhang J, He J, Xie Y, Chen J. Chicken LEAP2 Level Substantially Changes with Feed Intake and May Be Regulated by CDX4 in Small Intestine. Animals. 2022; 12(24):3496. https://doi.org/10.3390/ani12243496
Chicago/Turabian StyleZheng, Xiaotong, Ziwei Chen, Wuchao Zhuang, Jilong Zhang, Jiaheng He, Yinku Xie, and Jianfei Chen. 2022. "Chicken LEAP2 Level Substantially Changes with Feed Intake and May Be Regulated by CDX4 in Small Intestine" Animals 12, no. 24: 3496. https://doi.org/10.3390/ani12243496






