Preimplantation Developmental Competence of Bovine and Porcine Oocytes Activated by Zinc Chelation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Animal Welfare Statement
2.2. Bovine Cumulus-Oocyte Complexes Collection and In Vitro Maturation
2.3. Porcine Cumulus-Oocyte Complexes Collection and In Vitro Maturation
2.4. Bovine Oocyte Artificial Activation
2.5. Porcine Oocyte Artificial Activation
2.6. Bovine In Vitro Fertilization
2.7. In Vitro Culture of IVF and Parthenogenetic Bovine Embryos
2.8. In Vitro Culture of Parthenogenetic Porcine Embryos
2.9. Blastocyst Fixation, Immunofluorescence, and Cell Counting
2.10. Statistical Analyses
3. Results
3.1. Experiment 1: Effect of Two Culture Media on Bovine and Porcine Oocyte Activation Rates and Embryo Development after Oocyte Activation with PHEN
3.2. Experiment 2: Effects of Different PHEN Concentrations and Incubation Times on Bovine and Porcine Activation Rates and Parthenogenetic Developmental Competence
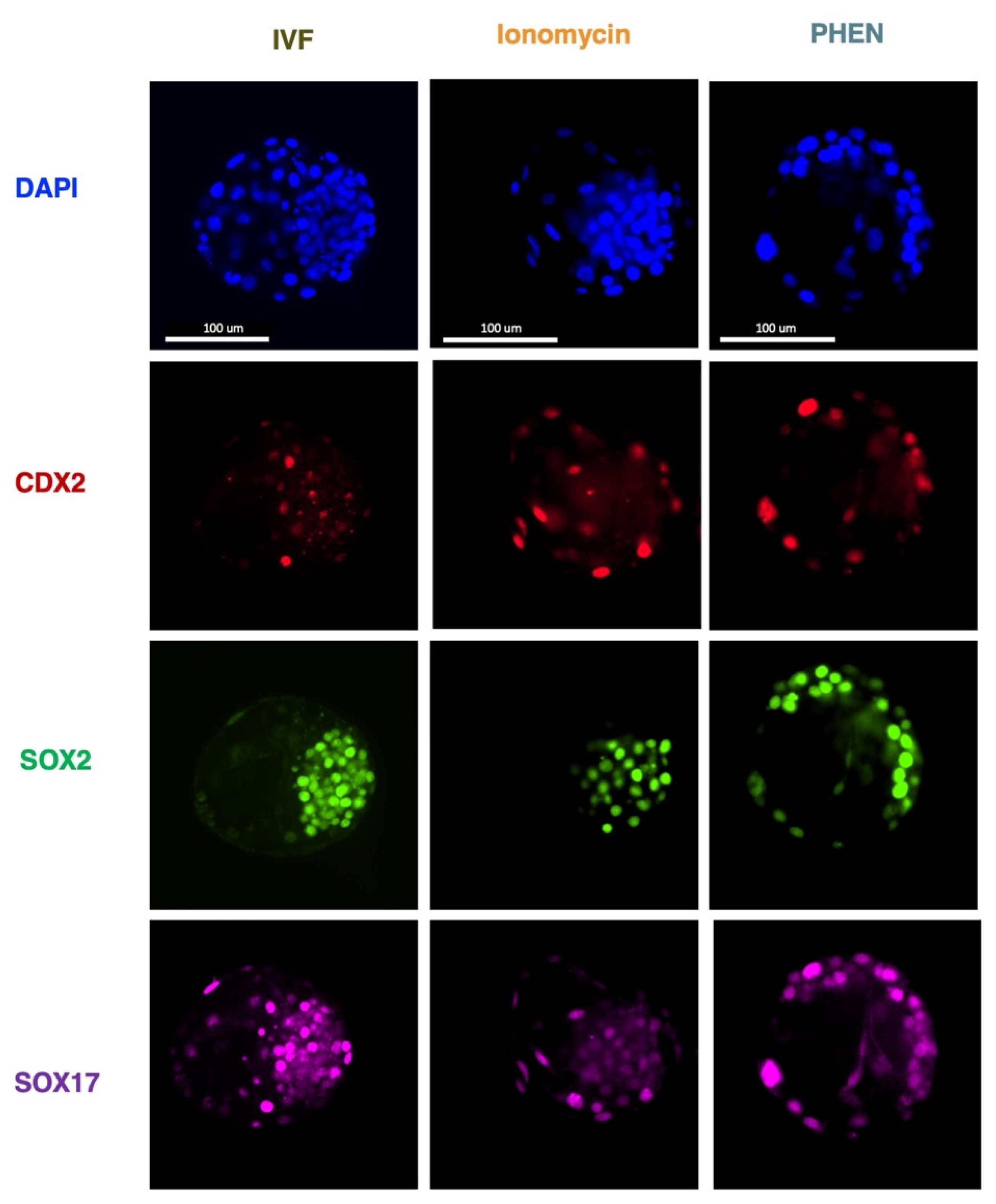
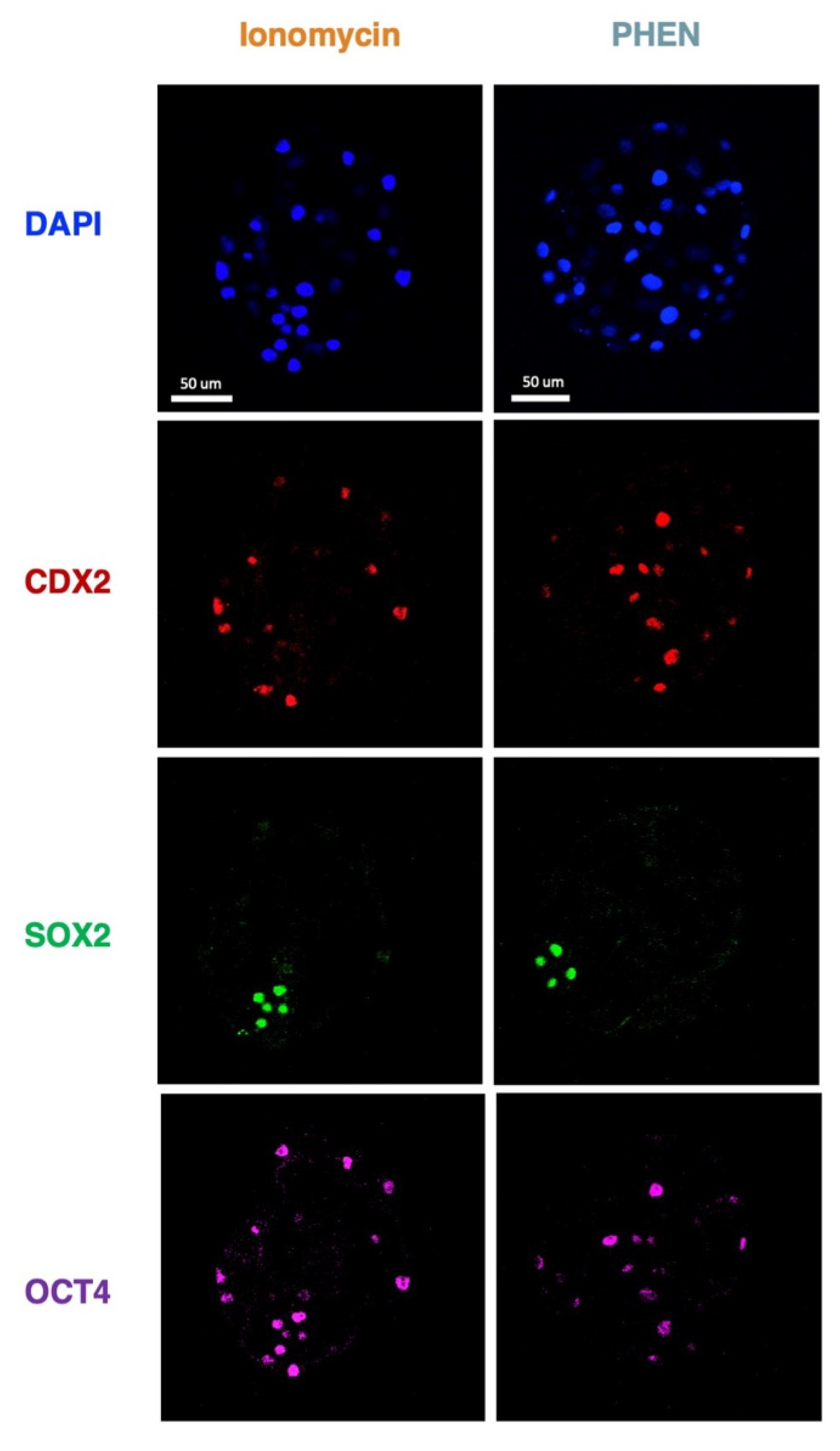
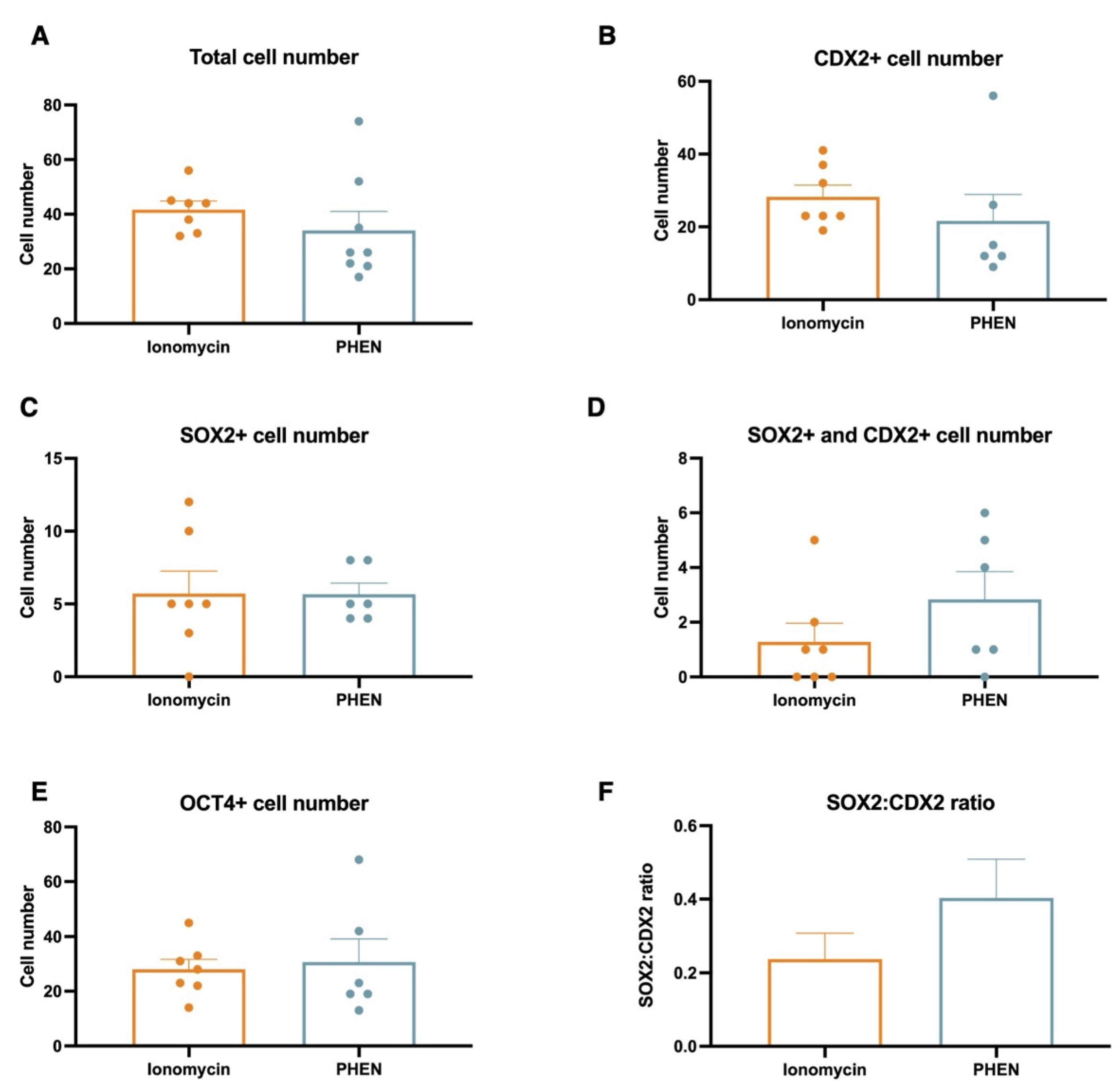
3.3. Experiment 3: Effects of Zn Chelation on the Quality and Cell Differentiation Markers Expression of Bovine and Porcine Blastocysts
3.4. Experiment 4: Effects of Combining Zn Chelation and Calcium Rise and Vice-Versa on Porcine Preimplantation Embryo Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Telford, W.G.; Fraker, P.J. Preferential induction of apoptosis in mouse CD4+CD8+ alpha beta TCRloCD3 epsilon lo thymocytes by zinc. J. Cell Physiol. 1995, 164, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambe, T.; Yamaguchi-Iwai, Y.; Sasaki, R.; Nagao, M. Overview of mammalian zinc transporters. Cell Mol. Life Sci. 2004, 61, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Metals on the move: Zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 2011, 24, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Duncan, F.E.; Lee, H.C.; Hornick, J.E.; Vogt, S.; Fissore, R.A.; O’Halloran, T.V.; Woodruff, T.K. Bovine eggs release zinc in response to parthenogenetic and sperm-induced egg activation. Theriogenology 2019, 127, 41–48. [Google Scholar] [CrossRef]
- Garner, T.B.; Hester, J.M.; Carothers, A.; Diaz, F.J. Role of zinc in female reproduction. Biol. Reprod. 2021, 104, 976–994. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, N.; Suzuki, E.; Okuda, E.; Perry, A.C. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development 2010, 137, 2659–2669. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Tokuhiro, K.; Dean, J. Glycan-Independent Gamete Recognition Triggers Egg Zinc Sparks and ZP2 Cleavage to Prevent Polyspermy. Dev. Cell 2018, 46, 627–640. [Google Scholar] [CrossRef] [Green Version]
- Que, E.L.; Duncan, F.E.; Bayer, A.R.; Philips, S.J.; Roth, E.W.; Bleher, R.; Gleber, S.C.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr. Biol. (Camb.) 2017, 9, 135–144. [Google Scholar] [CrossRef]
- Sun, L.; Chai, Y.; Hannigan, R.; Bhogaraju, V.K.; Machaca, K. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J. Cell Physiol. 2007, 213, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.E.; Que, E.L.; Zhang, N.; Feinberg, E.C.; O’Halloran, T.V.; Woodruff, T.K. The zinc spark is an inorganic signature of human egg activation. Sci. Rep. 2016, 6, 24737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uh, K.; Ryu, J.; Zhang, L.; Errington, J.; Machaty, Z.; Lee, K. Development of novel oocyte activation approaches using Zn(2+) chelators in pigs. Theriogenology 2019, 125, 259–267. [Google Scholar] [CrossRef]
- Lee, K.; Davis, A.; Zhang, L.; Ryu, J.; Spate, L.D.; Park, K.W.; Samuel, M.S.; Walters, E.M.; Murphy, C.N.; Machaty, Z.; et al. Pig oocyte activation using a Zn(2)(+) chelator, TPEN. Theriogenology 2015, 84, 1024–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uh, K.; Hay, A.; Chen, P.; Reese, E.; Lee, K. Design of novel oocyte activation methods: The role of zinc. Biol. Reprod. 2022, 106, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Gambini, A.; Briski, O.; Canel, N.G. State of the art of nuclear transfer technologies for assisting mammalian reproduction. Mol. Reprod. Dev. 2022, 89, 230–242. [Google Scholar] [CrossRef]
- Stein, P.; Savy, V.; Williams, A.M.; Williams, C.J. Modulators of calcium signalling at fertilization. Open Biol. 2020, 10, 200118. [Google Scholar] [CrossRef]
- Ozil, J.P.; Banrezes, B.; Toth, S.; Pan, H.; Schultz, R.M. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev. Biol. 2006, 300, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Shafqat, A.; Kashir, J.; Alsalameh, S.; Alkattan, K.; Yaqinuddin, A. Fertilization, Oocyte Activation, Calcium Release and Epigenetic Remodelling: Lessons From Cancer Models. Front. Cell Dev. Biol. 2022, 10, 781953. [Google Scholar] [CrossRef]
- Ciapa, B.; Arnoult, C. Could modifications of signalling pathways activated after ICSI induce a potential risk of epigenetic defects? Int. J. Dev. Biol. 2011, 55, 143–152. [Google Scholar] [CrossRef]
- Holm, P.; Booth, P.J.; Schmidt, M.H.; Greve, T.; Callesen, H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999, 52, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Suzuki, C.; Tanaka, A.; Anas, I.M.; Iwamura, S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol. Reprod. 2002, 66, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brackett, B.G.; Oliphant, G. Capacitation of rabbit spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gambini, A.; Williams, C.J. LUTs of blastocyst nuclei cell for quantification. Mol. Reprod. Dev. 2016, 83, 575. [Google Scholar] [CrossRef]
- Swann, K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 1990, 110, 1295–1302. [Google Scholar] [CrossRef]
- Zhao, M.H.; Kim, N.H.; Cui, X.S. Zinc depletion activates porcine metaphase II oocytes independently of the protein kinase C pathway. In Vitro Cell Dev. Biol. Anim. 2014, 50, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Suva, M.; Canel, N.G.; Salamone, D.F. Effect of single and combined treatments with MPF or MAPK inhibitors on parthenogenetic haploid activation of bovine oocytes. Reprod. Biol. 2019, 19, 386–393. [Google Scholar] [CrossRef]
- Aguila, L.; Suzuki, J.; Hill, A.B.T.; Garcia, M.; de Mattos, K.; Therrien, J.; Smith, L.C. Dysregulated Gene Expression of Imprinted and X-Linked Genes: A Link to Poor Development of Bovine Haploid Androgenetic Embryos. Front. Cell Dev. Biol. 2021, 9, 640712. [Google Scholar] [CrossRef]
- Sirard, M.A. 40 years of bovine IVF in the new genomic selection context. Reproduction 2018, 156, R1–R7. [Google Scholar] [CrossRef]
- Fuentes, F.; Munoz, E.; Contreras, M.J.; Arias, M.E.; Felmer, R. Bovine ICSI: Limiting factors, strategies to improve its efficiency and alternative approaches. Zygote 2022, 30, 749–767. [Google Scholar] [CrossRef]
- Ock, S.A.; Bhak, J.S.; Balasubramanian, S.; Lee, H.J.; Choe, S.Y.; Rho, G.J. Different activation treatments for successful development of bovine oocytes following intracytoplasmic sperm injection. Zygote 2003, 11, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Saucedo, J.; Paramio-Nieto, M.T.; Fierro, R.; Pina-Aguilar, R.E. Intracytoplasmic sperm injection (ICSI) in small ruminants. Anim. Reprod. Sci. 2012, 133, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Briski, O.; Salamone, D.F. Past, present and future of ICSI in livestock species. Anim. Reprod. Sci. 2022, 246, 106925. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Hu, H.; Liu, Z.; Zhang, L.; Zhuan, Q.; Li, X.; Fu, X.; Zhu, S.; Hou, Y. The Role of Ca(2+) in Maturation and Reprogramming of Bovine Oocytes: A System Study of Low-Calcium Model. Front. Cell Dev. Biol. 2021, 9, 746237. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.H.; Deemeh, M.R.; Tavalaee, M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil. Steril. 2010, 94, 520–526. [Google Scholar] [CrossRef]
- Tao, Y. Oocyte activation during round spermatid injection: State of the art. Reprod. BioMed. Online 2022, 45, 211–218. [Google Scholar] [CrossRef]
- Savy, V.; Stein, P.; Shi, M.; Williams, C.J. PMCA1 depletion in mouse eggs amplifies calcium signaling and impacts offspring growth section sign. Biol. Reprod. 2022, 107, 1439–1451. [Google Scholar] [CrossRef]
- Kim, A.M.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 2010, 6, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Lodde, V.; Garcia Barros, R.; Dall’Acqua, P.C.; Dieci, C.; Robert, C.; Bastien, A.; Sirard, M.A.; Franciosi, F.; Luciano, A.M. Zinc supports transcription and improves meiotic competence of growing bovine oocytes. Reproduction 2020, 159, 679–691. [Google Scholar] [CrossRef]
- Kong, B.Y.; Duncan, F.E.; Que, E.L.; Xu, Y.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions. Dev. Dyn. 2015, 244, 935–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, Y.; Yoon, J.D.; Cai, L.; Hwang, S.U.; Kim, E.; Lee, E.; Jeung, E.B.; Hyun, S.H. Effect of zinc on in vitro development of porcine embryos. Theriogenology 2015, 84, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Gambini, A.; Stein, P.; Savy, V.; Grow, E.J.; Papas, B.N.; Zhang, Y.; Kenan, A.C.; Padilla-Banks, E.; Cairns, B.R.; Williams, C.J. Developmentally Programmed Tankyrase Activity Upregulates beta-Catenin and Licenses Progression of Embryonic Genome Activation. Dev. Cell 2020, 53, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Shi, Y.; Wang, H.; Wang, Z.; Dang, Y.; Li, S.; Wang, S.; Zhang, K. Base editing in bovine embryos reveals a species-specific role of SOX2 in regulation of pluripotency. PLoS Genet. 2022, 18, e1010307. [Google Scholar] [CrossRef]

| Group | n. oocytes | n. cleaved embryos (%) | n. d 8 blastocysts (%) |
|---|---|---|---|
| Ionomycin | 80 | 76 (95.00) a | 61 (76.25) a |
| PHEN–SOF | 73 | 45 (61.64) b | 15 (20.55) b |
| PHEN–Talp-h | 93 | 71 (76.34) c | 27 (29.03) b |
| Group | n. oocytes | n. cleaved embryos (%) | n. d 8 blastocysts (%) |
|---|---|---|---|
| Ionomycin | 62 | 29 (46.77) | 11 (17.74) a |
| PHEN–PZM | 92 | 47 (51.09) | 3 (3.26) b |
| PHEN–Talp-h | 93 | 64 (68.82) | 14 (15.05) a |
| Group | n. oocytes | n. cleaved embryos (%) | n. d 8 blastocysts (%) |
|---|---|---|---|
| IVF | 101 | 69 (68.31) a | 42 (41.58) a |
| Ionomycin | 83 | 69 (83.13) b | 42 (50.60) a |
| PHEN 0.5 mM for 30 min | 73 | 45 (61.64) a | 15 (20,55) b |
| PHEN 0.5 mM for 1 h | 82 | 62 (75.60) a,b | 23 (28.04) b |
| PHEN 1 mM for 30 min | 72 | 39 (54.17) a,c | 20 (27.78) b |
| PHEN 1 mM for 1 h | 72 | 28 (38.89) c | 14 (19.44) b |
| Group | n. oocytes | n. cleaved embryos (%) | n. d 8 blastocysts (%) |
|---|---|---|---|
| Ionomycin | 97 | 71 (73.20) a | 20 (20.62) a |
| PHEN 0.5 mM for 1 h | 146 | 111 (76.03) a | 27 (18.49) a |
| PHEN 1 mM for 30 min | 85 | 70 (82.35) a | 37 (44.71) b |
| PHEN 1 mM for 1 h | 63 | 31 (49.21) b | 9 (14.29) a |
| Group | n. oocytes | n. cleaved embryos (%) | n. d 8 blastocysts (%) |
|---|---|---|---|
| Ionomycin | 79 | 65 (82.28) | 22 (27.85) a |
| PHEN | 76 | 69 (90.79) | 35 (46.05) b |
| Ionomycin + PHEN | 103 | 85 (82.52) | 32 (31.07) a |
| PHEN + Ionomycin | 76 | 69 (90.79) | 16 (21.05) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabeza, J.P.; Cámera, J.; Briski, O.; Felipe, M.Y.; Salamone, D.F.; Gambini, A. Preimplantation Developmental Competence of Bovine and Porcine Oocytes Activated by Zinc Chelation. Animals 2022, 12, 3560. https://doi.org/10.3390/ani12243560
Cabeza JP, Cámera J, Briski O, Felipe MY, Salamone DF, Gambini A. Preimplantation Developmental Competence of Bovine and Porcine Oocytes Activated by Zinc Chelation. Animals. 2022; 12(24):3560. https://doi.org/10.3390/ani12243560
Chicago/Turabian StyleCabeza, Juan P., Juan Cámera, Olinda Briski, Minerva Yauri Felipe, Daniel F. Salamone, and Andrés Gambini. 2022. "Preimplantation Developmental Competence of Bovine and Porcine Oocytes Activated by Zinc Chelation" Animals 12, no. 24: 3560. https://doi.org/10.3390/ani12243560
APA StyleCabeza, J. P., Cámera, J., Briski, O., Felipe, M. Y., Salamone, D. F., & Gambini, A. (2022). Preimplantation Developmental Competence of Bovine and Porcine Oocytes Activated by Zinc Chelation. Animals, 12(24), 3560. https://doi.org/10.3390/ani12243560







