Protein Supplementation during Mid-Gestation Alters the Amino Acid Patterns, Hepatic Metabolism, and Maternal Skeletal Muscle Turnover of Pregnant Zebu Beef Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Management
2.2. Measurements
2.2.1. Blood Parameters
2.2.2. Skeletal Muscle and Liver Tissue Sampling
2.2.3. Gene Expressions Analysis
2.3. Statistical Analyses
3. Results
3.1. Maternal Performance and Voluntary Feed Intake
3.2. Blood Parameters
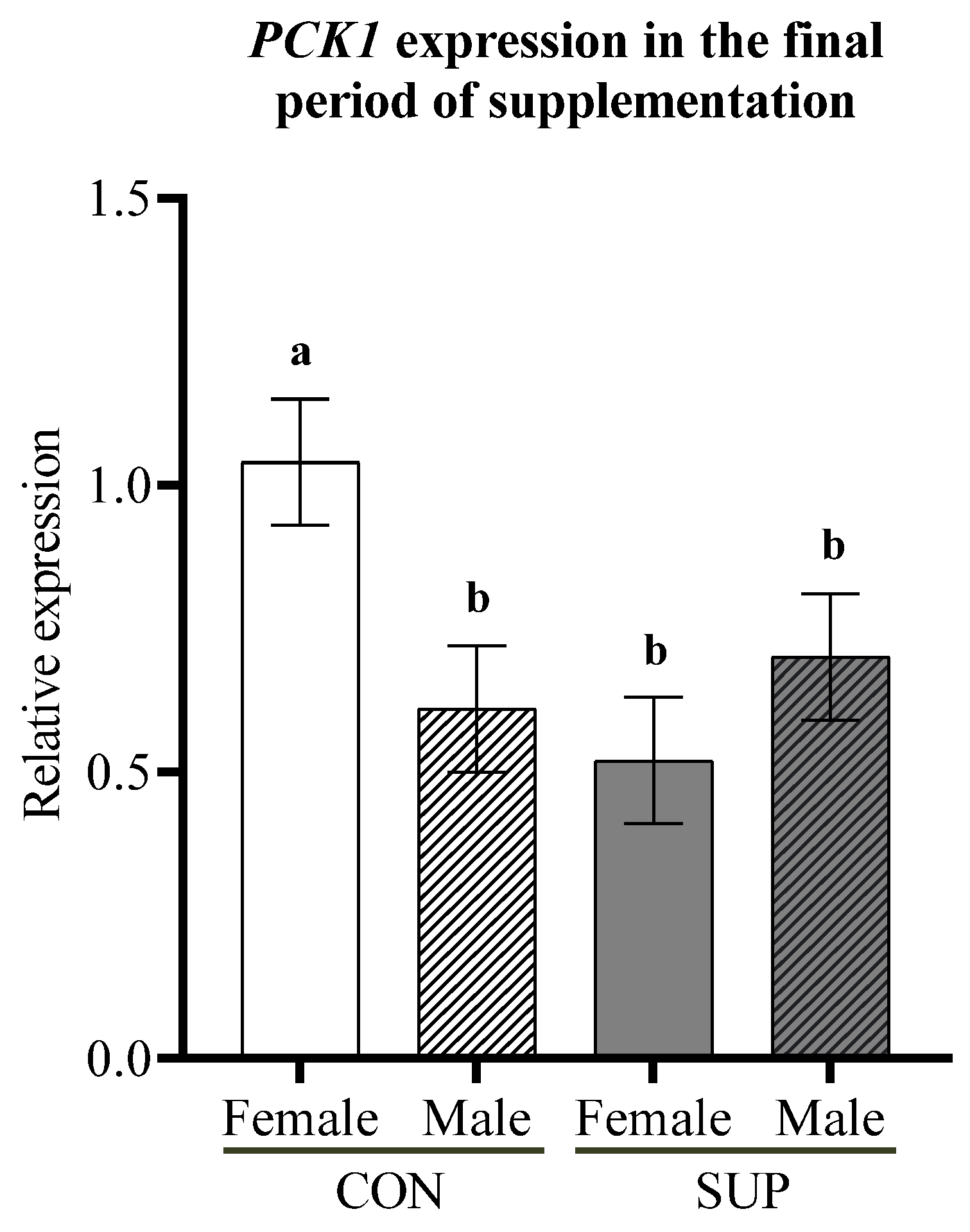
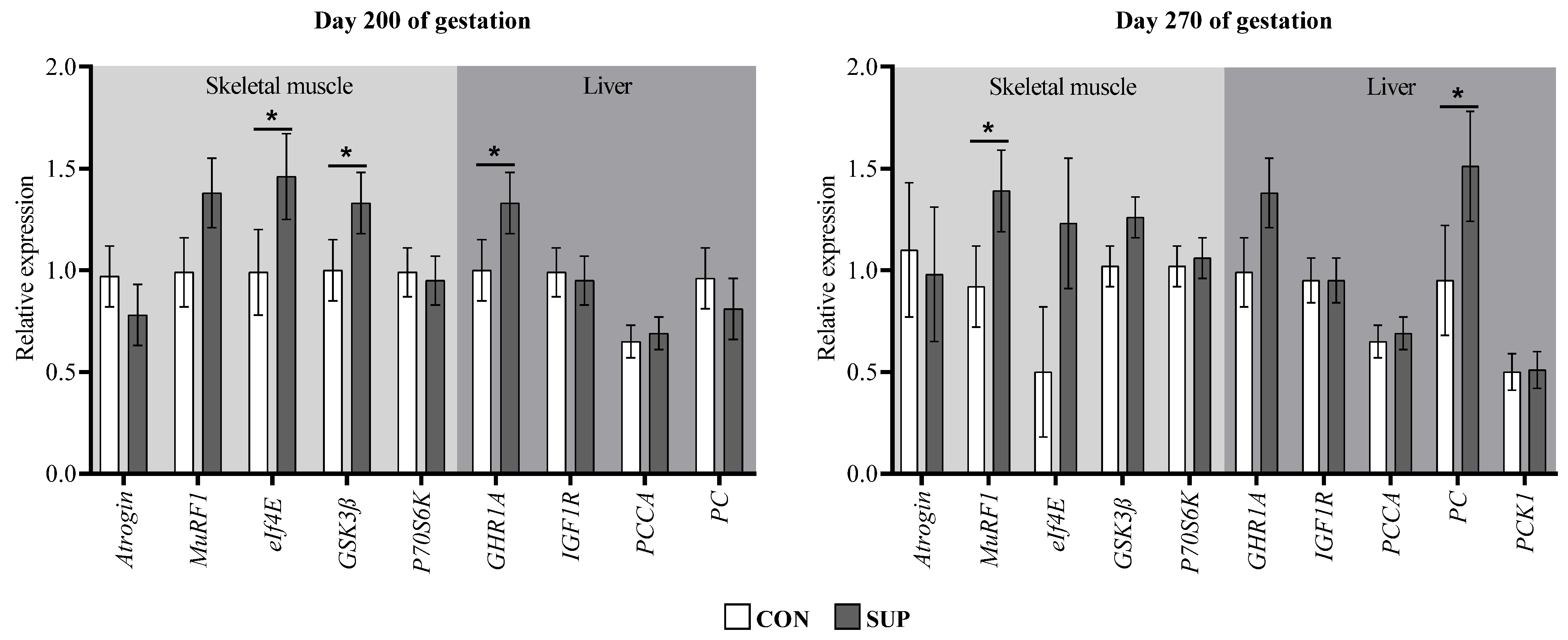
3.3. Gene Expression in Skeletal Muscle and Liver Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, J.M.; Rezende, C.d.P.; Ferreira Borges, A.M.; Homem, B.G.C.; Casagrande, D.R.; Macedo, T.M.; Alves, B.J.R.; Cabral de Sant’Anna, S.A.; Urquiaga, S.; Boddey, R.M. Production of beef cattle grazing on Brachiaria brizantha (Marandu grass)—Arachis pintoi (forage peanut cv. Belomonte) mixtures exceeded that on grass monocultures fertilized with 120 kg N/ha. Grass Forage Sci. 2020, 75, 28–36. [Google Scholar] [CrossRef]
- Paula, N.F.d.; Zervoudakis, J.T.; Cabral, L.d.S.; Carvalho, D.M.G.d.; Hatamoto-Zervoudakis, L.K.; Moraes, E.H.B.K.d.; Oliveira, A.A.d. Frequência de suplementação e fontes de proteína para recria de bovinos em pastejo no período seco: Desempenho produtivo e econômico. Rev. Bras. De Zootec. 2010, 39, 873–882. [Google Scholar] [CrossRef]
- Gionbelli, T.; Veloso, C.; Rotta, P.; Valadares Filho, S.C.; Carvalho, B.; Marcondes, M.I.; Cunha, C.S.; Novaes, M.; Prezotto, L.D.; Duarte, M. Foetal development of skeletal muscle in bovines as a function of maternal nutrition, foetal sex and gestational age. J. Anim. Physiol. Anim. Nutr. 2018, 102, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Zamudio, G.D.; da Cruz, W.F.; Schoonmaker, J.P.; de Resende, F.D.; Siqueira, G.R.; Neto, O.R.M.; Gionbelli, T.R.; Teixeira, P.D.; Rodrigues, L.M.; Gionbelli, M.P. Effect of rumen-protected fat on performance, carcass characteristics and beef quality of the progeny from Nellore cows fed by different planes of nutrition during gestation. Livest. Sci. 2022, 258, 104851. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Schoonmaker, J.P.; Resende, F.D.; Siqueira, G.R.; Neto, O.R.M.; Gionbelli, M.P.; Gionbelli, T.R.S.; Ladeira, M.M. Effects of protein supplementation on Nellore cows’ reproductive performance, growth, myogenesis, lipogenesis and intestine development of the progeny. Anim. Prod. Sci. 2020, 61, 371–380. [Google Scholar] [CrossRef]
- Lopes, R.; Sampaio, C.; Trece, A.; Teixeira, P.; Gionbelli, T.; Santos, L.; Costa, T.; Duarte, M.; Gionbelli, M. Impacts of protein supplementation during late gestation of beef cows on maternal skeletal muscle and liver tissues metabolism. Animal 2020, 14, 1867–1875. [Google Scholar] [CrossRef]
- Costa, T.C.; Du, M.; Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Schultz, E.B.; Gionbelli, M.P.; Duarte, M.d.S. Skeletal muscle development in postnatal beef cattle resulting from maternal protein restriction during mid-gestation. Animals 2021, 11, 860. [Google Scholar] [CrossRef]
- Bell, A.W.; Ehrhardt, R.A. Regulation of macronutrient partitioning between maternal and conceptus tissues in the pregnant ruminant. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; Cronjé, J.S., Ed.; CAB International: New York, NY, USA, 2000; pp. 275–293. [Google Scholar]
- Santos, M.C.; Costa, T.C.; Ramírez-Zamudio, G.D.; Nascimento, K.B.; Gionbelli, M.P.; Duarte, M.d.S. Prenatal origins of productivity and quality of beef. Rev. Bras. De Zootec. 2022, 51, e20220061. [Google Scholar] [CrossRef]
- Caton, J.; Bauer, M.; Hidari, H. Metabolic components of energy expenditure in growing beef cattle-review. Asian-Australas. J. Anim. Sci. 2000, 13, 702–710. [Google Scholar] [CrossRef]
- Micke, G.; Sullivan, T.; McMillen, I.; Gentili, S.; Perry, V. Protein intake during gestation affects postnatal bovine skeletal muscle growth and relative expression of IGF1, IGF1R, IGF2 and IGF2R. Mol. Cell. Endocrinol. 2011, 332, 234–241. [Google Scholar] [CrossRef]
- Costa, T.; Lourenço, P.; Souza, R.; Lopes, M.; Araújo, R.; Santos, M.; Luciano, L.; Massensini, J.; Chalfun, L.; Rennó, L. Ruminal undegradable protein enriched diet during late gestation of beef cows affects maternal metabolism and offspring’s skeletal muscle development. Anim. Feed Sci. Technol. 2022, 291, 115400. [Google Scholar] [CrossRef]
- Carvalho, E.B.; Costa, T.C.; Sanglard, L.P.; Nascimento, K.B.; Meneses, J.A.M.; Galvão, M.C.; Serão, N.V.L.; Duarte, M.S.; Gionbelli, M.P. Transcriptome profile in the skeletal muscle of cattle progeny as a function of maternal protein supplementation during mid-gestation. Livest. Sci. 2022, 263, 104995. [Google Scholar] [CrossRef]
- Egan, A. Nutritional status and intake regulation in sheep. III. The relationship between improvement of nitrogen status and increase in voluntary intake of low-protein roughages by sheep. Aust. J. Agric. Res. 1965, 16, 463–472. [Google Scholar] [CrossRef]
- Egan, A. The fate and effects of duodenally infused casein and urea nitrogen in sheep fed on a low-protein roughage. Aust. J. Agric. Res. 1965, 16, 169–177. [Google Scholar] [CrossRef]
- Egan, A.; Moir, R. Nutritional status and intake regulation in sheep. I. Effects of duodenally infused single doses of casein, urea, and propionate upon voluntary intake of a low-protein roughage by sheep. Aust. J. Agric. Res. 1965, 16, 437–449. [Google Scholar] [CrossRef]
- Barcelos, S.d.S.; Nascimento, K.B.; Silva, T.E.d.; Mezzomo, R.; Alves, K.S.; de Souza Duarte, M.; Gionbelli, M.P. The Effects of Prenatal Diet on Calf Performance and Perspectives for Fetal Programming Studies: A Meta-Analytical Investigation. Animals 2022, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Moreira, G.M.; Ramírez-Zamudio, G.D.; Souza, S.P.d.; Prezotto, L.D.; Chalfun, L.H.L.; Duarte, M.d.S.; Casagrande, D.R. Effects of Maternal Protein Supplementation at Mid-Gestation of Cows on Intake, Digestibility, and Feeding Behavior of the Offspring. Animals 2022, 12, 2865. [Google Scholar] [CrossRef]
- Valadares Filho, S.; Costa e Silva, L.; Gionbelli, M.; Rotta, P.; Marcondes, M.; Chizzotti, M.; Prados, L. Nutrient Requirements of Zebu and Crossbred Cattle-BR-CORTE, 3rd ed.; Suprema Gráfica Ltda: Viçosa, MG, Brazil, 2016. [Google Scholar]
- Crawford, M.; Doyle, W.; Meadows, N. Gender differences at birth and differences in fetal growth. Hum. Reprod. 1987, 2, 517–520. [Google Scholar] [CrossRef]
- Trivers, R.L.; Willard, D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef]
- Hinde, K.; Carpenter, A.J.; Clay, J.S.; Bradford, B.J. Holsteins favor heifers, not bulls: Biased milk production programmed during pregnancy as a function of fetal sex. PLoS ONE 2014, 9, e86169. [Google Scholar] [CrossRef]
- Ithurralde, J.; Pérez-Clariget, R.; Corrales, F.; Fila, D.; López-Pérez, Á.; de Jesús Marichal, M.; Saadoun, A.; Bielli, A. Sex-dependent effects of maternal undernutrition on growth performance, carcass characteristics and meat quality of lambs. Livest. Sci. 2019, 221, 105–114. [Google Scholar]
- Meneses, J.A.M. Impacts of Protein Supplementation during Mid-Gestation of Beef Cows on Maternal Physiology, Skeletal Muscle, and Liver Tissues Metabolism. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2021. [Google Scholar]
- Bergmeyer, H.-U. Principles of enzymatic analysis. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1965; pp. 3–13. [Google Scholar]
- McMurray, C.; Blanchflower, W.J.; Rice, D.A. Automated kinetic method for D-3-hydroxybutyrate in plasma or serum. Clin. Chem. 1984, 30, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Engle, T.; Spears, J.; Xi, L.; Edens, F. Dietary copper effects on lipid metabolism and circulating catecholamine concentrations in finishing steers. J. Anim. Sci. 2000, 78, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Wongkittichote, P.; Mew, N.A.; Chapman, K.A. Propionyl-CoA carboxylase–a review. Mol. Genet. Metab. 2017, 122, 145–152. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; St Maurice, M.; Rayment, I.; Cleland, W.W.; Wallace, J.C.; Attwood, P.V. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J. 2008, 413, 369–387. [Google Scholar] [CrossRef]
- Yu, S.; Meng, S.; Xiang, M.; Ma, H. Phosphoenolpyruvate carboxykinase in cell metabolism: Roles and mechanisms beyond gluconeogenesis. Mol. Metab. 2021, 53, 101257. [Google Scholar] [CrossRef]
- Coelho, T.C.; Chalfun-Junior, A.; Barreto, H.G.; Duarte, M.d.S.; Garcia, B.d.O.; Teixeira, P.D.; Gionbelli, T.R.S.; Ladeira, M.M. Reference gene selection for quantitative PCR in liver, skeletal muscle, and jejunum of Bos indicus cattle. Rev. Bras. De Zootec. 2022, 51. [Google Scholar] [CrossRef]
- Sampaio, C.B.; Detmann, E.; Lazzarini, I.; Souza, M.A.d.; Paulino, M.F.; Valadares Filho, S.d.C. Rumen dynamics of neutral detergent fiber in cattle fed low-quality tropical forage and supplemented with nitrogenous compounds. Rev. Bras. De Zootec. 2009, 38, 560–569. [Google Scholar] [CrossRef]
- Sampaio, C.B.; Detmann, E.; Paulino, M.F.; Valadares Filho, S.C.; de Souza, M.A.; Lazzarini, I.; Rodrigues Paulino, P.V.; de Queiroz, A.C. Intake and digestibility in cattle fed low-quality tropical forage and supplemented with nitrogenous compounds. Trop. Anim. Health Prod. 2010, 42, 1471–1479. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344S–1352S. [Google Scholar] [CrossRef]
- Xu, W.; van Knegsel, A.T.; Vervoort, J.J.; Bruckmaier, R.M.; van Hoeij, R.J.; Kemp, B.; Saccenti, E. Prediction of metabolic status of dairy cows in early lactation with on-farm cow data and machine learning algorithms. J. Dairy Sci. 2019, 102, 10186–10201. [Google Scholar] [CrossRef] [PubMed]
- Leury, B.; Chandler, K.; Bird, A.; Bell, A. Effects of maternal undernutrition and exercise on glucose kinetics in fetal sheep. Br. J. Nutr. 1990, 64, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Ehrhardt, R.A. Regulation of placental nutrient transport and implications for fetal growth. Nutr. Res. Rev. 2002, 15, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, M.; Chung, M.; Wilkening, R.B.; Fennessey, P.V.; Battaglia, F.C.; Meschia, G. Relationship of fetal alanine uptake and placental alanine metabolism to maternal plasma alanine concentration. Am. J. Physiol.-Endocrinol. Metab. 1998, 275, E942–E950. [Google Scholar] [CrossRef]
- Kwon, H.; Ford, S.P.; Bazer, F.W.; Spencer, T.E.; Nathanielsz, P.W.; Nijland, M.J.; Hess, B.W.; Wu, G. Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids. Biol. Reprod. 2004, 71, 901–908. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.A.; Moritz, K.M.; Walker, D.W.; Dickinson, H. Sexually dimorphic placental development throughout gestation in the spiny mouse (Acomys cahirinus). Placenta 2013, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Sex-specific placental responses in fetal development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xie, C.; Zhang, Y.; Fan, Z.; Yin, Y.; Blachier, F. Glutamate–glutamine cycle and exchange in the placenta–fetus unit during late pregnancy. Amino Acids 2015, 47, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Liu, C.; Feng, C.; Fan, Z.; Dai, Z.; Lai, C.; Li, Z.; Wu, G.; Wang, J. Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J. Nutr. 2012, 142, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, P.R.; Lobo, C.; Battaglia, F.C.; Fennessey, P.V.; Wilkening, R.B.; Meschia, G. Glutamine-glutamate exchange between placenta and fetal liver. Am. J. Physiol.-Endocrinol. Metab. 1995, 268, E705–E711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, L.; Wang, Z.; Deng, M.; Nie, H.; Zhang, G.; Ma, T.; Wang, F. N-carbamylglutamate and L-arginine improved maternal and placental development in underfed ewes. Reproduction 2016, 151, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.N.; Lopreiato, V.; Alharthi, A.; Loor, J.J. Amino acids and the regulation of oxidative stress and immune function in dairy cattle. J. Anim. Sci. 2020, 98, S175–S193. [Google Scholar] [CrossRef]
- Sadhu, M.J.; Guan, Q.; Li, F.; Sales-Lee, J.; Iavarone, A.T.; Hammond, M.C.; Cande, W.Z.; Rine, J. Nutritional control of epigenetic processes in yeast and human cells. Genetics 2013, 195, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Luke, T.D.; Pryce, J.E.; Wales, W.J.; Rochfort, S.J. A Tale of Two Biomarkers: Untargeted 1H NMR metabolomic fingerprinting of BHBA and NEFA in early lactation dairy cows. Metabolites 2020, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Zhang, Y.; Kinoshita, A.; Otomaru, K.; Oshima, K.; Gotoh, Y.; Oshima, I.; Sano, M.; Roh, S.; Oe, M. Maternal undernutrition during pregnancy alters amino acid metabolism and gene expression associated with energy metabolism and angiogenesis in fetal calf muscle. Metabolites 2021, 11, 582. [Google Scholar] [CrossRef]
- Lemons, J.A.; Schreiner, R.L. Amino acid metabolism in the ovine fetus. Am. J. Physiol.-Endocrinol. Metab. 1983, 244, E459–E466. [Google Scholar] [CrossRef]
- Faichney, G.; White, G. Effects of maternal nutritional status on fetal and placental growth and on fetal urea synthesis in sheep. Aust. J. Biol. Sci. 1987, 40, 365–378. [Google Scholar] [CrossRef]
- White, H.M. The role of TCA cycle anaplerosis in ketosis and fatty liver in periparturient dairy cows. Animals 2015, 5, 793–802. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Velez, J.; Donkin, S. Feed restriction induces pyruvate carboxylase but not phosphoenolpyruvate carboxykinase in dairy cows. J. Dairy Sci. 2005, 88, 2938–2948. [Google Scholar] [CrossRef]
- Cappel, D.A.; Deja, S.; Duarte, J.A.; Kucejova, B.; Iñigo, M.; Fletcher, J.A.; Fu, X.; Berglund, E.D.; Liu, T.; Elmquist, J.K. Pyruvate-carboxylase-mediated anaplerosis promotes antioxidant capacity by sustaining TCA cycle and redox metabolism in liver. Cell Metab. 2019, 29, 1291–1305.e8. [Google Scholar] [CrossRef] [PubMed]
- Hagopian, K.; Ramsey, J.J.; Weindruch, R. Caloric restriction increases gluconeogenic and transaminase enzyme activities in mouse liver. Exp. Gerontol. 2003, 38, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Feuers, R.J.; Duffy, P.H.; Leakey, J.A.; Turturro, A.; Mittelstaedt, R.A.; Hart, R.W. Effect of chronic caloric restriction on hepatic enzymes of intermediary metabolism in the male Fischer 344 rat. Mech. Ageing Dev. 1989, 48, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.; Heitzman, R.; Hibbitt, K. Effects of starvation on intermediary metabolism in the lactating cow. A comparison with metabolic changes occurring during bovine ketosis. Biochem. J. 1972, 128, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Young, J.; Beitz, D. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Boyd, C.; Bracken, C.; Lamberson, W.; Keisler, D.; Lucy, M. Reduced growth hormone receptor (GHR) messenger ribonucleic acid in liver of periparturient cattle is caused by a specific down-regulation of GHR 1A that is associated with decreased insulin-like growth factor I. Endocrinology 1999, 140, 3947–3954. [Google Scholar] [CrossRef]
- Pires, J.; Delavaud, C.; Faulconnier, Y.; Pomies, D.; Chilliard, Y. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows. J. Dairy Sci. 2013, 96, 6423–6439. [Google Scholar] [CrossRef]
- Naismith, D.; Morgan, B. The biphasic nature of protein metabolism during pregnancy in the rat. Br. J. Nutr. 1976, 36, 563–566. [Google Scholar] [CrossRef]
- Remesar, X.; Lopez-Tejero, D.; Pastor-Anglada, M. Some aspects of amino acid metabolism in the rat fetus. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 88, 719–725. [Google Scholar] [CrossRef]
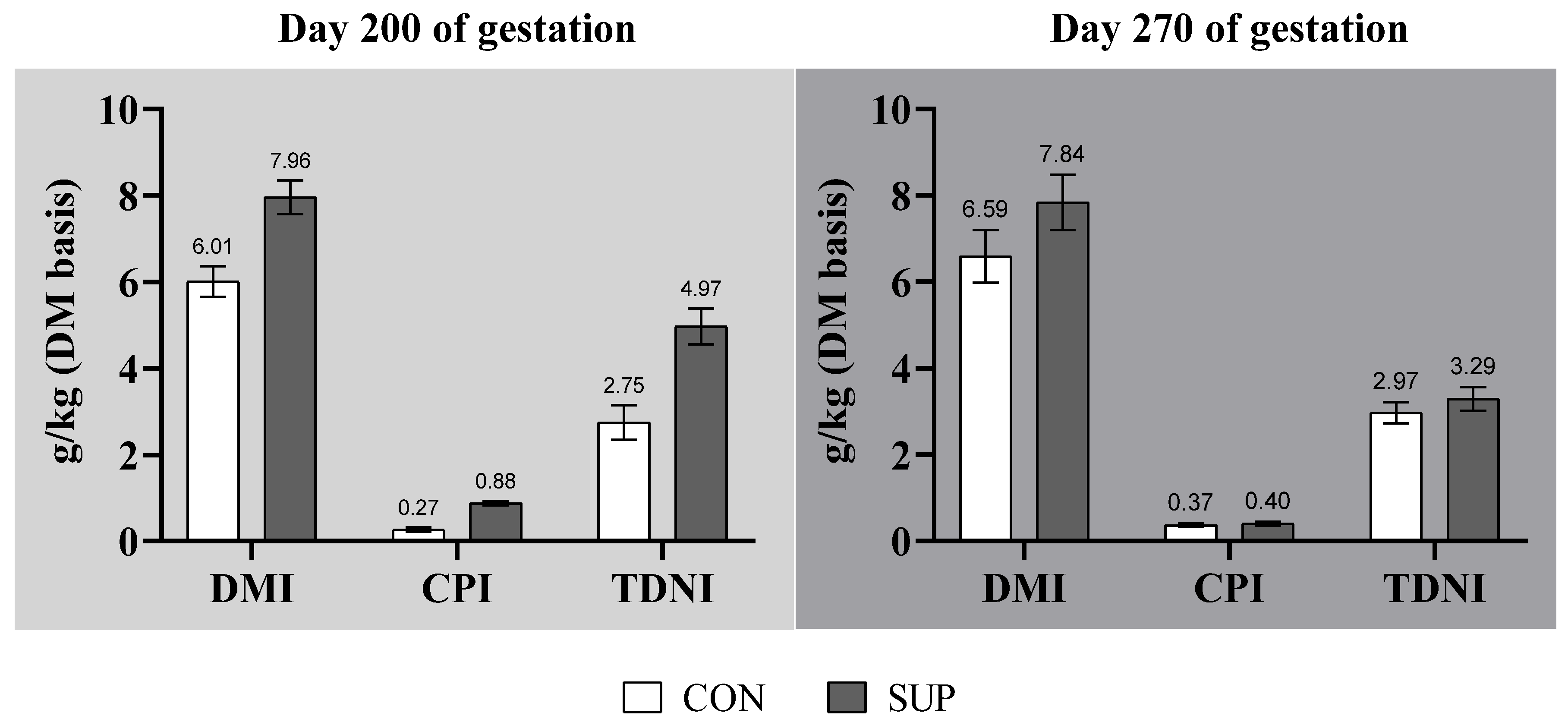


| Item | Mid-Gestation (Day 100 to 200) | Late-Gestation (Day 200 to Parturition) | |
|---|---|---|---|
| CON 1 | SUP 2 | ||
| Ingredients | |||
| Corn silage, % of DM | 75 | 75 | 100 |
| Sugarcane bagasse, % of DM | 25 | 25 | - |
| Supplement, kg of DM per day | - | 1.6 | - |
| Chemical composition | |||
| Dry matter, g/kg of DM | 418 | 492 | 330 |
| Organic matter, g/kg of DM | 951 | 960 | 941 |
| Crude protein, g/kg of DM | 53.3 | 125 | 72.2 |
| Ash and protein-free neutral detergent fiber, g/kg of DM | 631 | 51.6 | 549 |
| Ether extract, g/kg of DM | 24.1 | 29.4 | 29.2 |
| Total digestible nutrients, g/kg of DM | 614 | 647 | 677 |
| Metabolizable energy, Mcal/kg | 2.27 | 2.39 | 2.45 |
| Gene | Gene Abbreviation | Function | Access Number | Primer |
|---|---|---|---|---|
| Ribosomal protein S6 kinase | p70S6k | Protein Synthesis | AY396564.1 | F TTGAACCAAAAATCCGATCC |
| R AGCACCTCTTCCCCAGAAA | ||||
| Glycogen synthase kinase 3β | GSK3B | Protein Synthesis | NM_001101310.1 | F GCCCAGAACCACCTCCTTT |
| R TGCTGCCATCTTTGTCTCTG | ||||
| Eukaryotic translation initiation factor 4 E | eIf4E | Protein Synthesis | NM_174310 | F AAACCACCCCTACTCCGAAT |
| R TGCCCATCTGTTCTGTAAAGG | ||||
| Muscle ring finger 1 | MuRF1 | Protein Degradation | NM_001046155.1 | F GGGACAGATGAGGAAGAGGA |
| R CCTCATCATCGCCTTACTGG | ||||
| Muscle atrophy F-box protein | Atrogin-1 | Protein Degradation | NM_001046155.1 | F CCTTGAAGACCAGCAAAACA |
| R AGACTTGCCGACTCTTTGGA | ||||
| Growth hormone receptor 1A | GHR1A | Anabolism | AY748827 | F TCCAGCCTCTGTTTCAGGAG |
| R GCTGCCAGAGATCCATACCT | ||||
| Insulin-like growth factor 1 receptor | IGF1R | Anabolism/Energy Metabolism | NM_001077828 | F ATGTACTGCGCGCCTCTC |
| R CCCTCTACTTGTGTTCTTCAAATG | ||||
| Propionyl-CoA carboxylase | PCCA | Gluconeogenesis | NM_001083509.1 | F TTTGGTTTGCCGTCTGTTGG |
| R TTGAATGCCGCTGTCAACTC | ||||
| Pyruvate carboxylase | PC | Gluconeogenesis | NM_177946 | F GAGGTGGTCCGCAAGATG |
| R TCGTGCAGGGAAGTGATG | ||||
| Phosphoenolpyruvate carboxykinase 1 | PCK1 | Gluconeogenesis | NM_174737.2 | F GGGCTGATCGAAACCCTTAAT |
| R TTTCCTGGAGCCTGCTATTTC | ||||
| Β-actin | Β-actin | Endogenous Control | BC142413.1 | F GTCCACCTTCCAGCAGATGT |
| R CAGTCCGCCTAGAAGCATTT | ||||
| Cancer susceptibility candidate 3 | Casc3 | Endogenous Control | NM_001098069.1 | F GGACCTCCACCTCAGTTCAA |
| R GTCTTTGCCGTTGTGATGAA | ||||
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | Endogenous Control | NM_001034034.1 | F CGACTTCAACAGCGACACTC |
| R TTGTCGTACACAAGGAAATGAGC |
| Maternal Nutrition | Calf Sex | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | SUP | Female | Male | MN | CS | MN × CS | ||
| (n = 26) | (n = 26) | (n = 22) | (n = 30) | |||||
| Measurements at 200 days of gestation (end of supplementation period) | ||||||||
| IGF-1, Ng/mL | 61.9 | 66.9 | 62.6 | 66.2 | 2.00 | 0.06 | 0.18 | 0.64 |
| Insulin, µIU/mL | 6.70 | 10.4 | 8.77 | 8.37 | 0.45 | <0.01 | 0.51 | 0.43 |
| Glucose, mg/dL | 46.9 | 54.7 | 51.1 | 50.5 | 1.31 | <0.01 | 0.71 | 0.62 |
| Urea, mg/dL | 36.1 | 36.4 | 36.2 | 36.2 | 2.16 | 0.85 | 0.94 | 0.78 |
| NEFA, mmol/L | 0.11 | 0.10 | 0.11 | 0.10 | 0.01 | 0.56 | 0.50 | 0.63 |
| BHBA, mmol/L | 0.34 | 0.26 | 0.29 | 0.31 | 0.04 | 0.08 | 0.59 | 0.93 |
| Measurements at 270 days of gestation (pre-partum) | ||||||||
| IGF-1, Ng/mL | 58.8 | 62.09 | 59.6 | 61.3 | 3.20 | 0.20 | 0.48 | 0.33 |
| Insulin, µIU/mL | 6.58 | 9.05 | 7.78 | 7.85 | 0.59 | <0.01 | 0.87 | 0.14 |
| Glucose, mg/dL | 48.4 | 53.3 | 52.5 | 49.1 | 1.90 | 0.05 | 0.18 | 0.79 |
| Urea, mg/dL | 35.4 | 30.6 | 33.4 | 32.6 | 3.15 | 0.09 | 0.70 | 0.61 |
| NEFA, mmol/L | 0.10 | 0.08 | 0.09 | 0.10 | 0.90 | 0.01 | 0.42 | 0.07 |
| BHBA, mmol/L | 0.40 | 0.48 | 0.45 | 0.42 | 0.05 | 0.10 | 0.57 | 0.12 |
| Maternal Nutrition | Calf Sex | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | SUP | Female | Male | MN | CS | MN × CS | ||
| (n = 26) | (n = 26) | (n = 22) | (n = 30) | |||||
| Measurements at 200 days of gestation (end of supplementation period) | ||||||||
| Total AA | 1019 | 1533 | 1107 | 1445 | 128 | 0.03 | 0.34 | 0.10 |
| Essential AA | 611 | 662 | 647 | 626 | 66.1 | 0.11 | 0.66 | 0.31 |
| Non-essential AA | 579 | 834 | 621 | 791 | 66.7 | 0.02 | 0.16 | <0.01 |
| Glucogenic AA | 788 | 966 | 842 | 911 | 71.9 | 0.10 | 0.44 | 0.08 |
| Ketogenic AA | 141 | 132 | 152 | 120 | 17.6 | 0.69 | 0.15 | 0.13 |
| Glucogenic and ketogenic AA | 400 | 450 | 426 | 424 | 37.9 | 0.49 | 0.48 | 0.65 |
| Branched-chain AA | 355 | 351 | 383 | 323 | 47.1 | 0.95 | 0.30 | 0.26 |
| Measurements at 270 days of gestation (pre-partum) | ||||||||
| Total AA | 1121 | 1699 | 1226 | 1583 | 129 | 0.01 | 0.47 | 0.17 |
| Essential AA | 689 | 802 | 761 | 730 | 53.5 | 0.07 | 0.50 | 0.81 |
| Non-essential AA | 678 | 887 | 711 | 853 | 71.2 | 0.09 | 0.21 | 0.19 |
| Glucogenic AA | 981 | 1004 | 1027 | 957 | 62.7 | 0.76 | 0.36 | 0.69 |
| Ketogenic AA | 202 | 156 | 212 | 136 | 54.8 | 0.17 | 0.03 | 0.15 |
| Glucogenic and ketogenic AA | 417 | 529 | 455 | 490 | 31.1 | <0.01 | 0.35 | 0.51 |
| Branched-chain AA | 619 | 372 | 661 | 330 | 86.3 | 0.36 | 0.05 | 0.06 |
| Item | Maternal Nutrition | Calf Sex | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CON | SUP | Female | Male | MN | CS | MN × CS | ||
| (n = 26) | (n = 26) | (n = 22) | (n = 30) | |||||
| Measurements at 200 days of gestation (end of supplementation period) | ||||||||
| Essential AA | ||||||||
| Arginine | 70.8 | 93.7 | 79.8 | 84.7 | 13.0 | 0.16 | 0.75 | 0.59 |
| Phenylalanine | 78.0 | 74.4 | 88.5 | 63.9 | 11.7 | 0.80 | 0.10 | 0.08 |
| Histidine | 97.4 | 89.1 | 102 | 84.3 | 11.5 | 0.55 | 0.22 | 0.68 |
| Isoleucine | 125 | 122 | 138 | 108.3 | 18.3 | 0.90 | 0.19 | 0.31 |
| Methionine | 28.0 | 28.4 | 30.4 | 25.9 | 4.06 | 0.94 | 0.37 | 0.47 |
| Leucine | 122 | 117 | 129 | 110 | 12.2 | 0.76 | 0.21 | 0.19 |
| Tryptophan | 25.6 | 39.3 | 32.6 | 31.3 | 4.47 | 0.02 | 0.95 | 0.94 |
| Non-essential AA | ||||||||
| Aspartate | 15.5 | 21.5 | 18.8 | 18.2 | 4.39 | 0.26 | 0.90 | 0.73 |
| Glutamate | 142 | 122 | 132 | 131 | 19.4 | 0.41 | 0.98 | 0.81 |
| Asparagine | 6.87 | 8.24 | 8.16 | 6.95 | 1.23 | 0.39 | 0.42 | 0.90 |
| Serine | 82.2 | 88.0 | 76.1 | 94.2 | 12.4 | 0.71 | 0.24 | 0.10 |
| Glutamine | 177 | 257 | 191.3 | 233 | 45.0 | 0.11 | 0.45 | 0.09 |
| Tyrosine | 43.4 | 59.9 | 48.3 | 55.0 | 4.37 | <0.01 | 0.21 | 0.25 |
| Alanine | 229 | 282 | 257 | 255 | 19.3 | 0.03 | 0.94 | 0.15 |
| Measurements at 270 days of gestation (pre-partum) | ||||||||
| Essential AA | ||||||||
| Arginine | 65.3 | 85.3 | 69.5 | 81.1 | 10.4 | 0.12 | 0.36 | 0.23 |
| Phenylalanine | 47.7 | 63.2 | 57.3 | 53.7 | 2.88 | 0.03 | 0.58 | 0.84 |
| Histidine | 94.9 | 101 | 96.7 | 99.5 | 9.66 | 0.59 | 0.81 | 0.48 |
| Isoleucine | 191 | 121 | 196 | 116 | 22.5 | 0.69 | 0.20 | 0.03 |
| Methionine | 44.2 | 27.9 | 47.5 | 26.6 | 5.18 | 0.36 | 0.02 | 0.05 |
| Leucine | 122 | 156.2 | 142.6 | 136 | 5.97 | <0.01 | 0.52 | 0.67 |
| Tryptophan | 21.6 | 31.3 | 27.7 | 25.3 | 3.52 | 0.02 | 0.55 | 0.69 |
| Non-essential AA | ||||||||
| Aspartate | 9.22 | 9.31 | 8.54 | 8.90 | 4.62 | 0.89 | 0.64 | 0.96 |
| Glutamate | 286 | 317 | 299 | 304 | 26.0 | 0.33 | 0.87 | 0.12 |
| Asparagine | 25.9 | 27.3 | 25.4 | 26.5 | 17.4 | 0.70 | 0.71 | 0.72 |
| Serine | 107 | 104 | 100 | 108 | 13.8 | 0.84 | 0.51 | 0.24 |
| Glutamine | 98.3 | 87.7 | 87.9 | 98.2 | 12.2 | 0.47 | 0.49 | 0.89 |
| Tyrosine | 37.8 | 55.9 | 42.5 | 51.2 | 2.85 | <0.01 | 0.02 | 0.26 |
| Alanine | 211 | 287 | 244 | 253 | 25.2 | 0.02 | 0.77 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses, J.A.M.; Nascimento, K.B.; Galvão, M.C.; Ramírez-Zamudio, G.D.; Gionbelli, T.R.S.; Ladeira, M.M.; Duarte, M.d.S.; Casagrande, D.R.; Gionbelli, M.P. Protein Supplementation during Mid-Gestation Alters the Amino Acid Patterns, Hepatic Metabolism, and Maternal Skeletal Muscle Turnover of Pregnant Zebu Beef Cows. Animals 2022, 12, 3567. https://doi.org/10.3390/ani12243567
Meneses JAM, Nascimento KB, Galvão MC, Ramírez-Zamudio GD, Gionbelli TRS, Ladeira MM, Duarte MdS, Casagrande DR, Gionbelli MP. Protein Supplementation during Mid-Gestation Alters the Amino Acid Patterns, Hepatic Metabolism, and Maternal Skeletal Muscle Turnover of Pregnant Zebu Beef Cows. Animals. 2022; 12(24):3567. https://doi.org/10.3390/ani12243567
Chicago/Turabian StyleMeneses, Javier Andrés Moreno, Karolina Batista Nascimento, Matheus Castilho Galvão, German Darío Ramírez-Zamudio, Tathyane Ramalho Santos Gionbelli, Marcio Machado Ladeira, Marcio de Souza Duarte, Daniel Rume Casagrande, and Mateus Pies Gionbelli. 2022. "Protein Supplementation during Mid-Gestation Alters the Amino Acid Patterns, Hepatic Metabolism, and Maternal Skeletal Muscle Turnover of Pregnant Zebu Beef Cows" Animals 12, no. 24: 3567. https://doi.org/10.3390/ani12243567
APA StyleMeneses, J. A. M., Nascimento, K. B., Galvão, M. C., Ramírez-Zamudio, G. D., Gionbelli, T. R. S., Ladeira, M. M., Duarte, M. d. S., Casagrande, D. R., & Gionbelli, M. P. (2022). Protein Supplementation during Mid-Gestation Alters the Amino Acid Patterns, Hepatic Metabolism, and Maternal Skeletal Muscle Turnover of Pregnant Zebu Beef Cows. Animals, 12(24), 3567. https://doi.org/10.3390/ani12243567











