Tattoo Skin Disease in Cetacea: A Review, with New Cases for the Northeast Pacific
Abstract
:Simple Summary
Abstract
1. Introduction
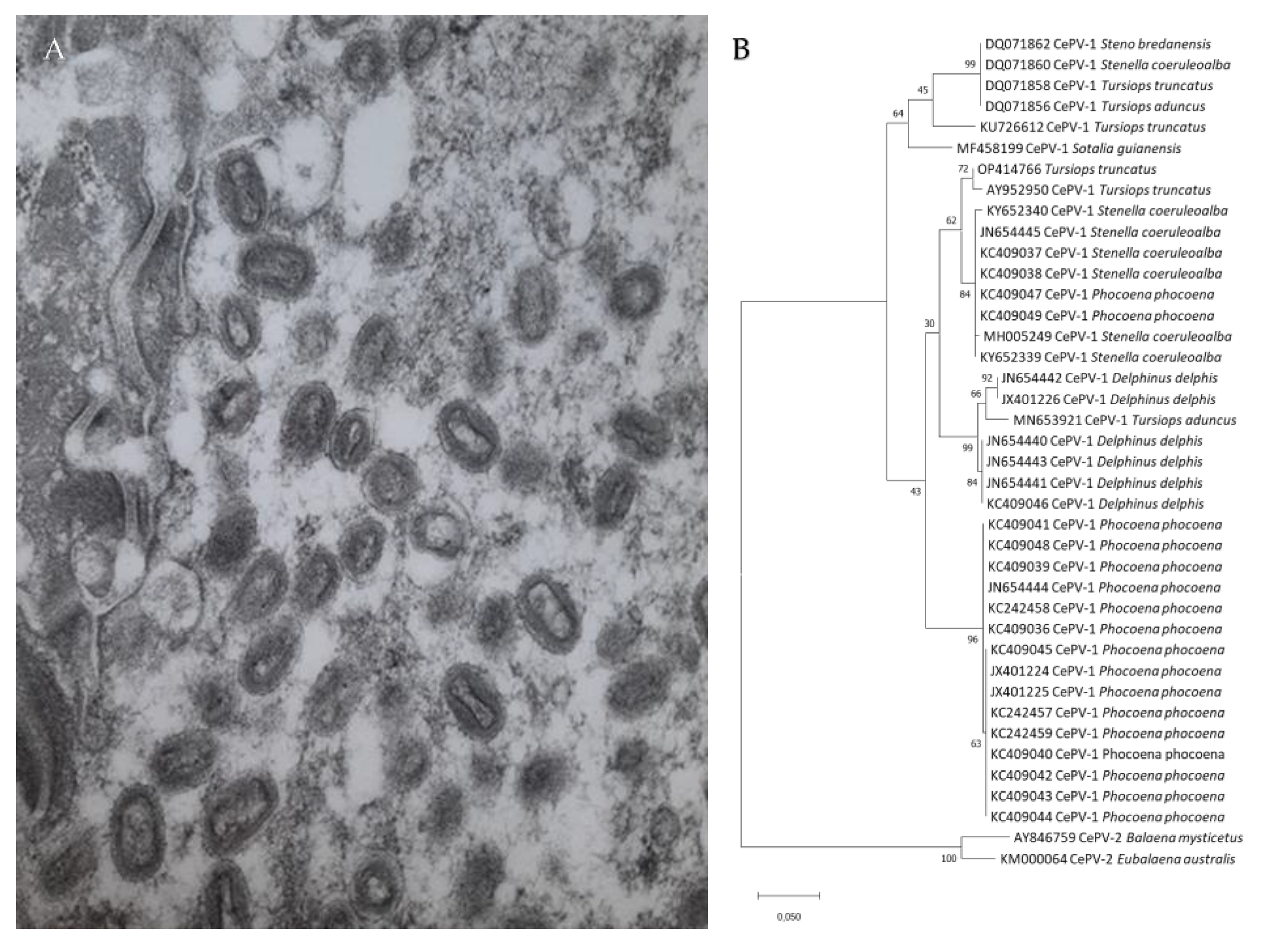
2. Aetiology
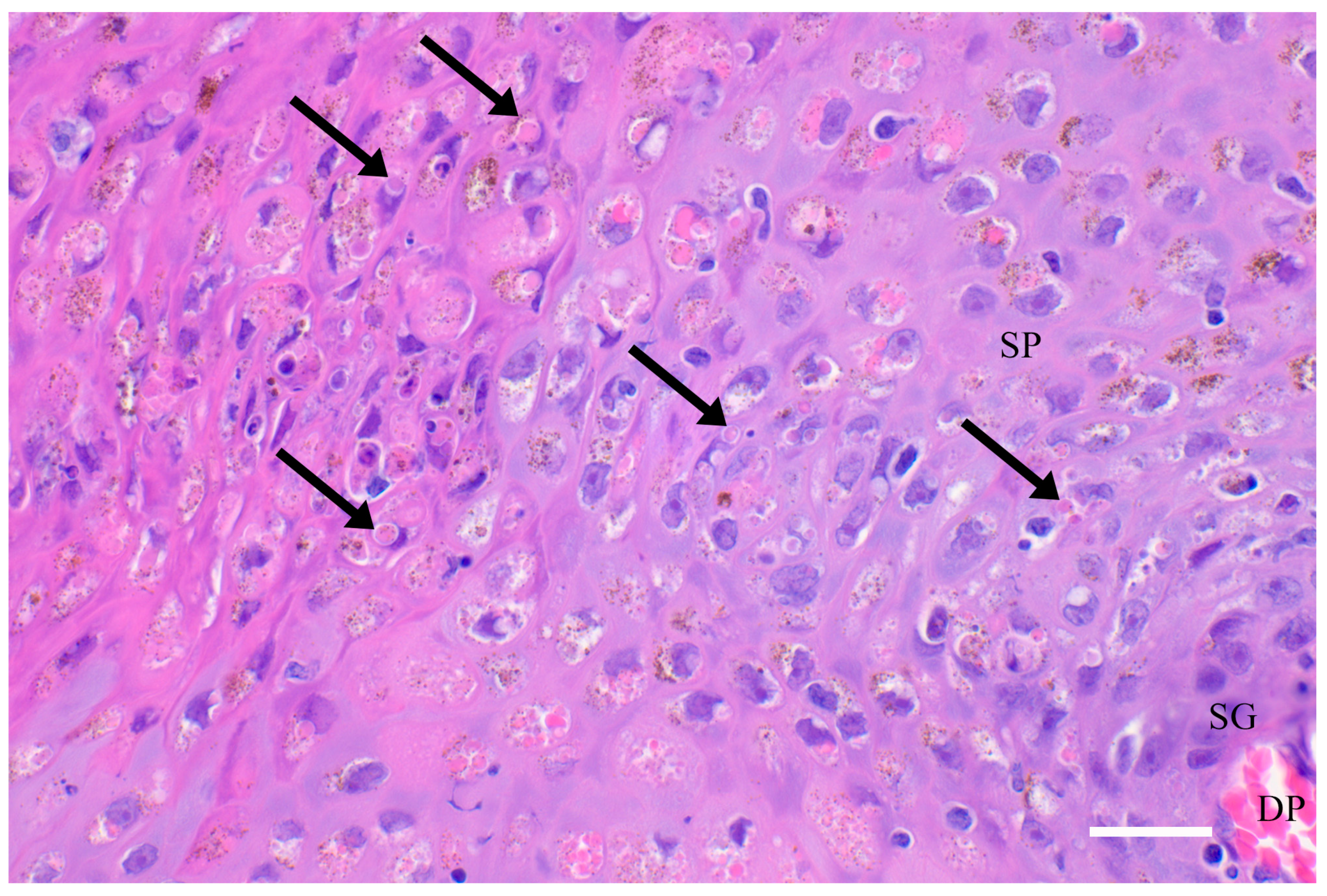
3. Pathology
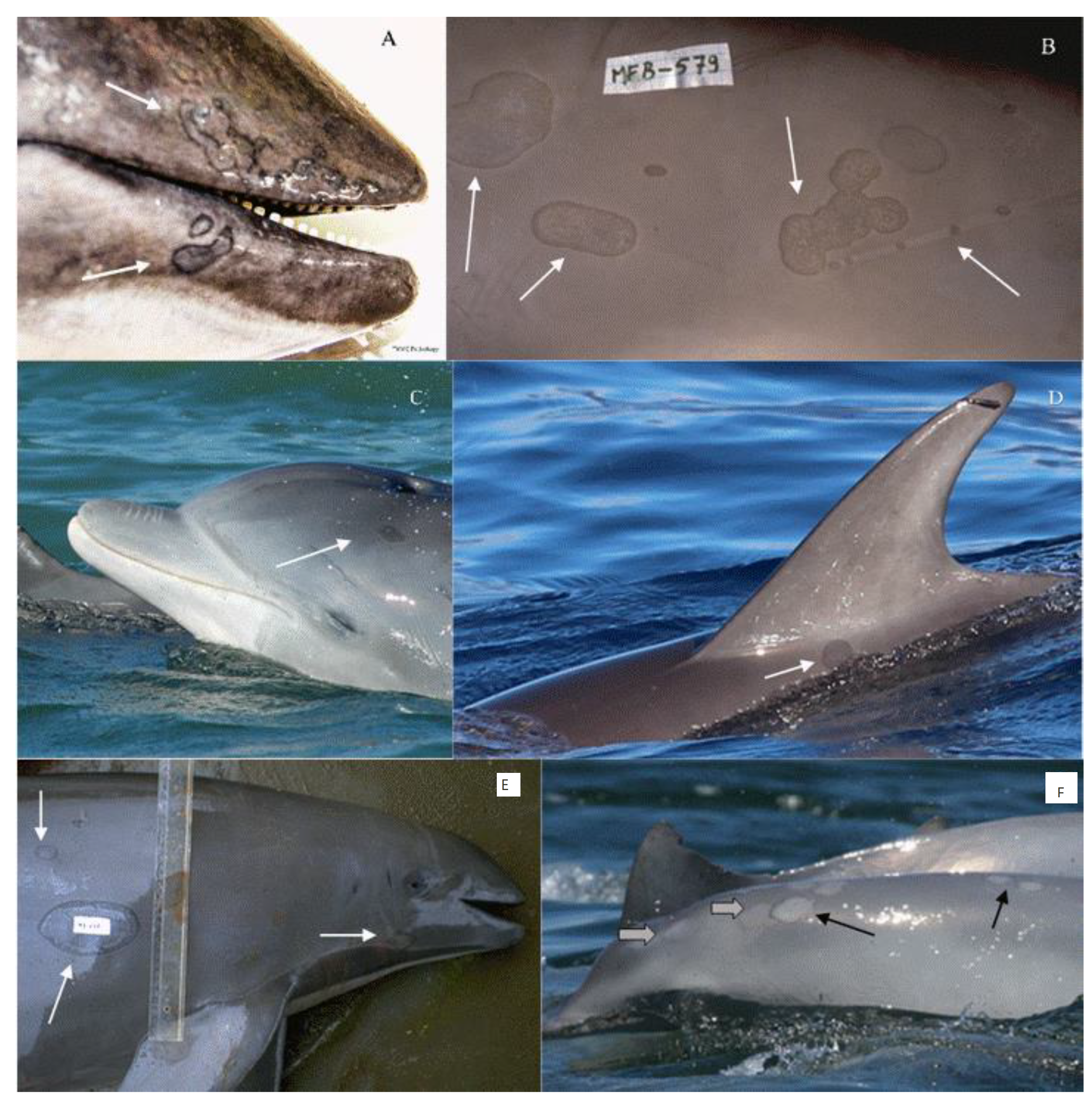
4. Diagnosis
5. Epidemiology
5.1. General Epidemiological Characteristics
5.2. Epidemiology of Tattoo Skin Disease in Odontocetes from the North Pacific
5.3. Role of Stress and Health Status
5.4. Influence of Environmental Factors
5.5. Impact of Immunotoxic Contaminants
5.6. Tattoo Skin Disease in Baleen Whales
6. Immune Response to CePVs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Geraci, J.R.; Hicks, B.D.; St Aubin, D.J. Dolphin pox: A skin disease of cetaceans. Can. J. Comp. Med. 1979, 43, 399–404. [Google Scholar] [PubMed]
- Van Bressem, M.F.; Van Waerebeek, K.; Reyes, J.C.; Dekegel, D.; Pastoret, P.P. Evidence of Poxvirus in Dusky Dolphin (Lagenorhynchus obscurus) and Burmeister’s Porpoise (Phocoena spinipinnis) from Coastal Peru. J. Wildl. Dis. 1993, 29, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanino, G.P.; Van Bressem, M.-F.; Van Waerebeek, K.; Pozo, N. Skin disorders of coastal dolphins at Añihue Reserve, Chilean Patagonia: A matter of concern. Bol. Mus. Nac. Hist. Nat. Chile 2014, 63, 127–158. [Google Scholar]
- Sweeney, J.C.; Ridgway, S.H. Common diseases of small cetaceans. J. Am. Vet. Med. Assoc. 1975, 167, 533–540. [Google Scholar] [PubMed]
- Flom, J.O.; Houk, E.J. Morphologic evidence of poxvirus in ‘tattoo’ lesions from captive bottlenosed dolphins. J. Wildl. Dis. 1979, 15, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Van Bressem, M.-F.; Van Waerebeek, K.; Aznar, F.J.; Raga, J.A.; Jepson, P.D.; Duignan, P.; Deaville, R.; Flach, L.; Viddi, F.; Baker, J.R.; et al. Epidemiological pattern of tattoo skin disease: A potential general health indicator for cetaceans. Dis. Aquat. Org. 2009, 85, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blacklaws, B.A.; Gajda, A.M.; Tippelt, S.; Jepson, P.D.; Deaville, R.; Van Bressem, M.-F.; Pearce, G.P. Molecular characterization of poxviruses associated with tattoo skin lesions in UK cetaceans. PLoS ONE 2013, 8, e71734. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.; Dastjerdi, A.; Davison, N.; Deaville, R.; Everest, D.; Peake, J.; Finnegan, C.; Jepson, P.; Steinbach, F. Identification of Novel Cetacean Poxviruses in Cetaceans Stranded in South West England. PLoS ONE 2015, 10, e0124315. [Google Scholar] [CrossRef]
- Sacristán, C.; Esperón, F.; Marigo, J.; Ewbank, A.C.; de Carvalho, R.R.; Groch, K.R.; de Castilho, P.V.; Sánchez-Sarmiento, A.M.; Costa-Silva, S.; Ferreira-Machado, E.; et al. Molecular identification and microscopic characterization of poxvirus in a Guiana dolphin and a common bottlenose dolphin, Brazil. Dis. Aquat. Org. 2018, 130, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Luciani, L.; Piorkowski, G.; De Lamballerie, X.; Van Waerebeek, K.; Van Bressem, M.-F. Detection of Cetacean Poxvirus in Peruvian Common Bottlenose Dolphins (Tursiops truncatus) Using a Pan-Poxvirus PCR. Viruses 2022, 23, 1850. [Google Scholar] [CrossRef]
- Bracht, A.J.; Brudek, R.L.; Ewing, R.Y.; Manire, C.A.; Burek, K.A.; Rosa, C.; Beckmen, K.B.; Maruniak, J.E.; Romero, C.H. Genetic identification of novel poxviruses of cetaceans and pinnipeds. Arch. Virol. 2006, 151, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.C.; Subramaniam, K.; Varsani, A.; McFadden, G.; Schaefer, A.M.; Bossart, G.D.; Romero, C.H.; Waltzek, T.B. Genome characterization of cetaceanpox virus from a managed Indo-Pacific bottlenose dolphin (Tursiops aduncus). Virus Res. 2020, 278, 197861. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, L. DNA-Sensing Antiviral Innate Immunity in Poxvirus Infection. Front. Immunol. 2020, 11, 1637. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, C.; Palacios, C.; Golemba, M.; Bratanich, A.; Argüelles, M.; Fazio, A.; Bertellotti, M.; Lombardo, D. Identification, molecular and phylogenetic analysis of poxvirus in skin lesions of southern right whale. Dis. Aquat. Org. 2015, 116, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duignan, P.J.; Van Bressem, M.F.; Cortés-Hinojosa, G.; Kennedy-Stoskopf, S. Viruses of marine mammals. In CRC Handbook of Marine Mammal Medicine: Health, Disease, and Rehabilitation, 3rd ed.; Dierauf, L.A., Gulland, F.M.D., Eds.; Taylor and Francis Publishers: Boca Raton, FL, USA, 2018. [Google Scholar]
- Powell, S.N.; Wallen, M.M.; Bansal, S.; Mann, J. Epidemiological Investigation of Tattoo-Like Skin Lesions among Bottlenose Dolphins in Shark Bay, Australia. Sci. Total Environ. 2018, 630, 774–780. [Google Scholar] [CrossRef]
- Smith, A.W.; Skilling, D.E.; Ridgway, S.H.; Fenner, C.A. Regression of cetacean tattoo lesions concurrent with conversion of precipitin antibody against a poxvirus. J. Am. Vet. Med. Assoc. 1983, 183, 1219–1222. [Google Scholar]
- Van Bressem, M.F.; van Waerebeek, K.; Bennett, M. Orthopoxvirus neutralising antibodies in small cetaceans from the Southeast Pacific. Lat. Am. J. Aquat. Mamm. 2006, 5, 49–54. [Google Scholar] [CrossRef]
- Van Bressem, M.-F.; Gaspar, R.; Aznar, J. Epidemiology of tattoo skin disease in bottlenose dolphins (Tursiops truncatus) from the Sado estuary, Portugal. Dis. Aquat. Org. 2003, 56, 171–179. [Google Scholar] [CrossRef]
- Hicks, B.D.; St Aubin, D.J.; Geraci, J.R.; Brown, W.R. Epidermal growth in the bottlenose dolphin, Tursiops truncatus. J. Investig. Dermatol. 1985, 85, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Segura-Göthlin, S.; Fernández, A.; Arbelo, M.; Felipe-Jiménez, I.; Colom-Rivero, A.; Almunia, J.; Sierra, E. The Validation of a Non-Invasive Skin Sampling Device for Detecting Cetacean Poxvirus. Animals 2021, 11, 2814. [Google Scholar] [CrossRef]
- Van Bressem, M.F.; van Waerebeek, K. Epidemiology of Poxvirus in Small Cetaceans from the Eastern South Pacific. Mar. Mammal. Sci. 1996, 12, 371–382. [Google Scholar] [CrossRef]
- Tomo, I.; Kemper, C.M. Strandings in St Vincent Gulf Bioregion, South Australia: 12-Year Study Monitors Biology and Pathology of Cetaceans. Oceans 2022, 3, 439–463. [Google Scholar] [CrossRef]
- Jiménez-Torres, C.; Verborgh, P.; de Stephanis, R.; Gauffier, P.; Esteban, R.; Giménez, J.; Van Bressem, M.-F. A visual health assessment of a resident community of bottlenose dolphins in the Strait of Gibraltar. In Proceedings of the 27th European Cetacean Society Conference, Setubal, Portugal, 8–10 April 2013. [Google Scholar]
- Hawkins, E.R.; Gustavsson, M.; Pogson-Manning, L.; Pheloung, H.; Jaehnichen, C. Prevalence of Skin Lesions and Injuries in Australian Humpback Dolphins (Sousa sahulensis) and Indo-Pacific Bottlenose Dolphins (Tursiops aduncus) in Moreton Bay, Queensland. Aquat. Mamm. 2022, 48, 297–313. [Google Scholar] [CrossRef]
- Chabanne, D.; Harrison, L.M.; Holyoake, C.; Finn, H.; Stephens, N.; Bejder, L. Final Report to the Swan River Trust for Project RSP10MUR Technical Report; Murdoch University Cetacean Research Unit: Perth, Australia, 2012; 95p, Available online: http://mucru.org/ (accessed on 10 November 2022).
- Burdett-Hart, L.; Rotstein, D.S.; Wells, R.S.; Allen, J.; Barleycorn, A.; Balmer, B.C.; Lane, S.M.; Speakman, T.; Zolman, E.S.; Stolen, M.; et al. Skin lesions on common bottlenose dolphins (Tursiops truncatus) from three sites in the Northwest Atlantic, USA. PLoS ONE 2012, 7, e33081. [Google Scholar]
- Bossart, G.D.; Schaefer, A.M.; McCulloch, S.; Goldstein, J.; Fair, P.A.; Reif, J.S. Mucocutaneous lesions in free-ranging Atlantic bottlenose dolphins Tursiops truncatus from the southeastern USA. Dis. Aquat. Organ. 2015, 115, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Kautek, G.; Van Bressem, M.-F.; Ritter, F. External Body Conditions in Cetaceans from La Gomera, Canary Islands, Spain. J. Mar. Anim. Ecol. 2018, 11, 4–17. [Google Scholar]
- Cocumelli, C.; Fichi, G.; Marsili, L.; Senese, M.; Cardeti, G.; Cersini, A.; Ricci, E.; Garibaldi, F.; Scholl, F.; Di Guardo, G.; et al. Cetacean Poxvirus in Two Striped Dolphins (Stenella coeruleoalba) Stranded on the Tyrrhenian Coast of Italy: Histopathological, Ultrastructural, Biomolecular, and Ecotoxicological Findings. Front. Veter. Sci. 2018, 5, 219. [Google Scholar] [CrossRef]
- Bearzi, M.; Rapoport, S.; Chau, J.; Saylan, C. Skin lesions and physical deformities of coastal and offshore common bottlenose dolphins (Tursiops truncatus) in Santa Monica Bay and adjacent areas, California. Ambio 2009, 38, 66–71. [Google Scholar] [CrossRef]
- Hamilton, P.K.; Marx, M.K. Skin lesions on North Atlantic right whales: Categories, prevalence and change in occurrence in the 1990s. Dis. Aquat. Org. 2005, 68, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Minton, G.; van Bressem, M.-F.; Willson, A.; Collins, T.; Al Harthi, S.; Sarrouf Willson, M.; Baldwin, R.; Leslie, M.S.; van Waerebeek, K. Visual health assessment and evaluation of anthropogenic threats to Arabian Sea humpback whales in Oman. J. Cetacean Res. Manag. 2022, 23, 59–79. [Google Scholar] [CrossRef]
- Powell, S.N.; Wallen, M.M.; Miketa, M.L.; Foroughirad, V.; Bansal, S.; Mann, J. Sociality and tattoo skin disease among bottlenose dolphins in Shark Bay, Australia. Behav. Ecol. 2020, 31, 459–466. [Google Scholar] [CrossRef]
- Cope, S.; Hines, E.; Bland, R.; Davis, J.D.; Tougher, B.; Zetterlind, V. Multi-sensor integration for an assessment of underwater radiated noise from common vessels in San Francisco Bay. The J. Acoust. Soc. Am. 2021, 149, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.; Chen, D.; Sun, J.; Greig, D.J.; Wu, Y. Characterization of brominated, chlorinated, and phosphate flame retardants in San Francisco Bay, an urban estuary. Sci. Total Environ. 2019, 652, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, S.M.; Cordaro, J.; Gulland, F.; Wheeler, E.; Dunkin, R.; Sigler, T.; Casper, D.; Berman, M.; Flannery, M.; Fire, S.; et al. An unusual mortality event of harbor porpoises (Phocoena phocoena) off central California: Increase in blunt trauma rather than an epizootic. Aquat. Mamm. 2012, 38, 301–310. [Google Scholar] [CrossRef]
- Wu, Y.; Tan, H.; Sutton, R.; Chen, D. From sediment to top predators: Broad exposure of polyhalogenated carbazoles in San Francisco Bay (USA). Environ. Sci. Technol. 2017, 51, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Jepson, P.D.; Bennett, P.M.; Deaville, R.; Allchin, C.R.; Baker, J.R.; Law, R.J. Relationships between polychlorinated biphenyls and health status in harbor porpoises (Phocoena phocoena) stranded in the United Kingdom. Environ. Toxicol. Chem. 2005, 24, 238–248. [Google Scholar] [CrossRef]
- Wells, R.S.; Tornero, V.; Borrell, A.; Aguilar, A.; Rowles, T.K.; Rhinehart, H.L.; Hofmann, S.; Jarman, W.M.; Hohn, A.A.; Sweeney, J.C. Integrating life-history and reproductive success data to examine potential relationships with organochlorine compounds for bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Sci. Total Environ. 2005, 349, 106–119. [Google Scholar] [CrossRef]
- Reif, J.S.; Peden-Adams, M.M.; Romano, T.A.; Rice, C.D.; Fair, P.A.; Bossart, G.D. Immune dysfunction in Atlantic bottlenose dolphins (Tursiops truncatus) with lobomycosis. Med. Mycol. 2009, 47, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Fair, P.A.; Schaefer, A.M.; Romano, T.; Bossart, G.D.; Lamb, S.V.; Reif, J.S. Stress response of wild bottlenose dolphins (Tursiops truncatus) during capture-release health assessment studies. Gen. Comp. Endocrinol. 2014, 206, 203–212. [Google Scholar] [CrossRef]
- Van Waerebeek, K.; van Bressem, M.-F.; Félix, F.; Alfaro, J.; García Godos, A.; Chavez, L.; Ontón, K.; Montes, D.; Bello, R. Mortality of dolphins and porpoises in coastal fisheries off Peru and southern Ecuador in 1994. Biol. Cons. 1997, 81, 43–49. [Google Scholar] [CrossRef]
- Van Bressem, M.-F.; van Waerebeek, K.; Duignan, P.J. Epidemiology of tattoo skin disease in captive common bottlenose dolphins (Tursiops truncatus): Are males more vulnerable than females? J. Appl. Anim. Welf. Sci. 2018, 21, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Croft, L.A.; Laughlin, R.; Manley, M.; Nollens, H.H. Water temperature fluctuations as a key driver of cetacean pox (tattoo) lesions in bottlenose dolphins Tursiops truncatus. Dis. Aquat. Organ. 2020, 139, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Shaul, N.J.; Dodder, N.G.; Aluwihare, L.I.; Mackintosh, S.A.; Maruya, K.A.; Chivers, S.J.; Danil, K.; Weller, D.W.; Hoh, E. Nontargeted biomonitoring of halogenated organic compounds in two ecotypes of bottlenose dolphins (Tursiops truncatus) from the Southern California Bight. Environ. Sci. Technol. 2015, 49, 1328–1338. [Google Scholar] [CrossRef] [Green Version]
- Mackintosh, S.A.; Dodder, N.G.; Shaul, N.J.; Aluwihare, L.I.; Maruya, K.A.; Chivers, S.J.; Danil, K.; Weller, D.W.; Hoh, E. Newly identified DDT-related compounds accumulating in Southern California bottlenose dolphins. Environ. Sci. Technol. 2016, 50, 12129–12137. [Google Scholar] [CrossRef]
- Cossaboon, J.M.; Hoh, E.; Chivers, S.J.; Weller, D.W.; Danil, K.; Maruya, K.A.; Dodder, N.G. Apex marine predators and ocean health: Proactive screening of halogenated organic contaminants reveals ecosystem indicator species. Chemosphere 2019, 221, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.B.; Maruya, K.A.; Dodder, N.G.; Lailson-Brito, J., Jr.; Azevedo, A.; Santos-Neto, E.; Torres, J.P.; Malm, O.; Hoh, E. Nontargeted screening of halogenated organic compounds in bottlenose dolphins (Tursiops truncatus) from Rio de Janeiro, Brazil. Environ. Sci. Technol. 2017, 51, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Vilela, R.; Huebner, M.; Vilela, C.; Vilela, G.; Pettersen, B.; Oliveira, C.; Mendoza, L. The taxonomy of two uncultivated fungal mammalian pathogens is revealed through phylogeny and population genetic analyses. Sci. Rep. 2021, 11, 18119. [Google Scholar] [CrossRef]
- Hall, A.J.; Hugunin, K.; Deaville, R.; Law, R.J.; Allchin, C.R.; Jepson, P. The risk of infection from polychlorinated biphenyl exposure in the harbor porpoise (Phocoena phocoena): A case-control approach. Environ. Health Perspect. 2006, 114, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Van Bressem, M.F.; Minton, G.; Collins, T.; Willson, A.; Baldwin, R.; Van Waerebeek, K. Tattoo-like skin disease in the endangered subpopulation of the Humpback Whale, Megaptera novaeangliae, in Oman (Cetacea: Balaenopteridae). Zool. Middle East 2014, 61, 1–8. [Google Scholar] [CrossRef]
- Pomilla, C.; Amaral, A.R.; Collins, T.; Minton, G.; Findlay, K.; Leslie, M.S.; Ponnampalam, L.; Baldwin, R.; Rosenbaum, R. The World’s Most Isolated and Distinct Whale Population? Humpback Whales of the Arabian Sea. PLoS ONE 2014, 9, e114162. [Google Scholar] [CrossRef] [Green Version]
- Dakhteh, S.M.H.; Ranjbar, S.; Moazeni, M.; Mohsenian, N.; Delshab, H.; Moshiri, H.; Nabavi, S.M.B.; Van Waerebeek, K. The Persian Gulf is part of the habitual range of the Arabian Sea Humpback whale population. J. Mar. Biol Oceanogr. 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Braun, B.A.; Marcovitz, A.; Camp, J.G.; Jia, R.; Bejerano, G. Mx1 and Mx2 key antiviral proteins are surprisingly lost in toothed whales. Proc. Natl. Acad. Sci. USA 2015, 112, 8036–8040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, O.; Jung, Y.E.; Lee, K.W.; An, Y.J.; Kim, J.; Roh, Y.R.; Bhak, J.; Park, K.; Weber, J.A.; Cheong, J.; et al. The Analyses of Cetacean Virus-Responsive Genes Reveal Evolutionary Marks in Mucosal Immunity-Associated Genes. Biochem. Genet. 2022, 60, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Kotwal, G.J. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 2002, 28, 149–185. [Google Scholar] [CrossRef]
- Baxby, D. Poxviruses in Principles and Practice of Clinical Virology, 2nd ed.; Zuckerman, A.J., Banatvala, J.E., Pattison, J.R., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 1990; pp. 411–434. [Google Scholar]
- Panchanathan, V.; Chaudhri, G.; Karupiah, G. Correlates of protective immunity in poxvirus infection: Where does antibody stand? Immunol. Cell Biol. 2008, 6, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Batley, K.C.; Sandoval-Castillo, J.; Kemper, C.M.; Zanardo, N.; Tomo, I.; Beheregaray, L.B.; Möller, L.M. Whole genomes reveal multiple candidate genes and pathways involved in the immune response of dolphins to a highly infectious virus. Mol. Ecol. 2021, 30, 6434–6448. [Google Scholar] [CrossRef]

| Scientific Name | Sample Type | TSD Prev (%) | Total Number | Ocean Province | Mean SST | Ref. |
|---|---|---|---|---|---|---|
| Delphinus delphis | BC | 3.6 | 28 | SE Pacific (Ecuador, central coast) | 23.8°C | [6] |
| Tursiops aduncus | S | 3.6 | 112 | Indian Ocean (Australia, St Vincent Gulf) | 17.7°C | [23] |
| Tursiops truncatus | FR | 4.5 | 334 | Gibraltar Strait (Spain) | 19.1°C | [24] |
| Tursiops truncatus | FR | 5 | 79 | SE Pacific (Peru, Paracas Bay) | 18.1°C | [6] |
| Sotalia guianensis | FR | 5.3 | 206 | SW Atlantic (Brazil, Sepetiba Bay) | 23.8°C | [6] |
| Delphinus delphis | BC | 5.6 | 18 | NE Atlantic (British Isles) | 11.8°C | [6] |
| Sousa sahulensis | FR | 9.9 | 91 | SE Pacific (Moreton Bay, Australia) | 23.1°C | [25] |
| Stenella coeruleoalba | S | 7.5 | 40 | Mediterranean Sea (Spain, Valencian Community) | 19.3°C | [6] |
| Tursiops aduncus | FR | 7 | 100 | SE Pacific (Moreton Bay, Australia) | 23.1°C | [25] |
| Phocoena phocoena | BC | 10 | 10 | North Sea (British Isles) | 10.8°C | [6] |
| Phocoena phocoena | S | 10.9 | 46 | NE Atlantic (British Isles) | 11.8°C | [6] |
| Cephalorhynchus h.hectori | S | 11.1 | 27 | SW Pacific & Tasman Sea (New Zealand, South Island) | 13.8°C | [6] |
| Delphinus delphis | S | 11.1 | 9 | NE Atlantic (British Isles) | 11.8°C | [6] |
| Tursiops truncatus | S | 12.5 | 8 | Mediterranean Sea (Spain, Valencian Community) | 19.3°C | [6] |
| Phocoena phocoena | S | 13.8 | 29 | North Sea (British Isles) | 10.8°C | [6] |
| Tursiops aduncus | FR | 13.9 | 36 | Indian Ocean (Australia, Swan-Canning Riverpark) | 20.8°C | [26] |
| Delphinus delphis | S | 16.7 | 6 | NE Pacific (USA, northern California) | 12.4°C | [This paper] |
| Cephalorhynchus eutropia | FR | 17.4 | 23 | SE Pacific (Chile, Guaitecas Archipelago, Aysén Region) | 12.1°C | [6] |
| Tursiops aduncus | FR | 19.4 | 247 | Indian Ocean (Australia, Shark Bay) | 22.5°C | [16] |
| Tursiops truncatus | FR | 19.9 | 171 | NW Atlantic (USA, South Carolina) | 21.1°C | [27] |
| Tursiops truncatus | FR | 20 | 35 | NE Atlantic (Portugal, Sado Estuary) | 17°C | [19] |
| Lagenorhynchus obliquidens | S | 20 | 5 | NE Pacific (USA, northern California) | 12.4°C | [This paper] |
| Tursiops truncatus | FR | 21 | 189 | NW Atlantic (USA, Georgia) | 20.7°C | [27] |
| Cephalorhynchus h. hectori | BC | 24.3 | 37 | SW Pacific & Tasman Sea (New Zealand, South Island) | 13.3°C | [6] |
| Lagenorhynchus australis | FR | 27.6 | 29 | SE Pacific (Chile, Guaitecas Archipelago, Aysén Region) | 12.1°C | [6] |
| Tursiops truncatus | FR | 27.9 | 43 | NW Atlantic (USA, FL, Indian River Lagoon) | 25.8°C | [28] |
| Grampus griseus | S | 33.3 | 3 | NE Pacific (USA, northern California) | 12.4°C | [This paper] |
| Lagenorhynchus obscurus | BC | 34.7 | 196 | SE Pacific (Peru, central coast) | 18°C | [22] |
| Lagenorhynchus australis | FR | 39.1 | 115 | SE Pacific (Chile, Añihué Reserve, Aysén Region) | 12.1°C | [3] |
| Stenella coeruleoalba | S | 40 | 5 | NE Pacific (USA, northern California) | 12.4°C | [This paper] |
| Tursiops truncatus | BC | 41.6 | 12 | SE Pacific (Peru, central coast) | 18°C | [22] |
| Tursiops truncatus | FR | 42.6 | 101 | NW Atlantic (USA, FL, Sarasota Bay) | 24.4°C | [27] |
| Phocoena phocoena | S | 43.6 | 55 | NE Pacific (USA, northern California) | 12.4°C | [This paper] |
| Delphinus capensis | BC | 61.1 | 54 | SE Pacific (Peru, central coast) | 18°C | [22] |
| Phocoena spinipinnis | BC | 62.3 | 77 | SE Pacific (Peru, central coast) | 18°C | [22] |
| Stenella frontalis | FR | (3) | NA | Central east Atlantic (La Gomera, Canary Islands) | 21.4°C | [29] |
| Stenella frontalis | S | (1) | NA | Central east Atlantic (Teneriffe, Canary Islands) | 21.1°C | [21] |
| Tursiops truncatus | FR | (2) | NA | Central east Atlantic (La Gomera, Canary Islands) | 21.4°C | [29] |
| Tursiops truncatus | S | (1) | NA | Central east Atlantic (Teneriffe, Canary Islands) | 21.1°C | [21] |
| Sotalia guianensis | FR & S | (5) | NA | SW Atlantic (Brazil, Guanabara Bay) | 23.7°C | [9] |
| Hyperoodon ampullatus | S | (1) | NA | North Sea (British Isles) | 10.8°C | [6] |
| Steno bredanensis | FR | (2) | NA | Central east Atlantic (La Gomera, Canary Islands) | 21.4°C | [29] |
| Delphinus delphis | FR | (1) | NA | Central east Atlantic (La Gomera, Canary Islands) | 21.4°C | [29] |
| Stenella coeruleoalba | S | (2) | NA | Mediterranean Sea (Italy, Anzio and Tuscany) | 19.2°C | [30] |
| Tursiops truncatus | FR | (1) | NA | NE Pacific (Santa Monica, California) | 16.6°C | [31] |
| Balaena mysticetus | H | (1) | NA | Beaufort Sea, Arctic (Kaktovik, Alaska) | 0°C | [11] |
| Eubalaena australis | S | (2) | NA | SE Atlantic (Chubut, Argentina) | 13.3°C | [14] |
| Eubalaena glacialis | FR | (1) | NA | NE Atlantic (New England, USA) | 10.7°C | [32] |
| Megaptera novaeangliae | FR | 41 | 93 | Arabian Sea (Oman) | 27.3°C | [33] |
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Nt | N pos | Prev % | Nt | N pos | Prev % | |
| Phocoena phocoena | ||||||
| calf | 8 | 0 | 0 | 7 | 1 | 14.3 |
| juvenile | 3 | 1 | 33.3 | 11 | 9 | 81.8 |
| subadult | 5 | 3 | 60.0 | 11 | 8 | 72.7 |
| adult | 8 | 2 | 25.0 | 2 | 0 | 0 |
| Total | 24 | 6 | 25.0 | 31 | 18 | 58.1 |
| Delphinidae | ||||||
| calf | 3 | 1 | 33.3 | 1 | 0 | 0 |
| juvenile | 2 | 1 | 50.0 | 5 | 1 | 20.0 |
| subadult | 0 | 0 | - | 3 | 2 | 66.7 |
| adult | 2 | 0 | 0 | 3 | 0 | 0 |
| Total | 7 | 2 | 28.6 | 12 | 3 | 25.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Bressem, M.-F.; Van Waerebeek, K.; Duignan, P.J. Tattoo Skin Disease in Cetacea: A Review, with New Cases for the Northeast Pacific. Animals 2022, 12, 3581. https://doi.org/10.3390/ani12243581
Van Bressem M-F, Van Waerebeek K, Duignan PJ. Tattoo Skin Disease in Cetacea: A Review, with New Cases for the Northeast Pacific. Animals. 2022; 12(24):3581. https://doi.org/10.3390/ani12243581
Chicago/Turabian StyleVan Bressem, Marie-Françoise, Koen Van Waerebeek, and Pádraig J. Duignan. 2022. "Tattoo Skin Disease in Cetacea: A Review, with New Cases for the Northeast Pacific" Animals 12, no. 24: 3581. https://doi.org/10.3390/ani12243581
APA StyleVan Bressem, M.-F., Van Waerebeek, K., & Duignan, P. J. (2022). Tattoo Skin Disease in Cetacea: A Review, with New Cases for the Northeast Pacific. Animals, 12(24), 3581. https://doi.org/10.3390/ani12243581




