Effects of Aerobic Exercise Training on the Growth, Swimming Performance, Antipredation Ability and Immune Parameters of Juvenile Rock Carp (Procypris rabaudi)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Aerobic Exercise Training Protocol
2.3. Parameter Measurements
2.3.1. Measurement of Growth Rate
2.3.2. Measurement of Ucat
2.3.3. Measurements of Ucrit and the Metabolic Rate
2.3.4. Measurement of the Immune Parameters
2.3.5. Measurement of Antipredation Ability
2.4. Data Analysis
3. Results
3.1. Growth Performance
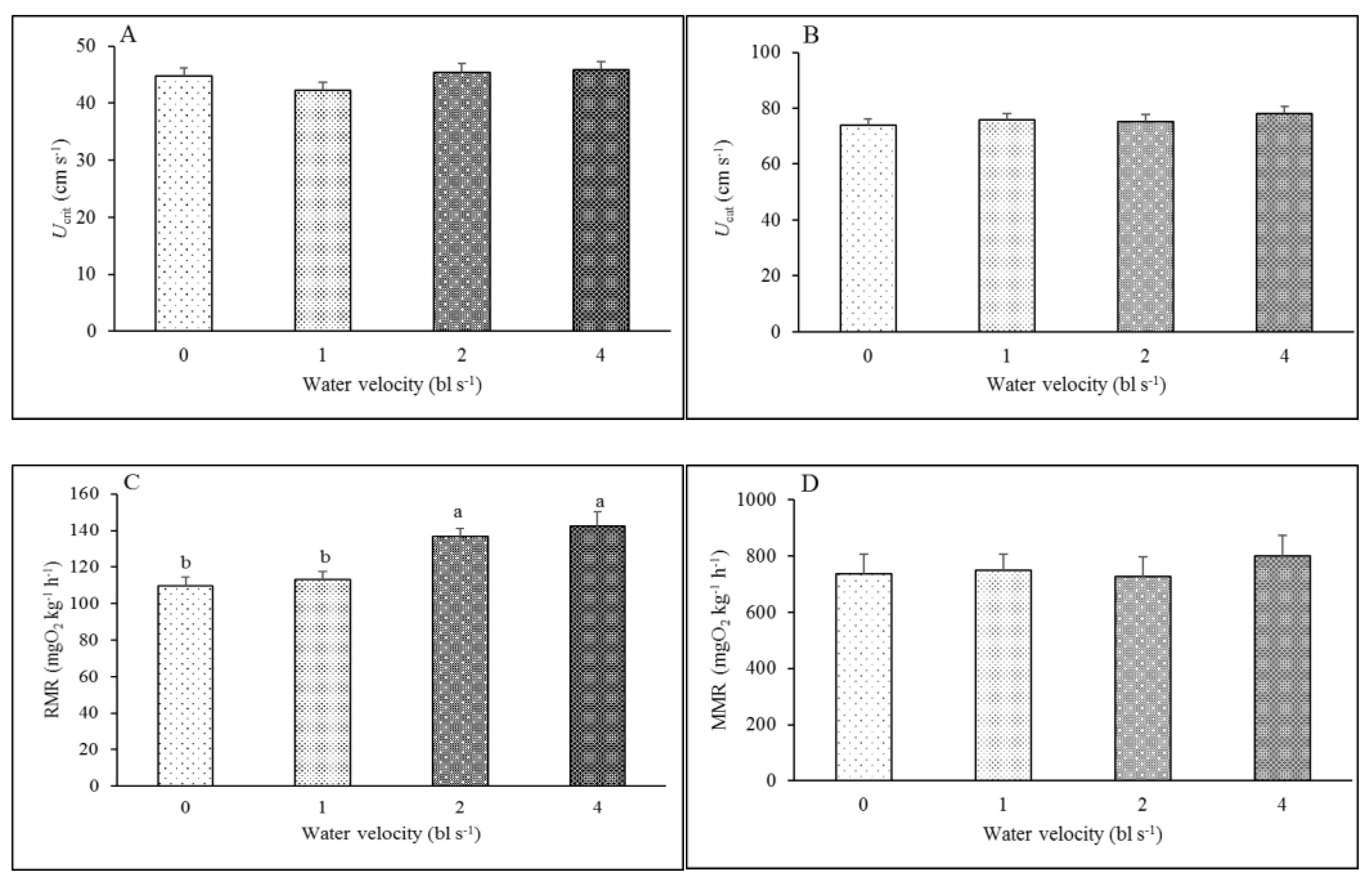
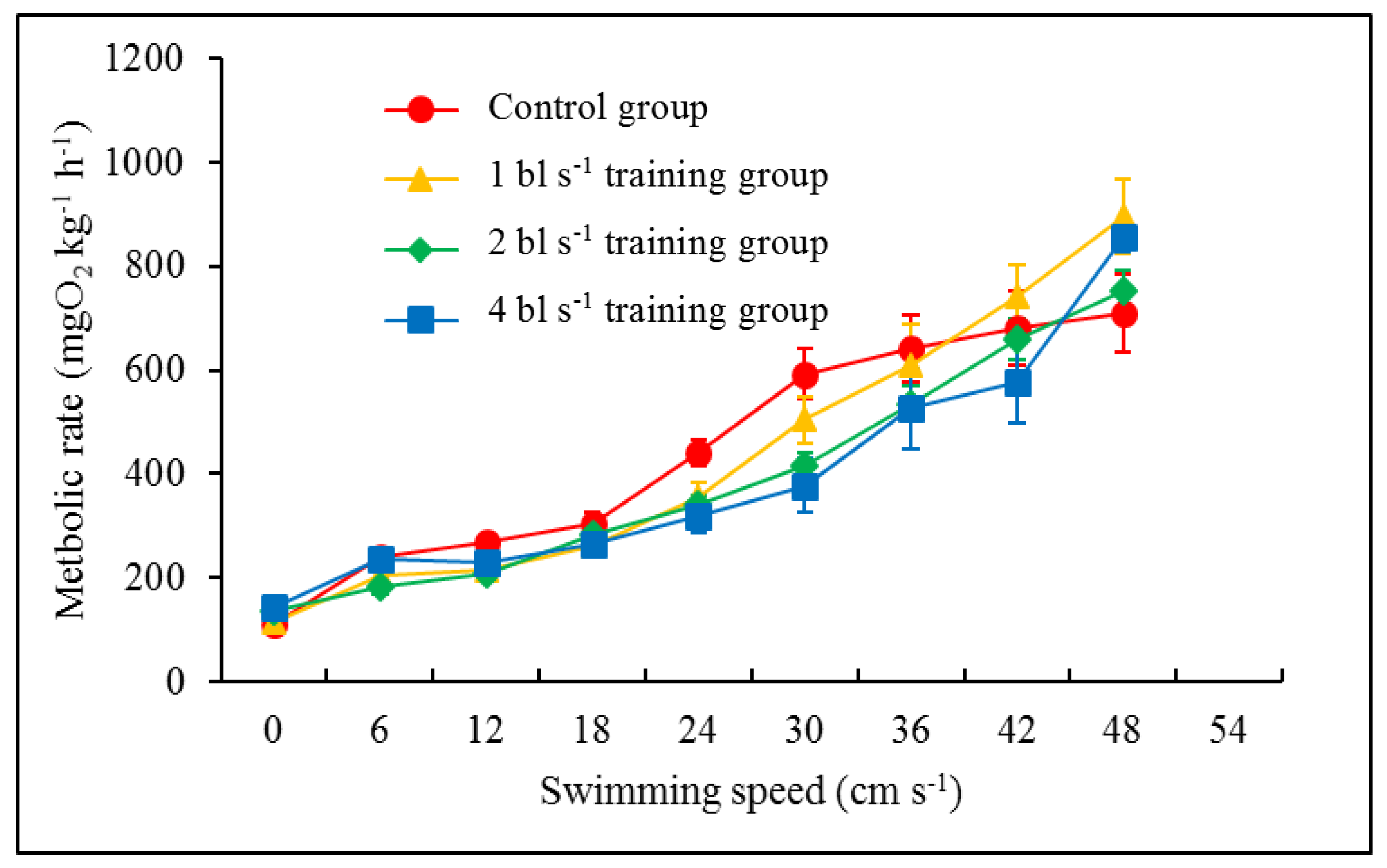
3.2. Swimming Ability and Metabolic Rate
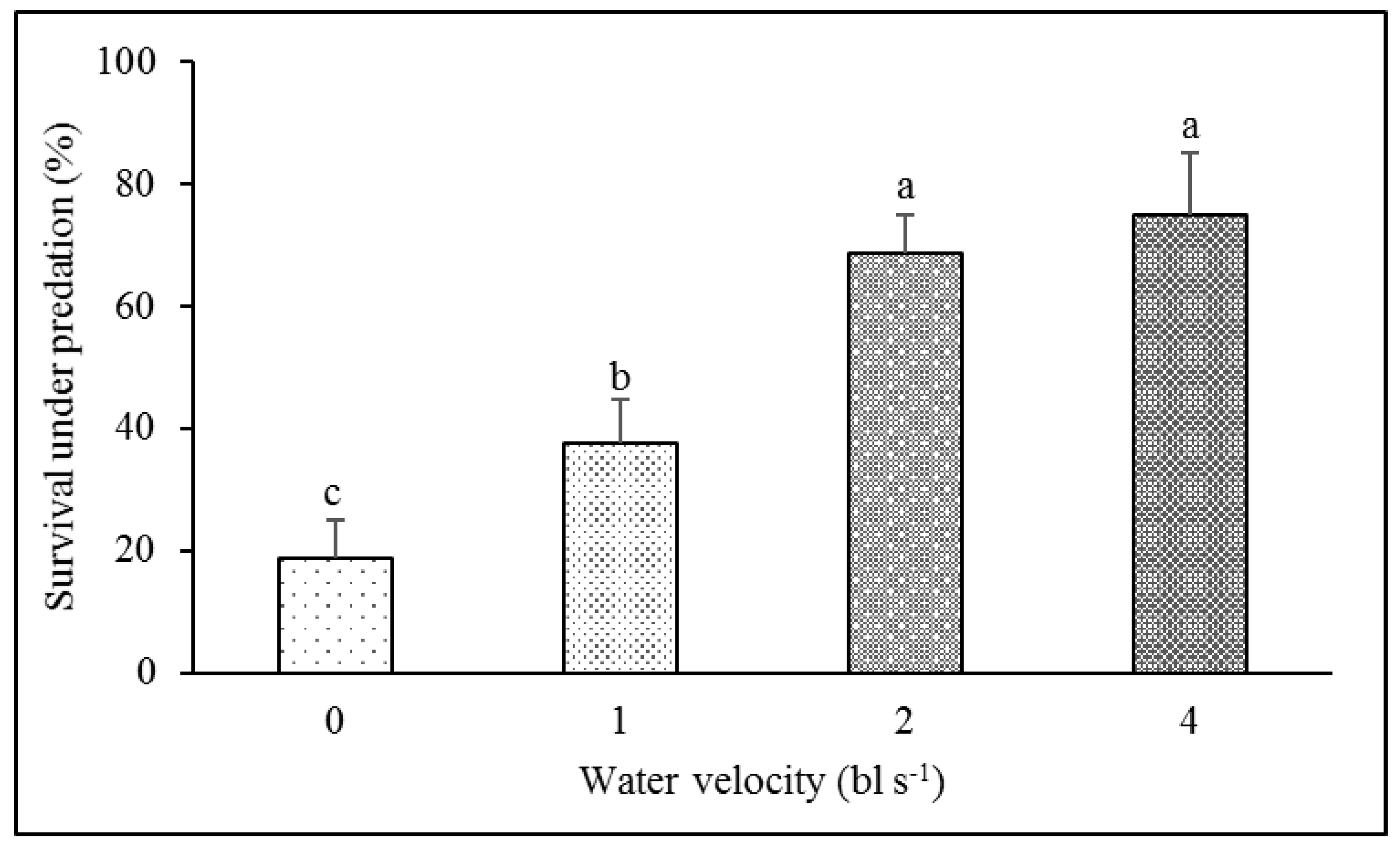
3.3. Antipredation Ability
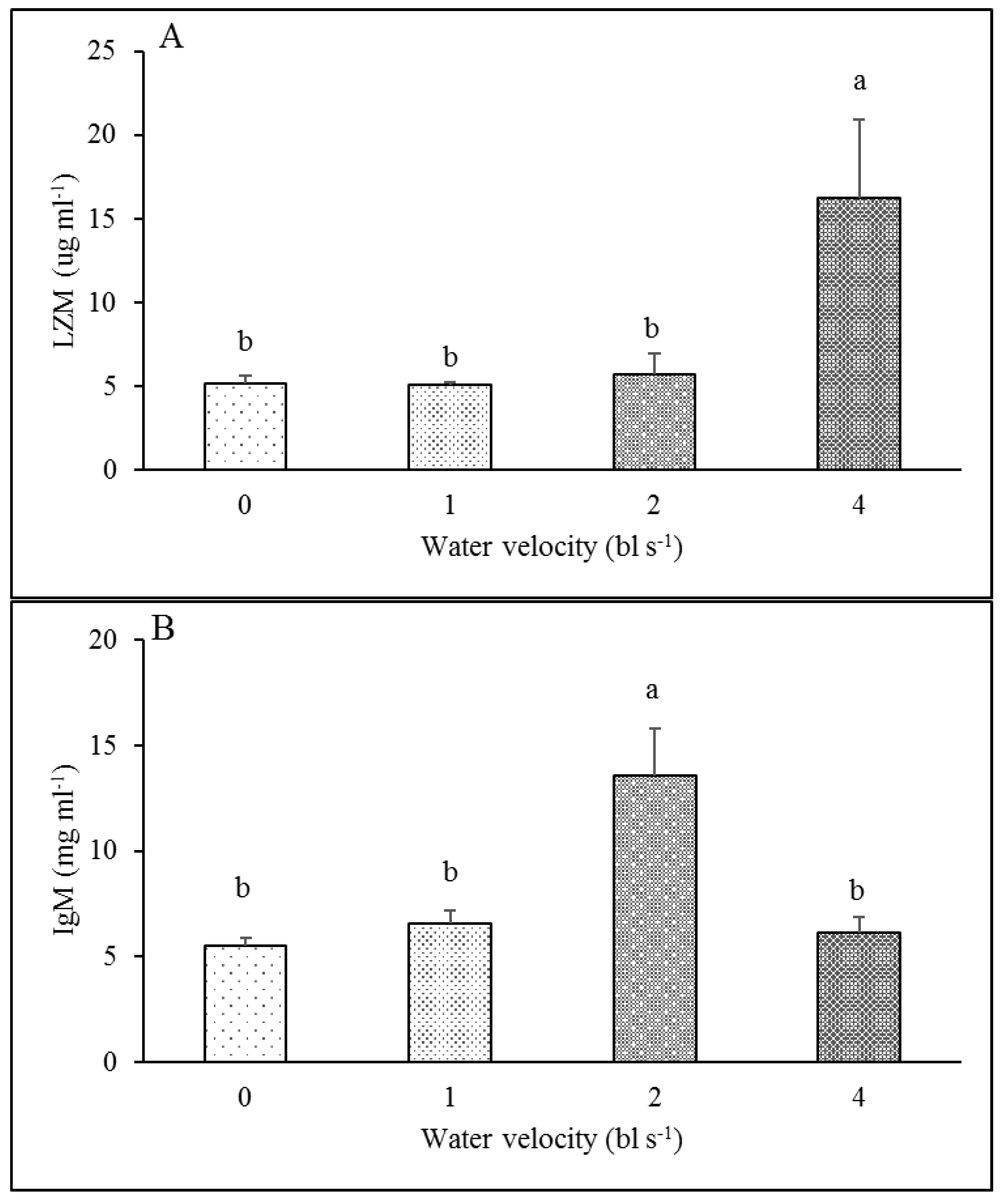
3.4. Immune Parameters
4. Discussion
4.1. Effect of Aerobic Exercise Training on the Growth of Juvenile Rock Carp
4.2. Effect of Aerobic Training on Swimming Performance
4.3. Effect of Aerobic Exercise Training on Antipredation Ability
4.4. Effect of Exercise Training on Immune Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaldúa, N.; Naya, D.E. Digestive flexibility during fasting in fish: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 169, 7–14. [Google Scholar] [CrossRef]
- Lee, C.G.; Farrell, A.P.; Lotto, A.; MacNutt, M.J.; Hinch, S.G.; Healey, M.C. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J. Exp. Biol. 2003, 206, 3239–3251. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.-J.; Cao, Z.; Yan, G.; Fu, C.; Pang, X. Integrating environmental variation, predation pressure, phenotypic plasticity and locomotor performance. Oecologia 2013, 173, 343–354. [Google Scholar] [CrossRef]
- Song, B.L.; Lin, X.T.; Xu, Z.N. Effects of upstream exercise training on feeding efficiency, growth and nutritional components of juvenile tinfoil barbs (Barbodes schwanenfeldi). J. Fish. China 2012, 36, 106–114. [Google Scholar] [CrossRef]
- Li, X.M.; Yu, L.J.; Wang, C.; Zeng, L.Q.; Cao, Z.D.; Fu, S.J.; Zhang, Y.G. The effect of aerobic exercise training on growth performance, digestive enzymes activities and postprandial metabolic response in juvenile qingbo (Spinibarbus sinensis). Comp. Biochem. Physiol. A Physiol. 2013, 166, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Pang, X.; Zheng, H.; Li, X.J.; Fu, S.J.; Zhang, Y.G. Effects of prolonged exercise training and exhaustive chasing training on the swimming performance of an endangered bream Megalobrama pellegrini. Aquat. Biol. 2017, 26, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Davison, W. The effects of exercise training on teleost fish, a review of recent literature. Comp. Biochem. Physiol. A Physiol. 1997, 117, 67–75. [Google Scholar] [CrossRef]
- Pearson, M.P.; Spriet, L.L.; Stevens, E.D. Effect of sprint training on swim performance and white muscle metabolism during exercise and recovery in rainbow trout (Salmo gairdneri). J. Exp. Biol. 1990, 149, 45–60. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Yan, X.Y.; Li, X.M.; Fu, X.; Xia, J.G.; Fu, S.J. Effect of exercise training on swimming performance, survival under predation and hypoxia tolerance in an endangered fish species in China. Mar. Freshw. Behav. Physiol. 2019, 52, 67–82. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, H.; Deng, L.J.; Li, T.C.; Yang, K.; Fu, S.J.; Song, Z.B. Improved aerobic and anaerobic swimming performance after exercise training and detraining in schizothorax wangchiachii: Implications for fisheries releases. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 245, 110698. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Yuan, X.Z.; Cao, Z.D.; Fu, S.J. The effects of temperature and exercise training on swimming performance in juvenile qingbo (Spinibarbus sinensis). J. Comp. Physiol. B 2012, 183, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, L.; Yuan, J.; Xiao, Y.; Fu, S.; Zhang, Y. The effect of aerobic exercise and starvation on growth performance and postprandial metabolic response in juvenile southern catfish (Silurus meridionalis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 193, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, G.; Urrutia, V. Growth in response to sustained swimming in young montezumae swordtails, Xiphophorus montezumae. Mar. Freshw. Behav. Physiol. 2008, 41, 65–72. [Google Scholar] [CrossRef]
- Arbeláez-Rojas, G.A.; Moraes, G. Optimization of sustaining swimming speed of matrinxã Brycon amazonicus: Performance and adaptive aspects. Sci. Agric. 2010, 67, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Plaut, I. Does pregnancy affect swimming performance of female Mosquitofish, Gambusia affinis? Funct. Ecol. 2010, 16, 290–295. [Google Scholar] [CrossRef]
- Marras, S.; Claireaux, G.; Mckenzie, D.J.; Nelson, J.A. Individual variation and repeatability in aerobic and anaerobic swimming performance of European sea bass, Dicentrarchus labrax. J. Exp. Biol. 2010, 213, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatry, C.; Thiem, J.D.; Binder, T.R.; Hatin, D.; Dumont, P.; Stamplecoskie, K.M.; Molina, J.M.; Smokorowski, K.E.; Cooke, S.J. Comparative physiology and relative swimming performance of three redhorse (moxostoma spp.) species: Associations with fishway passage success. Physiol. Biochem. Zool. 2014, 87, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Plaut, I. Critical swimming speed: Its ecological relevance. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef]
- Reidy, S.P.; Kerr, S.R.; Nelson, J.A. Aerobic and anaerobic swimming performance of individual Atlantic cod. J. Exp. Biol. 2000, 203, 347–357. [Google Scholar] [CrossRef]
- Oufiero, C.E.; Garland, T. Repeatability and correlation of swimming performances and size over varying time-scales in the guppy (Poecilia reticulata). Funct. Ecol. 2009, 23, 969–978. [Google Scholar] [CrossRef]
- He, W.; Xia, W.; Cao, Z.D.; Fu, S.J. The effect of prolonged exercise training on swimming performance and the underlying biochemical mechanisms in juvenile common carp (Cyprinus carpio). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 308–315. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.D.; Fu, S.J.; Peng, J.L.; Wang, Y.X. The effect of exhaustive chasing training and detraining on swimming performance in juvenile darkbarbel catfish (Peltebagrus vachelli). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2009, 179, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.J. Flow and stress acclimation both enhance predator avoidance in common cyprinid fish. Aquat. Biol. 2015, 24, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wu, Z.H. Advances in fish humoral immunity. Mar. Sci. 2001, 25, 20–22. [Google Scholar]
- Song, B.L. Effect of Water Current on Swimming Activity, Growth and Ecophysiological Aspect of Young Barbodes Schwanenfeldi; Jinan University: Guangzhou, China, 2008. [Google Scholar]
- Yu, L.J. The Effects of Exercise Training on Growth, Antioxidative Ablility and Immune Functionin Juvenile Spinibarbus Sinensis; Southwest University: Chongqing, China, 2014. [Google Scholar]
- Dong, P. Swimming Activities of Puffer Chrysanthemum and Its Effects on Respiratory Excretion and Nonspecific Immunity; Dalian Ocean University: Dalian, China, 2017. [Google Scholar]
- Castro, V.; Grisdale-Helland, B.; Helland, S.J.; Kristensen, T.; Jørgensen, S.M.J.; Helgerud, J.; Claireaux, G.; Farrell, A.P.; Krasnov, A.; Takle, H. Aerobic training stimulates growth and promotes disease resistance in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. A Physiol. 2011, 160, 278–290. [Google Scholar] [CrossRef]
- Azuma, T.; Noda, S.; Yada, T.; Ototake, M.; Nagoya, H.; Moriyama, S.; Yamada, H.; Nakanishi, T.; Lwata, M. Profiles in growth, smoltification, immune function and swimming performance of 1-year-old masu salmon Oncorhynchus masou masou reared in water flow. Fish. Sci. 2002, 68, 1282–1294. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Y.; Qin, X.; Shi, X.; Wang, X. The effect of aerobic exercise training on growth performance, innate immune response and disease resistance in juvenile Schizothorax prenanti. Aquaculture 2018, 486, 18–25. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, B.; Lin, X.; Xu, Z. Effects of water-current speed on hematological, biochemical and immune parameters in juvenile tinfoil barb, Barbonymus schwanenfeldii (Bleeker, 1854). J. Oceanol. Limnol. 2016, 34, 118–124. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Cai, Y.Q.; He, C.R.; Jiang, J. Preliminary studies on the biological characteristics of Procypris rabaudi (Tchang). J. Hydroecology 2003, 23, 17–21. [Google Scholar]
- Wang, S.; Le, P.Q.; Chen, Y.Y. Red Book of Endangered Animals in China; Science Press: Beijing, China, 1998; pp. 170–172. [Google Scholar]
- Yan, G.J.; He, X.K.; Cao, Z.D.; Fu, S.J. An interspecific comparison between morphology and swimming performance in cyprinids. J. Evol. Biol. 2013, 26, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Yu, L.J.; Cao, Z.D.; Fu, S.J.; Zhang, Y.G. The effects of exhaustive chasing training on the growth performance and postprandial metabolic response in Spinibarbus sinensis and Procypris rabaudi. Freshw. Fish. 2013, 43, 63–68. [Google Scholar]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Fuda, H.; Hara, A.; Kawamura, H.; Yamauchi, K. Changes in serum immunoglobulin M (IgM) concentrations during early development of chum salmon (Oncorhynchus keta) as determined by sensitive ELISA technique. Comp. Biochem. Physiol. A Physiol. 1993, 106, 69–74. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Bryant, J.; Stevens, E.D. Effect of a sprint training protocol on growth rate, conversion efficiency, food consumption and body composition of rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1988, 33, 861–870. [Google Scholar] [CrossRef]
- Hernandez, M.D.; Mendiola, P.; de Costa, J.D.; Zamora, S. Effects of intense exercise training on rainbow trout growth, body composition and metabolic responses. J. Physiol. Biochem. 2002, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yogata, H.Y.; Oku, H. The effects of swimming exercise on growth and whole-body protein and fat contents of fed and unfed fingerling yellowtail. Fish. Sci. 2000, 66, 1100–1105. [Google Scholar] [CrossRef] [Green Version]
- Yogata, H.Y.; Oku, H. Effects of water velocity on growth performance of juvenile Japanese flounder Paralichthys olivaceus. J. World Aquac. Soc. 2000, 31, 225–231. [Google Scholar] [CrossRef]
- Ghanawi, J.; Mohanna, C.; Saoud, I.P. Effect of continuous water movement on growth and body composition of juvenile Rabbitfish, Siganus rivulatus. J. World Aquac. Soc. 2010, 41, 834–839. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Jobling, M. The behavior and the relationship between food intake and growth of juvenile Arctic charr, Salvelinus alpinus, L., subjected to sustained exercise. Can. J. Zool. 1990, 68, 2185–2191. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Li, X.; Zheng, H.; Peng, J.; Fu, S. Sustained exercise-trained juvenile black carp (mylopharyngodon piceus) at a moderate water velocity exhibit improved aerobic swimming performance and increased postprandial metabolic responses. Biol. Open 2018, 7, bio032425. [Google Scholar] [CrossRef] [Green Version]
- Gruber, S.J.; Dickson, K.A. Effects of endurance training in the leopard shark, Triakis semifasciata. Physiol. Zool. 1997, 70, 481–492. [Google Scholar] [CrossRef]
- Gallaugher, P.E.; Thorarensen, H.; Kiessling, A.; Farrell, A.P. Effects of high intensity exercise training on cardiovascular function, oxygen uptake, internal oxygen transport and osmotic balance in chinook salmon (Oncorhynchus tshawytscha) during critical speed swimming. J. Exp. Biol. 2001, 204, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Cao, Z.D.; Peng, J.L.; Fu, S.J. The effect of exercise training on the metabolic interaction between digestion and locomotion in juvenile darkbarbel catfish (Peltebagrus vachelli). Comp. Biochem. Physiol. A Physiol. 2010, 156, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Young, P.S.; Cech, J.J. Effects of exercise conditioning on stress responses and recovery in cultured and wild young-of-the-year striped bass, Morone saxatilis. Can. J. Fish. Aquat. Sci. 1993, 50, 2094–2099. [Google Scholar] [CrossRef]
- Pakkasmaa, S.; Piironen, J. Water Velocity Shapes Juvenile Salmonids. Evol. Ecol. 2001, 14, 721–730. [Google Scholar] [CrossRef]
- Burrows, R.E. The influence of fingerling quality on adult salmon survivals. Trans. Am. Fish. Soc. 1969, 98, 777–784. [Google Scholar] [CrossRef]
- Cresswell, R.C.; Williams, R. Post-stocking movements and recapture of hatchely-reared trout released into flowing waters-effect of prior acclimation to flow. J. Fish Biol. 1983, 23, 265–276. [Google Scholar] [CrossRef]
- Wang, M.; Yi, M.M.; Lu, M.X. Review on the fish health assessment. Acta Hydrobiol. Sin. 2019, 43, 226–232. [Google Scholar]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Arnold, J.N.; Dwek, R.A.; Rudd, P.M.; Sim, R.B. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol. Lett. 2006, 106, 103–110. [Google Scholar] [CrossRef]
- Leya, T.; Ahmad, I.; Valappil, R.K.; Kurcheti, P.P.; Tripathi, G.; Sharma, R.; Bedekar, M.K. Development of species-specific IgM antibodies and elevation of mucosal immune response in Labeo rohita using recombinant bicistronic nano DNA vaccine priming. Fish Shellfish Immunol. 2021, 113, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, L.Q.; Fu, S.J.; Peng, J.L.; Cao, Z.D. Intraspecific differences in standard metabolic rate and its correlations with locomotion and feeding metabolism in juvenile common carp (Cyprinus carpio). Chin. J. Zool. 2016, 51, 384–394. [Google Scholar]


| Parameters | Water Velocity | ||||
|---|---|---|---|---|---|
| Control | 1 bl s−1 | 2 bl s−1 | 4 bl s−1 | ||
| Ucrit | |||||
| Body mass (g) | n = 9 | 4.47 ± 0.15 | 4.25 ± 0.18 | 4.07 ± 0.14 | 4.22 ± 0.28 |
| Body length (cm) | n = 9 | 6.66 ± 0.09 | 6.67 ± 0.12 | 6.43 ± 0.11 | 6.48 ± 0.09 |
| Ucat | |||||
| Body mass (g) | n = 9 | 4.45 ± 0.10 | 4.54 ± 0.26 | 4.05 ± 0.16 | 4.57 ± 0.23 |
| Body length (cm) | n = 9 | 6.62 ± 0.07 | 6.53 ± 0.11 | 6.53 ± 0.08 | 6.72 ± 0.08 |
| Survival rate | |||||
| Body mass (g) | n = 4 | 4.38 ± 0.16 | 3.77 ± 0.17 | 3.69 ± 0.17 | 3.15 ± 0.09 |
| Body length (cm) | n = 4 | 6.44 ± 0.08 | 6.26 ± 0.08 | 6.19 ± 0.08 | 6.01 ± 0.14 |
| LZM | |||||
| Body mass (g) | n = 6 | 4.13 ± 0.17 | 4.84 ± 0.18 | 4.40 ± 0.12 | 3.99 ± 0.29 |
| Body length (cm) | n = 6 | 6.45 ± 0.13 | 6.71 ± 0.09 | 6.31 ± 0.07 | 6.15 ± 0.18 |
| IgM | |||||
| Body mass (g) | n = 6 | 4.40 ± 0.32 | 5.07 ± 0.39 | 4.18 ± 0.36 | 3.75 ± 0.19 |
| Body length (cm) | n = 6 | 6.51 ± 0.13 | 6.70 ± 0.19 | 6.37 ± 0.18 | 6.18 ± 0.09 |
| Spleen | |||||
| Body mass (g) | n = 6 | 4.23 ± 0.15 | 4.08 ± 0.28 | 4.52 ± 0.06 | 4.92 ± 0.54 |
| Body length (cm) | n = 6 | 6.58 ± 0.09 | 6.48 ± 0.13 | 6.60 ± 0.07 | 6.90 ± 0.09 |
| Parameters | Training | Results of One-Way ANOVA | |||
|---|---|---|---|---|---|
| Control Group | 1 bl s−1 Training Group | 2 bl s−1 Training Group | 4 bl s−1 Training Group | Training Effect | |
| Initial body weight (g) | 4.12 ± 0.23 | 4.04 ± 0.32 | 3.83 ± 0.20 | 3.82 ± 0.27 | F3,11 = 0.068; p = 0.396 |
| Initial body length (cm) | 6.36 ± 0.09 | 6.29 ± 0.11 | 6.32 ± 0.07 | 6.23 ± 0.09 | F3,11 = 0.368; p = 0.788 |
| Final body weight (g) | 4.38 ± 0.04 | 4.31 ± 0.28 | 4.08 ± 0.19 | 4.20 ± 0.11 | F3,11 = 0.518; p = 0.681 |
| Final body length (cm) | 6.53 ± 0.06 | 6.52 ± 0.13 | 6.36 ± 0.08 | 6.41 ± 0.09 | F3,11 = 0.022; p = 0.800 |
| Length-specific growth rate (% d−1) | 0.08 ± 0.02 | 0.09 ± 0.04 | 0.05 ± 0.03 | 0.08 ± 0.05 | F3,11 = 0.362; p = 0.782 |
| Weight-specific growth rate (% d−1) | 0.18 ± 0.13 | 0.19 ± 0.08 | 0.18 ± 0.08 | 0.28 ± 0.18 | F3,11 = 0.179; p = 0.907 |
| Parameters | Covariate Effect | Training Effect |
|---|---|---|
| Critical swimming speed (Ucrit) | F1,35 = 13.745, p = 0.001 | F3,35 = 2.127, p = 0.117 |
| Constant acceleration speed (Ucat) | F1,35 = 1.150, p = 0.292 | F3,35 = 0.445, p = 0.723 |
| Resting metabolic rate (RMR) | F1,35 = 0.031, p = 0.860 | F3,35 = 8.330, p < 0.001 |
| Maximum metabolic rate (MMR) | F1,35 = 9.620, p = 0.004 | F3,35 = 0.479, p = 0.699 |
| Metabolic scope (MS) | F1,35 = 10.220, p = 0.003 | F3,35 = 0.245, p = 0.864 |
| Survival under predation | F1,15 = 1.697, p = 0.219 | F3,15 = 8.407, p = 0.003 |
| LZM | F1,23 = 0.303, p = 0.588 | F3,23 = 4.629, p = 0.014 |
| IgM | F1,23 = 1.067, p = 0.315 | F3,23 = 9.547, p < 0.001 |
| Spleen index | F1,23 = 8.151, p = 0.010 | F3,23 = 1.945, p = 0.157 |
| Parameters | Training | Results of Two-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| Control Group | 1 bl s−1 Training Group | 2 bl s−1 Training Group | 4 bl s−1 Training Group | Velocity Effect | Training Effect | Interaction Effect | ||
| Metabolic rate (mg O2 kg−1 h−1) | 6 cm s−1 | 239.15 ± 4.57 Ca | 203.78 ± 8.51 Dab | 180.95 ± 14.22 Gb | 236.93 ± 15.71 Da | F7,265 = 83.262; p < 0.001 | F3,265 = 4.490; p = 0.004 | F21,265 = 1.287; p = 0.185 |
| 12 cm s−1 | 267.27 ± 13.14 Cb | 215.08 ± 10.57 Da | 206.20 ± 10.32 Ga | 228.25 ± 10.85 Da | ||||
| 18 cm s−1 | 303.11 ± 22.49 Ca | 262.61 ± 13.70 CDa | 283.02 ± 10.54 Fa | 264.20 ± 16.61 CDa | ||||
| 24 cm s−1 | 439.12 ± 24.84 Bb | 355.75 ± 27.61 Ca | 340.54 ± 18.81 Ea | 317.48 ± 29.67 CDa | ||||
| 30 cm s−1 | 592.48 ± 47.96 Ac | 503.90 ± 44.1 Bbc | 414.52 ± 26.42 Dab | 377.45 ± 50.72 Ca | ||||
| 36 cm s−1 | 640.93 ± 65.21 Aa | 609.56 ± 76.59 Ba | 534.31 ± 33.81 Ca | 525.07 ± 78.09 Ba | ||||
| 42 cm s−1 | 679.92 ± 71.63 Aa | 743.45 ± 58.74 Aa | 659.74 ± 39.38 Ba | 577.67 ± 78.71 Ba | ||||
| 48 cm s−1 | 708.89 ± 75.65 Aa | 895.67 ± 72.71 Aa | 751.12 ± 39.69 Aa | 856.23 ± 28.69 Aa | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Q.; Fu, S.; Huang, T.; Li, X.; Shi, X. Effects of Aerobic Exercise Training on the Growth, Swimming Performance, Antipredation Ability and Immune Parameters of Juvenile Rock Carp (Procypris rabaudi). Animals 2022, 12, 257. https://doi.org/10.3390/ani12030257
Hou Q, Fu S, Huang T, Li X, Shi X. Effects of Aerobic Exercise Training on the Growth, Swimming Performance, Antipredation Ability and Immune Parameters of Juvenile Rock Carp (Procypris rabaudi). Animals. 2022; 12(3):257. https://doi.org/10.3390/ani12030257
Chicago/Turabian StyleHou, Qimiao, Shijian Fu, Tiji Huang, Xiuming Li, and Xiaotao Shi. 2022. "Effects of Aerobic Exercise Training on the Growth, Swimming Performance, Antipredation Ability and Immune Parameters of Juvenile Rock Carp (Procypris rabaudi)" Animals 12, no. 3: 257. https://doi.org/10.3390/ani12030257
APA StyleHou, Q., Fu, S., Huang, T., Li, X., & Shi, X. (2022). Effects of Aerobic Exercise Training on the Growth, Swimming Performance, Antipredation Ability and Immune Parameters of Juvenile Rock Carp (Procypris rabaudi). Animals, 12(3), 257. https://doi.org/10.3390/ani12030257







