Out of Sight, Out of Mind or Just Something in the Way? Visual Barriers Do Not Reduce Intraspecific Agonism in an All-Male Group of Nile Crocodiles (Crocodylus niloticus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
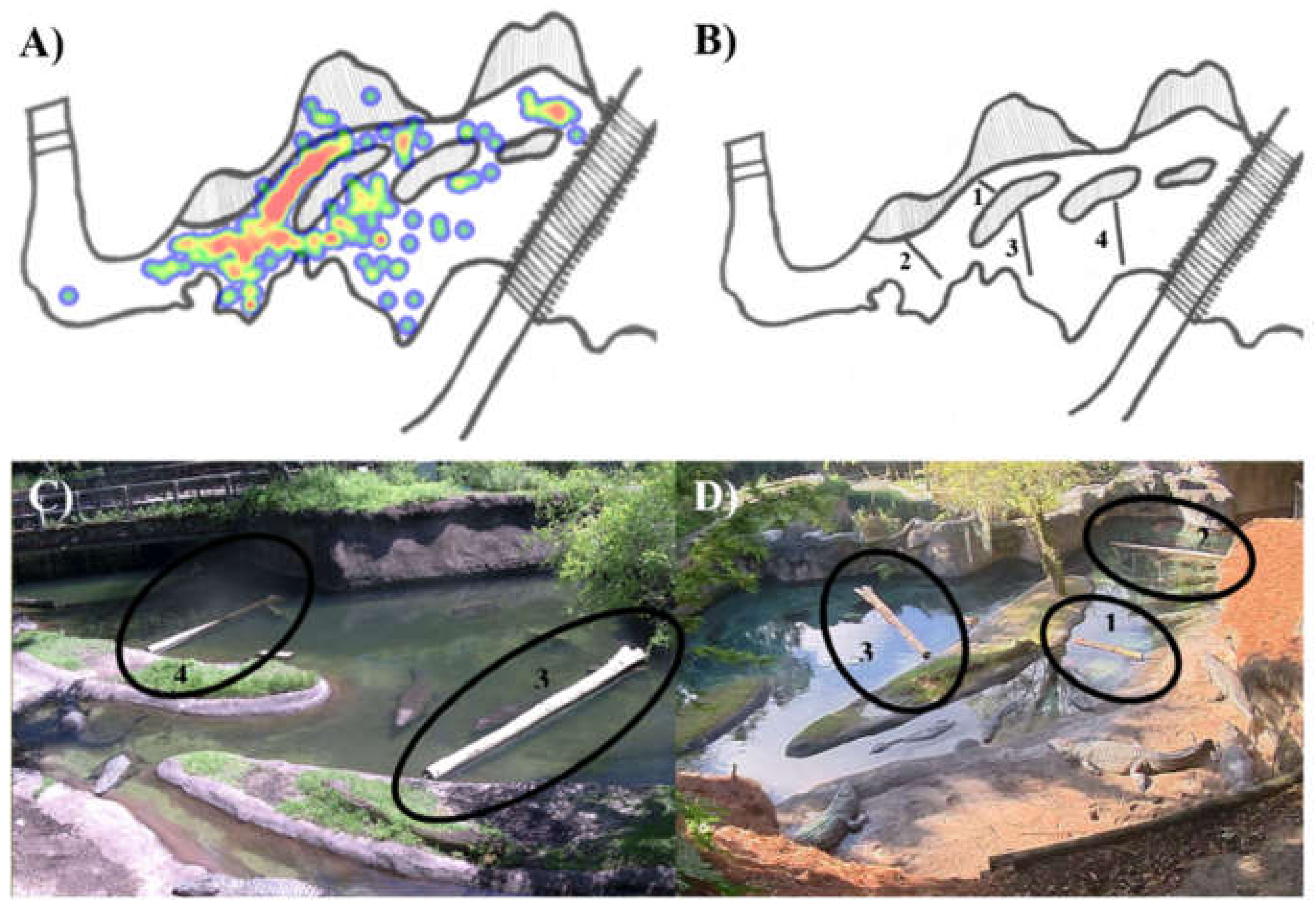
2.1. Study Subjects and Visual Barriers
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Short-Term Effects
3.2. Long-Term Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Short-Term Effect Model Outputs
| Model Estimate Parameters | Analysis of Variance Type III Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Dependent Variable | Fixed Effects | Estimate | S.E. | Z | p | χ2 | Df | p |
| Rate of Total Agonistic Bouts | Intercept | 7.261 | 6.135 | 1.184 | 0.237 | 1.401 | 1 | 0.237 |
| Barrier Presence (Pre) | 0.371 | 0.517 | 0.717 | 0.473 | 0.514 | 1 | 0.473 | |
| Time of Day (Morning) | −1.186 | 0.917 | −1.294 | 0.196 | 3.228 | 2 | 0.199 | |
| Time of Day (Midday) | −1.219 | 0.680 | −1.792 | 0.073 | - | - | - | |
| Temperature | −0.075 | 0.072 | −1.043 | 0.297 | 1.089 | 1 | 0.297 | |
| Rate of Agonistic Bouts on Land | Intercept | 14.698 | 12.451 | 1.180 | 0.238 | 1.393 | 1 | 0.238 |
| Barrier Presence (Pre) | −0.253 | 1.069 | −0.237 | 0.813 | 0.056 | 1 | 0.813 | |
| Time of Day (Morning) | −1.790 | 2.170 | −0.825 | 0.410 | 1.364 | 2 | 0.506 | |
| Time of Day (Midday) | −2.112 | 1.808 | −1.168 | 0.243 | - | - | - | |
| Temperature | −0.175 | 0.145 | −1.210 | 0.226 | 1.465 | 1 | 0.226 | |
| Proportion of Group in Water | Intercept | −6.604 | 1.518 | −4.351 | <0.001 * | 18.932 | 1 | <0.001 * |
| Barrier Presence (Pre) | 1.195 | 0.257 | 4.647 | <0.001 * | 21.590 | 1 | <0.001 * | |
| Time of Day (Morning) | 1.374 | 0.208 | 6.598 | <0.001 * | 43.543 | 2 | <0.001 * | |
| Time of Day (Midday) | 0.713 | 0.168 | 4.240 | <0.001 * | - | - | - | |
| Temperature | 0.068 | 0.018 | 3.723 | <0.001 * | 13.858 | 1 | <0.001 * | |
| Proportion of Group in Proximity | Intercept | 1.831 | 1.764 | 1.038 | 0.300 | 1.077 | 1 | 0.299 |
| Barrier Presence (Pre) | 0.168 | 0.360 | 0.466 | 0.641 | 0.217 | 1 | 0.641 | |
| Time of Day (Morning) | 0.024 | 0.237 | 0.100 | 0.921 | 42.525 | 2 | <0.001 * | |
| Time of Day (Midday) | 0.980 | 0.182 | 5.393 | <0.001 * | - | - | - | |
| Temperature | −0.045 | 0.021 | −2.114 | 0.035 * | 4.468 | 1 | 0.035 * | |
Appendix B. Long-Term Effect Model Outputs
| Model Estimate Parameters | Analysis of Variance Type III Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Dependent Variable | Fixed Effects | Estimate | S.E. | Z | p | χ2 | Df | p |
| Rate of Total Agonistic Bouts | Intercept | 0.851 | 0.966 | 0.880 | 0.379 | 0.775 | 1 | 0.379 |
| Barrier Presence (Pre) | −0.358 | 0.300 | −1.560 | 0.119 | 2.433 | 1 | 0.119 | |
| Time of Day (Morning) | 0.664 | 0.416 | 1.597 | 0.110 | 26.010 | 2 | <0.001 * | |
| Time of Day (Midday) | −0.633 | 0.404 | −1.565 | 0.117 | - | - | - | |
| Temperature | −0.004 | 0.013 | −0.337 | 0.736 | 0.114 | 1 | 0.736 | |
| Month (November) | −0.391 | 0.239 | −1.635 | 0.102 | 2.672 | 1 | 0.102 | |
| Rate of Agonistic Bouts on Land | Intercept | −1.206 | 2.013 | −0.599 | 0.549 | 0.359 | 1 | 0.549 |
| Barrier Presence (Pre) | −0.654 | 0.509 | −1.284 | 0.199 | 1.649 | 1 | 0.199 | |
| Time of Day (Morning) | 0.402 | 0.770 | 0.521 | 0.602 | 2.673 | 2 | 0.263 | |
| Time of Day (Midday) | −0.637 | 0.811 | −0.786 | 0.432 | - | - | - | |
| Temperature | −0.014 | 0.028 | −0.494 | 0.622 | 0.244 | 1 | 0.622 | |
| Month (November) | −0.575 | 0.565 | −1.017 | 0.309 | 1.034 | 1 | 0.310 | |
| Proportion of Group in Water | Intercept | 1.478 | 0.464 | 3.184 | 0.001 * | 10.140 | 1 | 0.001 * |
| Barrier Presence (Pre) | 0.372 | 0.138 | 2.693 | 0.007 * | 7.251 | 1 | 0.007 * | |
| Time of Day (Morning) | 0.928 | 0.126 | 7.337 | <0.001 * | 166.543 | 2 | <0.001 * | |
| Time of Day (Midday) | −0.294 | 0.098 | −3.015 | 0.003 * | - | - | - | |
| Temperature | −0.036 | 0.006 | −5.769 | <0.001 * | 33.278 | 1 | <0.001 * | |
| Month (November) | −0.035 | 0.146 | −0.242 | 0.809 | 0.059 | 1 | 0.809 | |
| Proportion of Group in Proximity | Intercept | 0.194 | 0.408 | 0.475 | 0.635 | 0.225 | 1 | 0.635 |
| Barrier Presence (Pre) | −0.409 | 0.110 | −3.702 | <0.001 * | 13.707 | 1 | <0.001 * | |
| Time of Day (Morning) | −0.798 | 0.112 | −7.104 | <0.001 * | 57.583 | 2 | <0.001 * | |
| Time of Day (Midday) | −0.198 | 0.087 | −2.283 | 0.022 * | - | - | - | |
| Temperature | −0.008 | 0.006 | −1.389 | 0.165 | 1.929 | 1 | 0.165 | |
| Month (November) | 0.122 | 0.118 | 1.031 | 0.303 | 1.063 | 1 | 0.303 | |
References
- Lawrence, K.; Sherwen, S.L.; Larsen, H. Natural habitat design for zoo-housed elasmobranch and teleost fish species improves behavioural repertoire and space use in a visitor facing exhibit. Animals 2021, 11, 2979. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, S.S.; Shepherdson, D.; Lewis, K.; Prado, N.; Brown, J.L.; Lee, B.; Wielebnowski, N. Supporting zoo Asian elephant (Elephas maximus) welfare and herd dynamics with a more complex and expanded habitat. Animals 2021, 11, 2566. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.R.; Calcutt, S.; Schapiro, S.J.; Hau, J. Space use selectivity by chimpanzees and gorillas in an indoor-outdoor enclosure. Am. J. Primatol. 2011, 73, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.F.; Aureli, F. Social network changes during space restriction in zoo chimpanzees. Primates 2019, 60, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.R.; Wagner, K.E.; Schaprio, S.J.; Hau, J. Ape behavior in two alternating environments: Comparing exhibit and short-term holding areas. Am. J. Primatol. 2010, 72, 951–959. [Google Scholar] [CrossRef]
- Ritzler, C.P.; Lukas, K.E.; Bernstein-Kurtycz, L.M.; Koester, D.C. The effects of choice-based design and management on the behavior and space use of zoo-housed amur tigers (Panthera tigris altaica). J. Appl. Anim. Welf. Sci. 2021, 1–14. [Google Scholar] [CrossRef]
- Lukas, K.E.; Hoff, M.P.; Maple, T.L. Gorilla behavior in response to systematic alternation between zoo enclosures. Appl. Anim. Behav. Sci. 2003, 81, 367–386. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Effects of environmental enrichment on reproduction. Zoo Biol. 1994, 13, 447–458. [Google Scholar] [CrossRef]
- Carlstead, K.; Shepherdson, D. Alleviating stress in zoo animals with environmental enrichment. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Publishing: New York, NY, USA, 2000; pp. 337–349. [Google Scholar]
- Arey, D.S.; Edwards, S.A. Factors influencing aggression between sows after mixing and the consequences for welfare and production. Livest. Prod. Sci. 1998, 1, 61–70. [Google Scholar] [CrossRef]
- Deeming, C.; Cooper, J.; Hodges, H. Effect of sight barriers in pens of breeding ring-necked pheasants (Phasianus colicus): Behavior and welfare. Br. Poult. Sci. 2011, 52, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Torrezani, C.S.; Pinho-Neto, C.F.; Miyai, C.A.; Sanches, F.H.C.; Barreto, R.E. Structural enrichment reduces aggression in Tilapia rendalli. Mar. Freshw. Behav. Physiol. 2013, 46, 183–190. [Google Scholar] [CrossRef]
- Valuska, A.J.; Leighty, K.A.; Shutz, P.J.; Ferrie, G.M.; Sky, C.C.; Bettinger, T.L. The use of visual barriers to reduce aggression among a group of marabou storks (Leptoptilos crumeniferus). Zoo Biol. 2013, 32, 648–651. [Google Scholar] [CrossRef]
- Troxell-Smith, S.M.; Miller, L.J. Using natural history information for zoo animal management: A case study with okapi. J. Zoo Aquar. Res. 2016, 4, 38–41. [Google Scholar]
- Larson, J.M. Mitigation of Alarm Calls and Vigilant Behavior in Captive Brown Capuchin Monkeys (Cebus paella): Using a Visual Barrier to Reduce Stress from a Nearby Canada Lynx (Lynx canadensis). Master’s Thesis, Western Illinois University, Macomb, IL, USA, 2017. [Google Scholar]
- Flanagan, A.M.; Rutz, C.; Farabaugh, S.; Greggor, A.L.; Masuda, B.; Swaisgood, R.R. Inter-aviary distance and visual access influence conservation breeding outcomes in a territorial, endangered bird. Biol. Conserv. 2020, 242, 108429. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The visitor effect on zoo animals: Implications and opportunities for zoo animal welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Chiew, S.J.; Butler, K.L.; Sherwen, S.L.; Coleman, G.J.; Fanson, K.V.; Hemsorth, P.H. Effects of regulating visitor viewing proximity and the intensity of visitor behaviour on the little penguin (Eudyptula minor) behaviour and welfare. Animals 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Blaney, E.C.; Wells, D.L. The influence of a camouflage net barrier on the behavior, welfare and public perceptions of zoo-housed gorillas. Anim. Welf. 2004, 13, 111–118. [Google Scholar]
- Clark, F.E.; Fitzpatrick, M.; Hartley, A.; King, A.J.; Lee, T.; Routh, A.; Walker, S.L.; George, K. Relationship between behavior, adrenal activity, and environment in zoo-housed western lowland gorillas (Gorilla gorilla gorilla). Zoo Biol. 2012, 31, 306–321. [Google Scholar] [CrossRef]
- Kuhar, C.W.; Bettinger, T.L.; Sironen, A.L.; Shaw, J.H.; Lasley, B.L. Factors affecting reproduction in zoo-housed Geoffroys’ tamarins (Saguinus geoffroyi). Zoo Biol. 2003, 22, 545–559. [Google Scholar] [CrossRef]
- Bashaw, M.J.; Kelling, A.S.; Bloomsmith, M.A.; Maple, T.L. Environmental effects on the behavior of zoo-housed lions and tigers, with a case study of the effects of a visual barrier on pacing. J. Appl. Anim. Welf. Sci. 2007, 10, 95–109. [Google Scholar] [CrossRef]
- Martin, O.; Vinyoles, D.; Garcia-Galea, E.; Maté, C. Improving the welfare of a zoo-housed male drill (Mandrillus leucophaeus poensis) aggressive towards visitors. J. Appl. Anim. Welf. Sci. 2016, 19, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crofoot, M.C.; Rubenstein, D.I.; Maiya, A.S.; Berger-Wolf, T.Y. Aggression, grooming and group-level cooperation in white-faced capuchins (Cebus capucinus): Insights from social networks. Am. J. Primatol. 2011, 73, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Tank, A.; Ness, T.; Leahy, M.; Ross, S.R. Sex and season predict wounds in zoo-housed Japanese macaques (Macaca fuscata): A multi-institutional study. Zoo Biol. 2020, 39, 147–155. [Google Scholar] [CrossRef]
- Leeds, A.; Boyer, B.; Ross, S.R.; Lukas, K.E. The effects of group type and young silverbacks on wounding rates in western lowland gorilla (Gorilla gorilla gorilla) groups in North American Zoos. Zoo Biol. 2015, 34, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Leeds, A.; Boyer, B.; Ross, S.R.; Lukas, K.E. Patterns of wounding in mixed-sex social groups of western lowland gorillas (Gorilla gorilla gorilla). Appl. Anim. Behav. Sci. 2021, 236, 105226. [Google Scholar] [CrossRef]
- Ross, S.R.; Bloomsmith, M.A.; Bettinger, T.L.; Wagner, K.E. The influence of captive adolescent male chimpanzees on wounding: Management and welfare implications. Zoo Biol. 2009, 28, 623–634. [Google Scholar] [CrossRef]
- Wiley, J.N.; Leeds, A.; Carpenter, K.D.; Kendall, C.J. Patterns of wounding in hamadryas baboons (Papio hamadryas) in North American zoos. Zoo Biol. 2018, 37, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Brien, M.L.; Gienger, C.M.; Webb, G.J.; McGuinness, K.; Christian, K.A. Out of sight or in too deep: Effect of visual barriers and water depth on agonistic behavior and growth in hatchling saltwater crocodiles (Crocodylus porosus). Appl. Anim. Behav. Sci. 2014, 158, 102–110. [Google Scholar] [CrossRef]
- Nagloo, N.; Collin, S.P.; Hemmi, J.M.; Hart, N.S. Spatial resolving power and spectral sensitivity of the saltwater crocodile, Crocodylus porosus, and the freshwater crocodile, Crocodylus johnstoni. J. Exp. Biol. 2016, 219, 1394–1404. [Google Scholar] [CrossRef] [Green Version]
- Garrick, L.D.; Lang, J.W. Social signals and behaviors of adult alligators and crocodiles. Am. Zool. 1977, 17, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Fergusson, R.A. Nile Crocodile Crocodylus niloticus. In Crocodiles: Status Survey and Conservation Action Plan, 3rd ed.; Manolis, S.C., Stevenson, C., Eds.; Crocodile Specialist Group: Darwin, Australia, 2010; pp. 84–89. [Google Scholar]
- Kofron, C.P. Behavior of Nile crocodiles in a seasonal river in Zimbabwe. Copeia 1993, 1993, 463–469. [Google Scholar] [CrossRef]
- Modha, M.L. The ecology of the Nile crocodile (Crocodylus niloticus laurenti) on Central Island, Lake Rudolf. E. Afr. Wildl. J. 1967, 5, 74–95. [Google Scholar] [CrossRef]
- Pooley, A.C.; Gans, C. The Nile crocodile. Sci. Am. 1976, 234, 114–125. [Google Scholar] [CrossRef]
- Riley, A.; Terry, M.; Freeman, H.; Alba, A.C.; Soltis, J.; Leeds, A. Evaluating the effect of visitor presence on Nile crocodile (Crocodylus niloticus) behavior. J. Zool. Bot. Gard. 2021, 2, 115–129. [Google Scholar] [CrossRef]
- Wark, J.D.; Cronin, K.A.; Niemann, T.; Shender, M.A.; Horrigan, A.; Kao, A.; Ross, M.R. Monitoring the behavior and habitat use of animals to enhance welfare using the ZooMonitor app. Anim. Behav. Cogn. 2019, 6, 158–167. [Google Scholar] [CrossRef]
- Brien, M.L.; Lang, J.W.; Webb, G.J.; Stevenson, C.; Christian, K.A. The good, the bad, and the ugly: Agonistic behaviour in juvenile crocodilians. PLoS ONE 2013, 8, e80872. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 20 January 2022).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University of Press: Cambridge, UK, 2002. [Google Scholar]
- Weldon, P.J.; Ferguson, M.W.J. Chemoreception in crocodilians: Anatomy, natural history and empirical results. Brain Behav. Evol. 1993, 41, 239–245. [Google Scholar] [CrossRef]
- Kofron, C.P. The reproductive cycle of the Nile crocodile (Crocodylus niloticus). J. Zool. 1990, 221, 477–488. [Google Scholar] [CrossRef]
- Detoeuf-Boulade, A.S. Reproductive Cycle and Sexual Size Dimorphism of the Nile Crocodile (Crocodylus niloticus) in the Okavango Delta, Botswana. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2006. [Google Scholar]
- Seebacher, F.; Grigg, G.C.; Beard, L.A. Crocodiles as dinosaurs: Behavioural thermoregulation in very large ectotherms leads to high and stable body temperatures. J. Exp. Biol. 1999, 202, 77–86. [Google Scholar] [CrossRef]
- Pough, F.H. Amphibians and reptiles as low-energy systems. In Behavioural Energetics: The Cost of Survival in Vertebrates; Aspey, W.P., Lustick, S., Eds.; Ohio State University Press: Columbus, OH, USA, 1983; pp. 141–188. [Google Scholar]
- Agha, M.; Price, S.J.; Nowakowski, J.; Augustine, B.; Todd, B.D. Mass mortality of eastern box turtles with upper respiratory disease following atypical cold weather. Dis. Aquat. Org. 2017, 124, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estep, D.Q.; Baker, S.C. The effects of temporary cover on the behaviour of socially housed stump-tailed macaques (Macaca arctoides). Zoo Biol. 1991, 10, 465–472. [Google Scholar] [CrossRef]
- Species360 Zoological Information Management System. 2021. Available online: Zims.Species360.org (accessed on 1 November 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leeds, A.; Riley, A.; Terry, M.; Mazorra, M.; Wick, L.; Krug, S.; Wolfe, K.; Leonard, I.; Daneault, A.; Alba, A.C.; et al. Out of Sight, Out of Mind or Just Something in the Way? Visual Barriers Do Not Reduce Intraspecific Agonism in an All-Male Group of Nile Crocodiles (Crocodylus niloticus). Animals 2022, 12, 269. https://doi.org/10.3390/ani12030269
Leeds A, Riley A, Terry M, Mazorra M, Wick L, Krug S, Wolfe K, Leonard I, Daneault A, Alba AC, et al. Out of Sight, Out of Mind or Just Something in the Way? Visual Barriers Do Not Reduce Intraspecific Agonism in an All-Male Group of Nile Crocodiles (Crocodylus niloticus). Animals. 2022; 12(3):269. https://doi.org/10.3390/ani12030269
Chicago/Turabian StyleLeeds, Austin, Alex Riley, Megan Terry, Marcus Mazorra, Lindsay Wick, Scott Krug, Kristen Wolfe, Ike Leonard, Andy Daneault, Andrew C. Alba, and et al. 2022. "Out of Sight, Out of Mind or Just Something in the Way? Visual Barriers Do Not Reduce Intraspecific Agonism in an All-Male Group of Nile Crocodiles (Crocodylus niloticus)" Animals 12, no. 3: 269. https://doi.org/10.3390/ani12030269







