Sertoli, Leydig, and Spermatogonial Cells’ Specific Gene and Protein Expressions as Dog Testes Evolve from Immature into Mature States
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Testes Collection, and Testicular Tissue Preparation
2.2. Real-Time Polymerase Chain Reaction
2.2.1. RNA Isolation
2.2.2. Complementary DNA (cDNA) Synthesis
2.2.3. Real-Time PCR of Cells’ Specific Markers
2.2.4. Determination of mRNA Expression Using Real-Time PCR
2.3. Western Blot Analysis
2.4. Protein Blot Analysis
2.5. Immunohistochemistry Localization of FSHR, AMH, LHR, INSL3, THY1, and CDH1 on Testis
Evaluation of Mean Intensity and Mean Density of the Area Occupied by Chromogenic Labeling for Cell-Specific Proteins in Immature and Mature Testis
2.6. Stereology
2.6.1. Tissue Preparation and Sampling
2.6.2. Cell Identification
2.6.3. Cell Number Calculation
2.7. Statistical Analysis
3. Results
3.1. The mRNA Expression of Cell-Specific Markers
3.2. Protein Expressions of Cell-Specific Markers
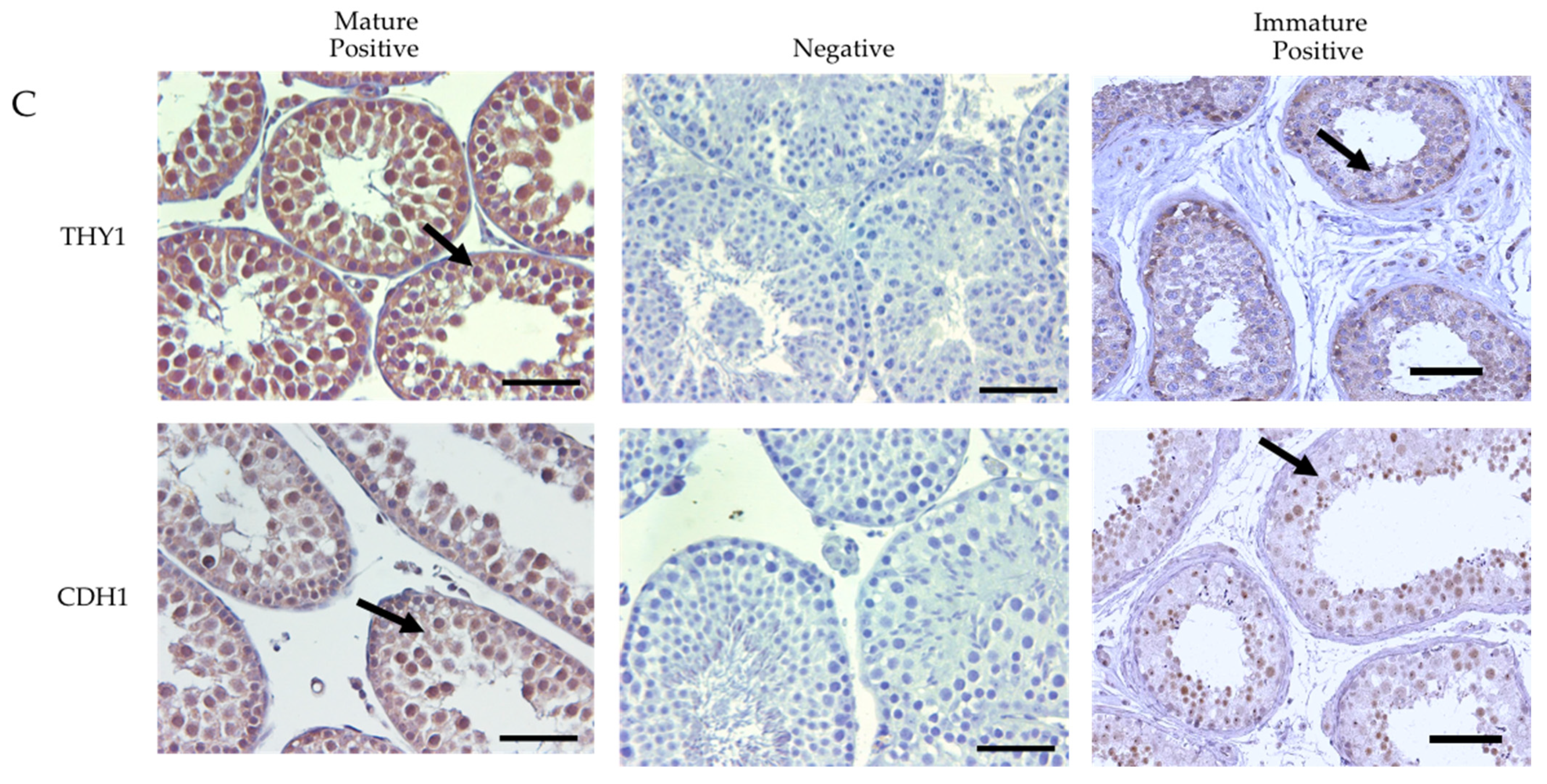
3.3. Immunolocalization of the Cells-Specific Markers
The Semi-Quantitative Analysis of the Mean Intensity and Density of the Area Occupied by the Chromogenic Labeling for Cell-Specific Protein Markers in Immature and Mature Dog Testes
3.4. The Testis Stereology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellvé, A.R.; Cavicchia, J.C.; Millette, C.F.; O’Brien, D.A.; Bhatnagar, Y.M.; Dym, M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 1977, 74, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Malkov, M.; Fisher, Y.; Don, J. Developmental schedule of the postnatal rat testis determined by flow cytometry. Biol. Reprod. 1998, 59, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Kluin, P.M.; Kramer, M.F.; de Rooij, D.G. Proliferation of spermatogonia and Sertoli cells in maturing mice. Anat. Embryol. (Berl) 1994, 169, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolussi, M.; Zanchetta, R.; Belvedere, P.; Colombo, L. Sertoli and Leydig cell numbers and gonadotropin receptors in rat testis from birth to puberty. Cell Tissue Res. 1990, 260, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Wreford, N.G.; de Kretser, D.M. A quantitative study of spermatogenesis in the developing rat testis. Biol. Reprod. 1990, 43, 629–635. [Google Scholar]
- França, L.R.; Silva, V.A., Jr.; Chiarini-Garcia, H.; Garcia, S.K.; Debeljuk, L. Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol. Reprod. 2000, 63, 1629–1636. [Google Scholar] [CrossRef]
- Harkey, M.A.; Asano, A.; Zoulas, M.E.; Torok-Storb, B.; Nagashima, J.; Travis, A. Isolation, genetic manipulation, and transplantation of canine spermatogonial stem cells: Progress toward transgenesis through the male germ-line. Reproduction 2013, 146, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6487–6492. [Google Scholar] [CrossRef] [Green Version]
- Lacerda, S.M.S.N.; Aponte, P.M.; Costa, G.M.J.; Campos-Junior, P.H.A.; Segatelli, T.M.; Silva, M.A.; França, L.R. An overview on spermatogonial stem cell physiology, niche and transplantation. Anim. Reprod. 2012, 9, 798–808. [Google Scholar]
- Tseng, Y.T.; Liao, H.F.; Yu, C.Y.; Mo, C.F.; Lin, S.P. Epigenetic factors in the regulation of prospermatogonia and spermatogonial stem cells. Reproduction 2015, 150, R77–R91. [Google Scholar] [CrossRef]
- Liang, S.; Liu, D.; Li, X.; Wei, M.; Yu, X.; Li, Q.; Ma, H.; Zhang, Z.; Qin, Z. SOX2 participates in spermatogenesis of Zhikong scallop Chlamys farreri. Sci. Rep. 2019, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.; Su, B.; Lipke, E.A.; Perera, D.A.; Li, C.; Qin, Z.; Li, Y.; Dunn, D.A.; Cek, S.; Peatman, E.; et al. Spermatogonial stem cells specific marker identification in channel catfish, Ictalurus punctatus and blue catfish, I. furcatus. Fish Physiol. Biochem. 2015, 41, 1545–1556. [Google Scholar] [CrossRef]
- Vigier, B.; Tran, D.; du Mesnil du Buisson, F.; Heyman, Y.; Josso, N. Use of monoclonal antibody techniques to study the ontogeny of bovine anti-Müllerian hormone. J. Reprod. Fertil. 1983, 69, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, B.A.; Conley, A.J.; Grundy, S.A.; Sabeur, K.; Liu, I.K. Expression of anti-Müllerian hormone (AMH) in the equine testis. Theriogenology 2008, 69, 624–631. [Google Scholar] [CrossRef]
- Banco, B.; Veronesi, M.C.; Giudice, C.; Rota, A.; Grieco, V. Immunohistochemical evaluation of the expression of anti-Müllerian hormone in mature, immature and neoplastic canine Sertoli cells. J. Comp. Pathol. 2012, 146, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ano, H.; Hidaka, Y.; Katamoto, H. Evaluation of anti-Müllerian hormone in a dog with a Sertoli cell tumour. Vet. Dermatol. 2014, 25, 142-e41. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.R. Expression of retinoic acid-metabolizing enzymes, ALDH1A1, ALDH1A2, ALDH1A3, CYP26A1, CYP26B1 and CYP26C1 in canine testis during post-natal development. Reprod. Domest. Anim. 2016, 51, 901–909. [Google Scholar] [CrossRef]
- Dobrinski, I. Germ cell transplantation and testis tissue xenografting in domestic animals. Anim. Reprod. Sci. 2005, 89, 137–145. [Google Scholar] [CrossRef]
- Kasimanickam, V.R.; Kasimanickam, R.K.; Rogers, H.A. Immunolocalization of retinoic acid receptor-alpha, -beta, and -gamma, in bovine and canine sperm. Theriogenology 2013, 79, 1010–1018. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, R. Exogenous retinoic acid and cytochrome P450 26B1 inhibitor modulate meiosis-associated genes expression in canine testis, an in vitro model. Reprod. Domest. Anim. 2014, 49, 315–323. [Google Scholar] [CrossRef]
- Hejmej, A.; Kopera, I.; Kotula-Balak, M.; Lydka, M.; Lenartowicz, M.; Bilinska, B. Are expression and localization of tight and adherens junction proteins in testes of adult boar affected by foetal and neonatal exposure to flutamide? Int. J. Androl. 2012, 35, 340–352. [Google Scholar] [CrossRef]
- Kotula-Balak, M.; Hejmej, A.; Lydka, M.; Cierpich, A.; Bilinska, B. Detection of aromatase, androgen, and estrogen receptors in bank vole spermatozoa. Theriogenology 2012, 78, 385–392. [Google Scholar] [CrossRef]
- Tsai, K.L.; Clark, L.A.; Murphy, K.E. Understanding hereditary diseases using the dog and human as companion model systems. Mamm. Genome Soc. 2007, 18, 444–451. [Google Scholar]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Rodriguez, J.S.; Pelzer, K.D.; Sponenberg, P.D.; Thatcher, C.D. Tocopherol induced angiogenesis in placental vascular network in late pregnant ewes. Reprod. Biol. Endocrinol. 2010, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayhew, T.M. A review of recent advances in stereology for quantifying neural structure. J. Neurocytol. 1992, 21, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A. Stereology as a valuable tool in the toolbox of testicular research. Ann. Anat. 2014, 196, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Wreford, N.G. Theory and practice of stereological techniques applied to the estimation of cell number and nuclear volume in the testis. Microsc. Res. Tech. 1995, 32, 423–436. [Google Scholar] [CrossRef]
- Petersen, P.M.; Seierøe, K.; Pakkenberg, B. The total number of Leydig and Sertoli cells in the testes of men across various age groups—A stereological study. J Anat. 2015, 226, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Hytönen, M.K.; Lohi, H. Canine models of human rare disorders. Rare Dis. 2016, 4, e1241362. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, C.; Nathar, T.J.; Ghosh, A.; Hickstein, D.D.; Nelson, E. Contemporary animal models for human gene therapy applications. Curr. Gene Ther. 2015, 15, 531–540. [Google Scholar] [CrossRef]
- Lee, W.Y.; Lee, R.; Park, H.J.; Do, J.T.; Park, C.; Kim, J.H.; Jhun, H.; Lee, J.H.; Hur, T.; Song, H. Characterization of male germ cell markers in canine testis. Anim. Reprod. Sci. 2017, 182, 1–8. [Google Scholar] [CrossRef]
- Aksglaede, L.; Sørensen, K.; Boas, M.; Mouritsen, A.; Hagen, C.P.; Jensen, R.B.; Petersen, J.H.; Linneberg, A.; Andersson, A.M.; Main, K.M.; et al. Changes in anti-Mullerian hormone (AMH) throughout the life span: A population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J. Clin. Endocrinol. Metab. 2010, 95, 5357–5364. [Google Scholar] [CrossRef]
- Rebourcet, D.; O’Shaughnessy, P.J.; Monteiro, A.; Milne, L.; Cruickshanks, L.; Jeffrey, N.; Guillou, F.; Freeman, T.C.; Mitchell, R.T.; Smith, L.B. Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. PLoS ONE 2014, 9, e105687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelsztein, N.Y.; Grinspon, R.P.; Schteingart, H.F.; Rey, R.A. Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int. J. Pediat. Endocrinol. 2016, 2016, 20. [Google Scholar] [CrossRef] [Green Version]
- Steger, K.; Rey, R.; Kliesch, S.; Louis, F.; Schleicher, G.; Bergmann, M. Immunohistochemical detection of immature Sertoli cell markers in testicular tissue of infertile adult men: A preliminary study. Int. J. Androl. 1996, 19, 122–128. [Google Scholar] [CrossRef]
- Rey, R.; Sabourin, J.C.; Venara, M.; Long, W.Q.; Jaubert, F.; Zeller, W.P.; Duvillard, P.; Chemes, H.; Bidart, J.M. Anti-Müllerian hormone is a specific marker of sertoli- and granulosa-cell origin in gonadal tumors. Hum. Pathol. 2000, 31, 1202–1208. [Google Scholar] [CrossRef]
- Albert, S.; Wistuba, J.; Eildermann, K.; Ehmcke, J.; Schlatt, S.; Gromoll, J.; Kossack, N. Comparative marker analysis after isolation and culture of testicular cells from the immature marmoset. Cells Tissues Organs 2012, 196, 543–554. [Google Scholar] [CrossRef]
- Griswold, S.L.; Behringer, R.R. Fetal Leydig cell origin and development. Sex Dev. 2009, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Li, X.; Li, L.; Chen, H.; Ge, R.S. Insights into the Development of the Adult Leydig Cell Lineage from Stem Leydig Cells. Front. Physiol. 2017, 8, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendis-Handagama, S.M.; Ariyaratne, H.B. Differentiation of the adult Leydig cell population in the postnatal testis. Biol. Reprod. 2001, 65, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Ewing, L.L. Leydig cell differentiation during maturation of the rat testis: A stereological study of cell number and ultrastructure. Anat. Rec. 1987, 219, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Klonisch, T.; Ivell, R.; Balvers, M.; Kliesch, S.; Fischer, B.; Bergmann, M.; Steger, K. Expression of relaxin-like factor is down-regulated in human testicular Leydig cell neoplasia. Mol. Hum. Reprod. 1999, 5, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivell, R.; Wade, J.D.; Anand-Ivell, R. INSL3 as a biomarker of Leydig cell functionality. Biol. Reprod. 2013, 88, 147. [Google Scholar] [CrossRef] [PubMed]
- Anand-Ivell, R.; Wohlgemuth, J.; Haren, M.T.; Hope, P.J.; Hatzinikolas, G.; Wittert, G.; Ivell, R. Peripheral INSL3 concentrations decline with age in a large population of Australian men. Int. J. Androl. 2006, 29, 618–626. [Google Scholar] [CrossRef]
- Shinohara, T.; Avarbock, M.R.; Brinster, R.L. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 1999, 96, 5504–5509. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, T.; Orwig, K.E.; Avarbock, M.R.; Brinster, R.L. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc. Natl. Acad. Sci. USA 2000, 97, 8346–8351. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Takakura, A.; Ohbo, K.; Abe, K.; Wakabayashi, J.; Yamamoto, M.; Suda, T.; Nabeshima, Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev. Biol. 2004, 269, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.C.; Braydich-Stolle, L.; Dym, M. Isolation of male germ-line stem cells; influence of GDNF. Dev. Biol. 2005, 279, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, M.; Kadokawa, Y.; Kurahashi, H.; Marunouchi, T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol. Reprod. 2007, 76, 130–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, H.; Brinster, R.L. Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol. 2008, 86, 59–84. [Google Scholar] [PubMed] [Green Version]
- Abbasi, H.; Tahmoorespur, M.; Hosseini, S.M.; Nasiri, Z.; Bahadorani, M.; Hajian, M.; Nasiri, M.R.; Nasr-Esfahani, M.H. THY1 as a reliable marker for enrichment of undifferentiated spermatogonia in the goat. Theriogenology 2013, 80, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, M.; Jorsaraei, S.; Fattahi, E.; Tabari, M.G.; Neyshaburi, E.Z. SCF improves in vitro differentiation of SSCs through transcriptionally up-regulating PRTM1, STRA8, c-KIT, PIWIL2, and OCT4 genes. Reprod. Sci. 2021, 28, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Goedken, M.J.; Kerlin, R.L.; Morton, D. Spontaneous and age-related testicular findings in beagle dogs. Toxicol. Pathol. 2008, 36, 465–471. [Google Scholar] [CrossRef] [PubMed]






| Gene | Primer | Sequence 5′ to 3′ | Product Size (Nucleotides) | Accession Number |
|---|---|---|---|---|
| FSHR | Forward Reverse | CGTGTTCTCCAACCTGTCCA TCAGCCATAGTAGAACCTTTTGA | 188 | XM_003431533.2 |
| AMH | Forward Reverse | GGAGGAAGTGACATGGGAGC CAAAGGTTCTGGGTGCCTGG | 159 | XM_542190.4 |
| LHR | Forward Reverse | AACGGTTTCTGCTCACCCAA ATGAGAAAACGGGGTACTGTCA | 197 | AF389885.1 |
| INSL3 | Forward Reverse | GACCGTGAGCTGTTGCAGTG CAGTAGTGTGCGGGATTGGT | 155 | NM_001002962.1 |
| THY1 | Forward Reverse | GAGCCCAGATCAAGGACTGAG CTGGATGGGCAAGGTGGTAG | 179 | XM_844606.3 |
| CDH1 | Forward Reverse | AGGTCTCATCGGGGCTCTG ACACCATCTGTGCCCACTTT | 199 | XM_536807.4 |
| ACTB | Forward Reverse | TCCCTGGAGAAGAGCTACGA CTTCTGCATCCTGTCAGCAA | 243 | AF021873 |
| Protein | Primary Antibody | Secondary Antibody |
|---|---|---|
| FSHR | Anti-FSH-R antibody (ab137695); rabbit polyclonal | Goat anti-rabbit IgG-FITC (sc-2012) |
| AMH | Anti-AMH antibody (ab84952: rabbit polyclonal | Goat anti-rabbit IgG-FITC (sc-2012) |
| LHR | Anti- LHR antibody (ab96603); rabbit polyclonal | Goat anti-rabbit IgG-FITC (sc-2012) |
| INSL3 | Anti-INSL3 antibody (ab199536); recombinant | Goat anti-rabbit IgG-FITC (sc-2012) |
| THY1 | Anti-Thy1 antibody (ab92574); recombinant | Goat anti-rabbit IgG-FITC (sc-2012) |
| CDH1 | Anti-CDH1 antibody (ab226779); rabbit polyclonal | Goat anti-rabbit IgG-FITC (sc-2012) |
| ACTB | Anti-beta Actin antibody (ab6276); mouse monoclonal | Goat anti-mouse IgG-FITC (sc-2010) |
| Protein | Immature | Mature |
|---|---|---|
| FSHR | 52.19 ± 3.43 a | 31.48 ± 2.27 |
| AMH | 49.23 ± 3.34 a | 18.42 ± 2.92 b |
| LHR | 31.43 ± 3.84 a | 26.38 ± 2.42 b |
| INSL3 | 24.19 ± 1.25 a | 1912 ± 1.60 b |
| THY1 | 51.31 ± 2.24 a | 45.43 ± 2.13 b |
| CDH1 | 53.44 ± 3.41 a | 48.13 ± 1.56 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasimanickam, V.R.; Kasimanickam, R.K. Sertoli, Leydig, and Spermatogonial Cells’ Specific Gene and Protein Expressions as Dog Testes Evolve from Immature into Mature States. Animals 2022, 12, 271. https://doi.org/10.3390/ani12030271
Kasimanickam VR, Kasimanickam RK. Sertoli, Leydig, and Spermatogonial Cells’ Specific Gene and Protein Expressions as Dog Testes Evolve from Immature into Mature States. Animals. 2022; 12(3):271. https://doi.org/10.3390/ani12030271
Chicago/Turabian StyleKasimanickam, Vanmathy R., and Ramanathan K. Kasimanickam. 2022. "Sertoli, Leydig, and Spermatogonial Cells’ Specific Gene and Protein Expressions as Dog Testes Evolve from Immature into Mature States" Animals 12, no. 3: 271. https://doi.org/10.3390/ani12030271
APA StyleKasimanickam, V. R., & Kasimanickam, R. K. (2022). Sertoli, Leydig, and Spermatogonial Cells’ Specific Gene and Protein Expressions as Dog Testes Evolve from Immature into Mature States. Animals, 12(3), 271. https://doi.org/10.3390/ani12030271






