The Effect of Photoperiodic Conditions on GnRH/LH Secretion in Ewes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. LH Assay
2.3. Melatonin Assay
2.4. GnRH Peptide Assay
2.5. Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. The Influence of the Day Time on the Secretion of LH in Anestrous and Follicular Phase Ewes
3.2. The Effect of the Day Time on the Circulating Melatonin Concentration in Anestrous and Follicular Phase Ewes
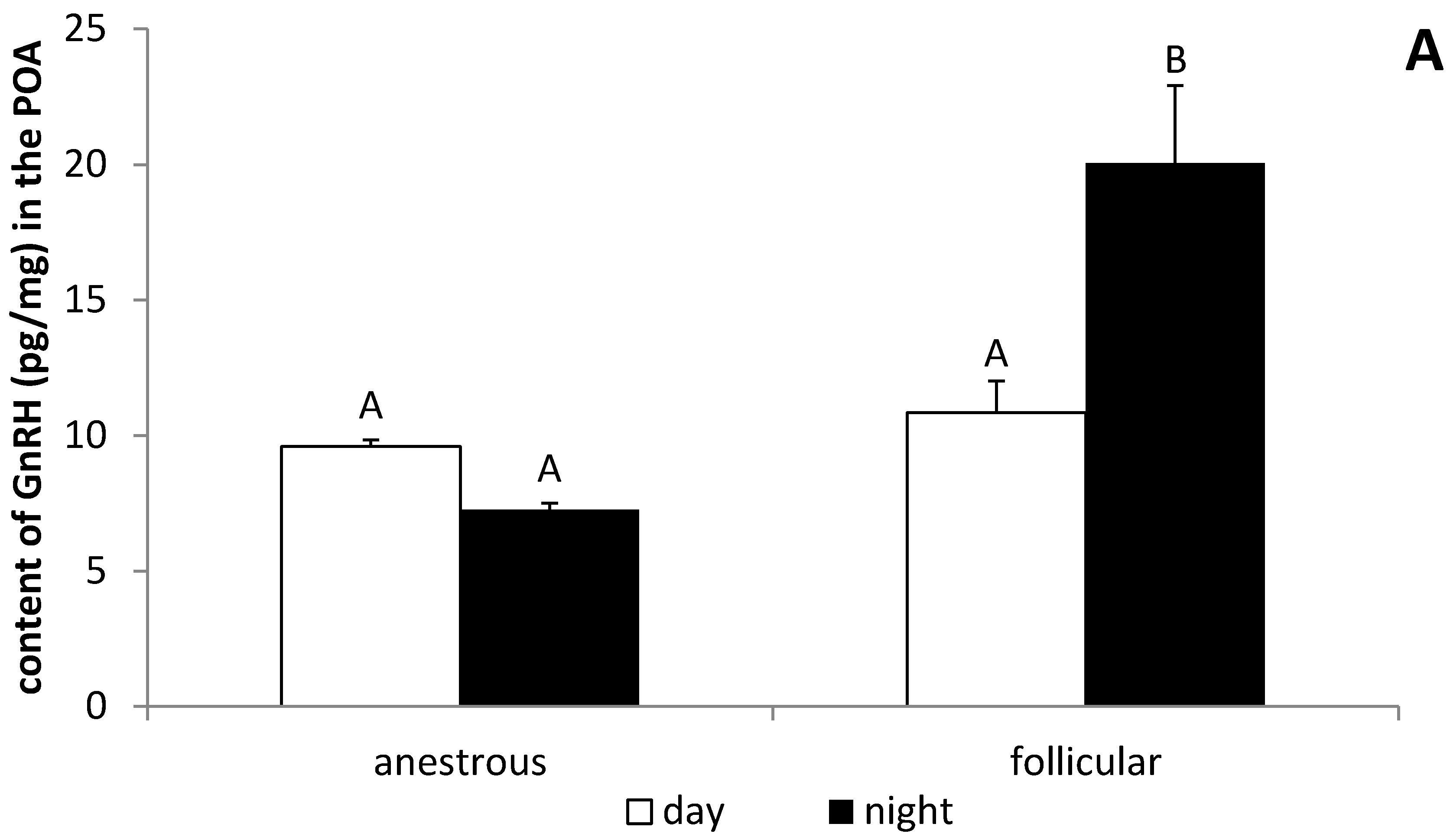
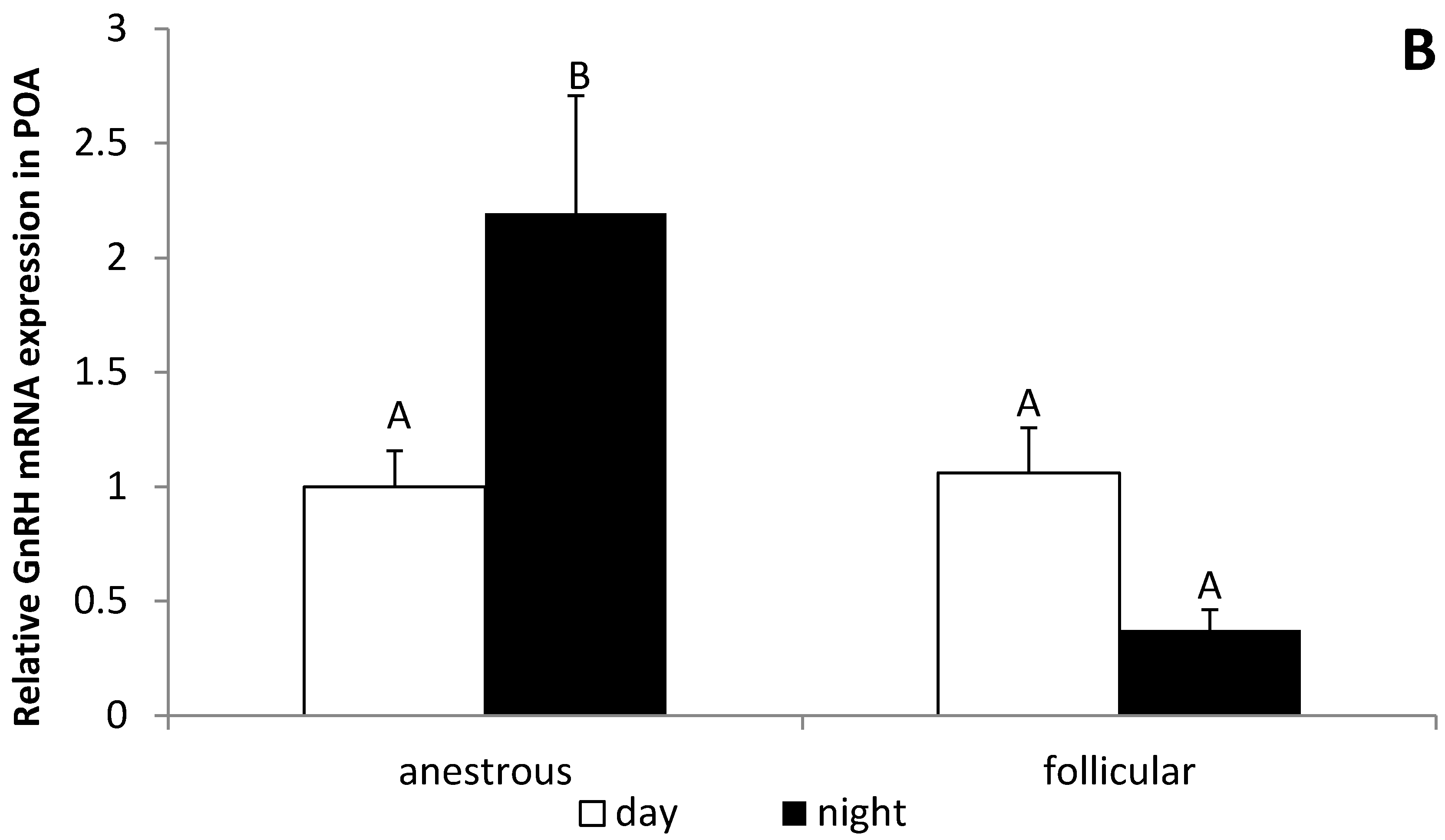
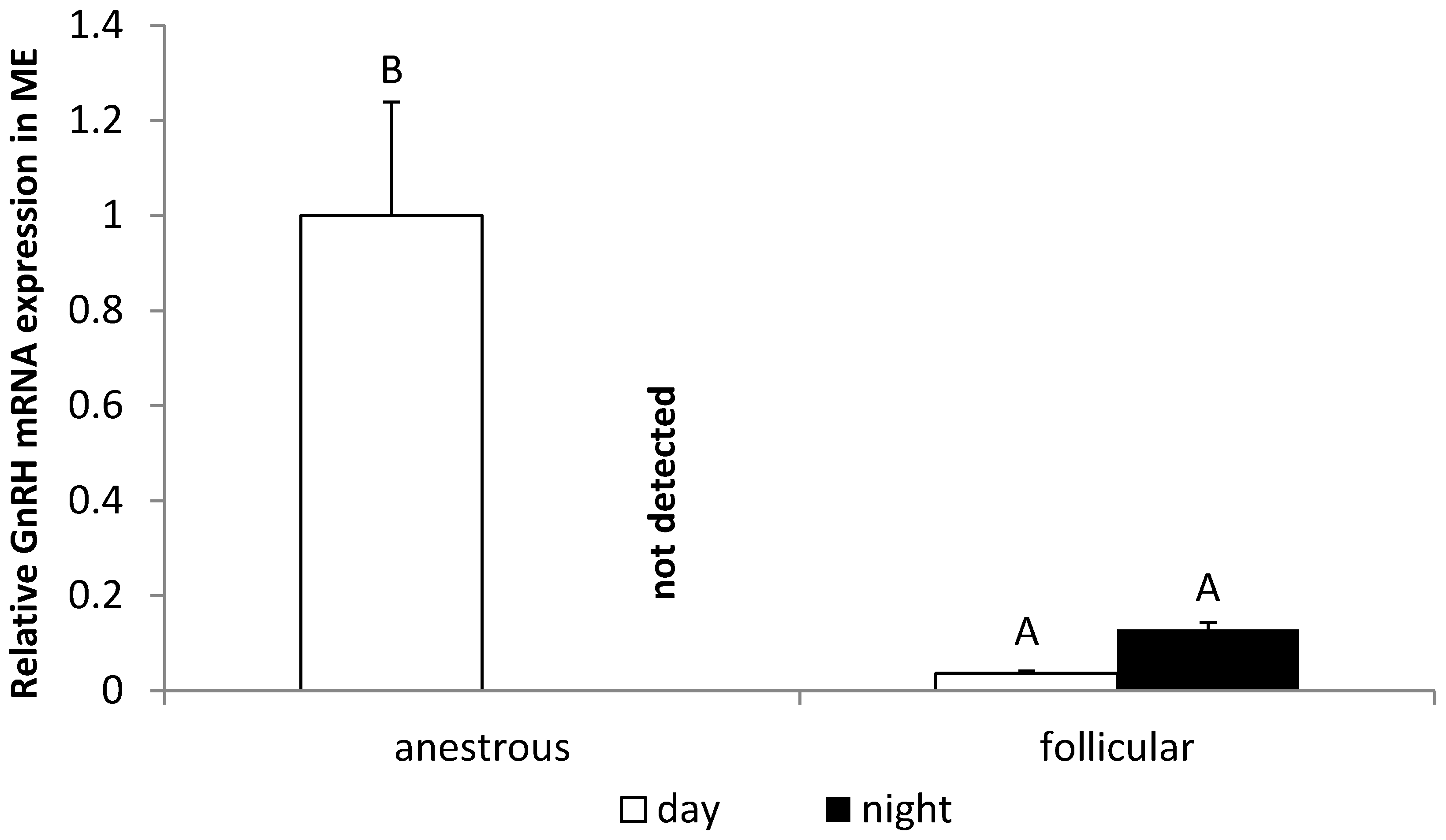
3.3. The Influence of the Day Time on the GnRH Expression in the POA and ME in Anestrous and Follicular Phase Ewes
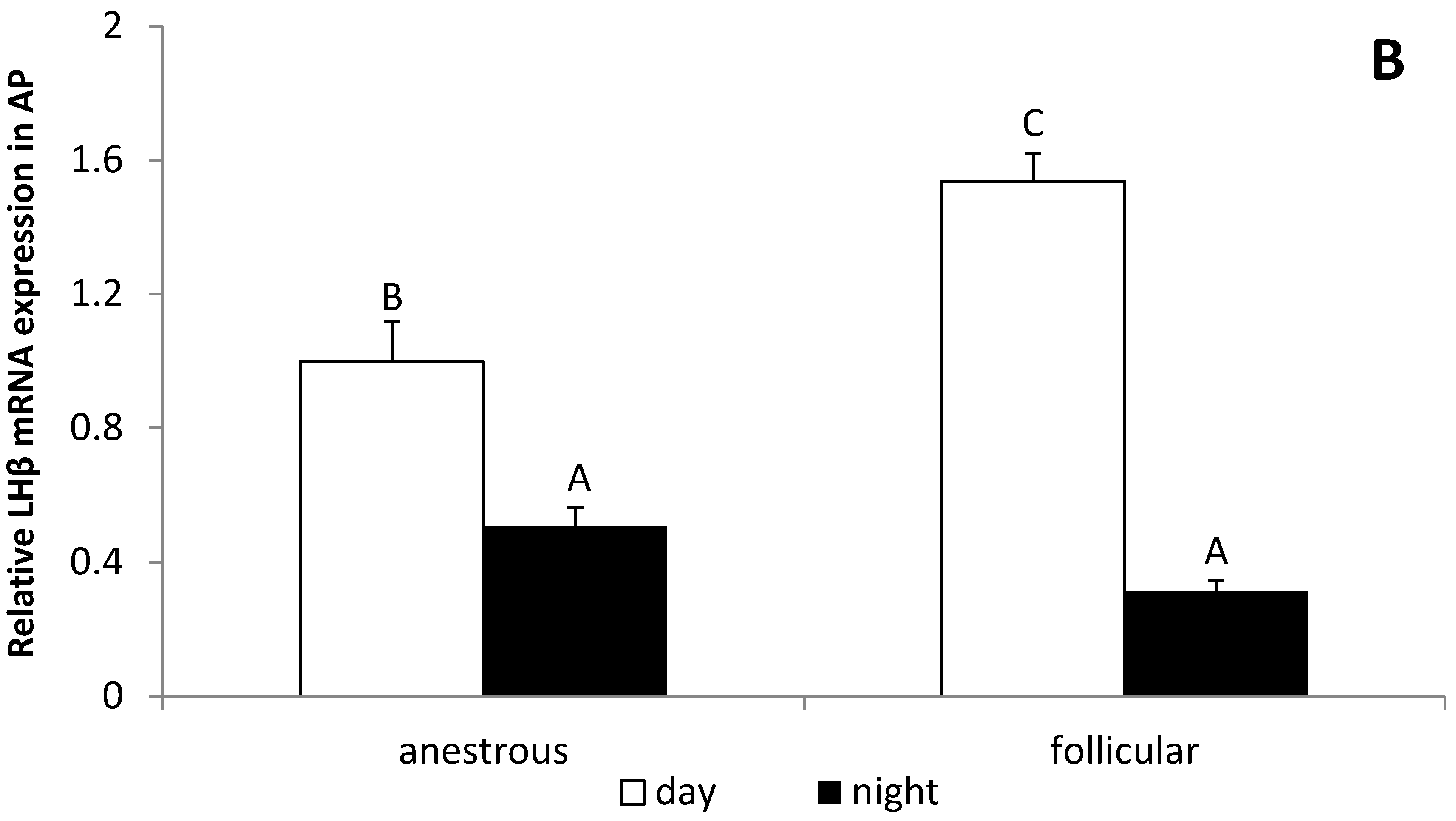
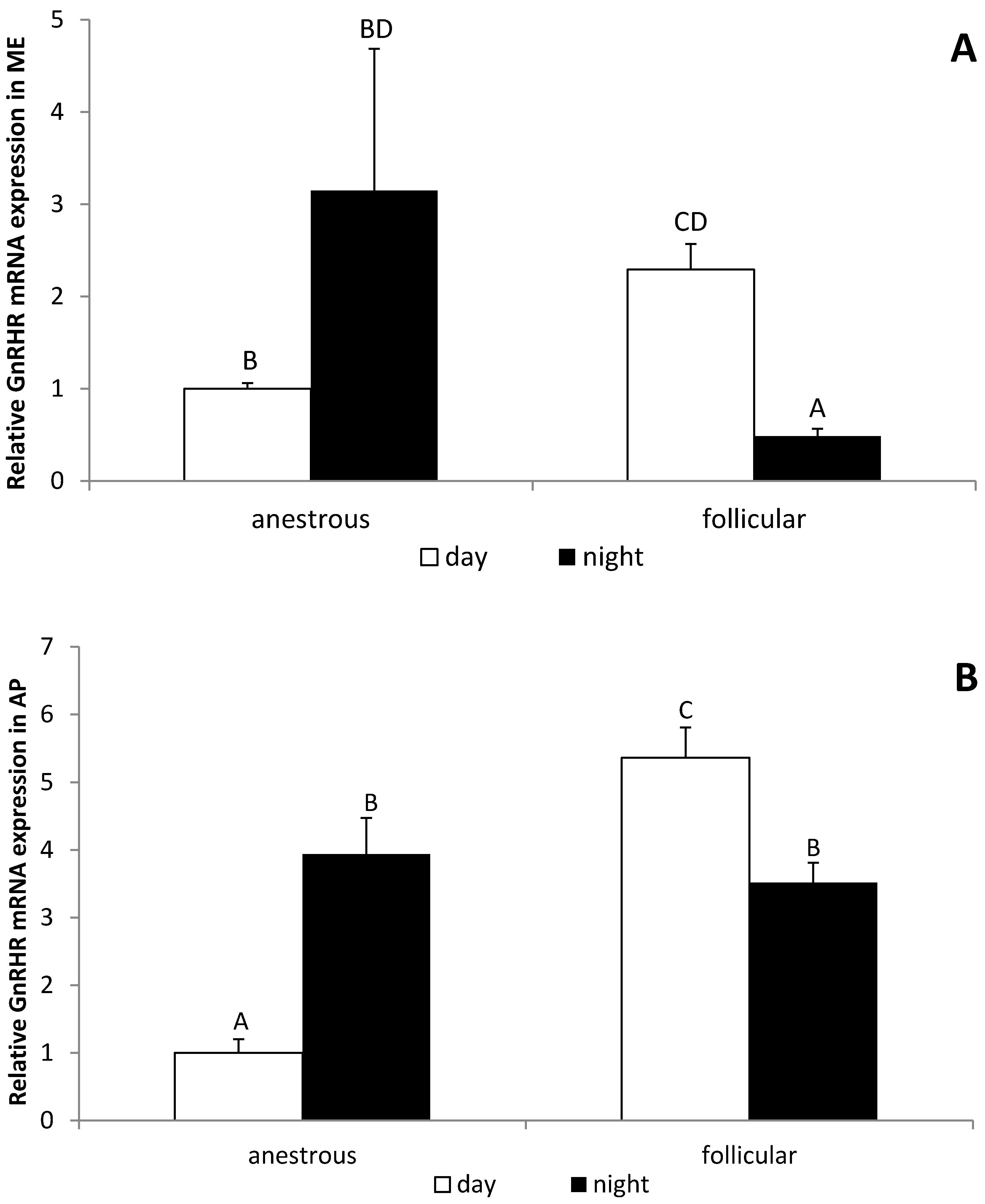
3.4. The Influence of the Day Time on the GnRHR Gene Expression in the ME and AP in Anestrous and Follicular Phase Ewes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herman, A.P.; Bochenek, J.; Król, K.; Krawczyńska, A.; Antushevich, H.; Pawlina, B.; Herman, A.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central Interleukin-1 β Suppresses the Nocturnal Secretion of Melatonin. Mediat. Inflamm. 2016, 2016, 2589483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, R.L.; Jansen, H.T.; Billings, H.J.; Coolen, L.M.; Lehman, M.N. Neural Systems Mediating Seasonal Breeding in the Ewe. J. Neuroendocrinol. 2010, 22, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Van der Beek, E.M.; Horvath, T.L.; Wiegant, V.M.; Van den Hurk, R.; Buijs, R.M. Evidence for a Direct Neuronal Pathway from the Suprachiasmatic Nucleus to the Gonadotropin-Releasing Hormone System: Combined Tracing and Light and Electron Microscopic Immunocytochemical Studies. J. Comp. Neurol. 1997, 384, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Chemineau, P.; Bodin, L.; Migaud, M.; Thiéry, J.; Malpaux, B. Neuroendocrine and Genetic Control of Seasonal Reproduction in Sheep and Goats: Seasonality of Reproduction in Small Ruminants. Reprod. Domest. Anim. 2010, 45, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Migaud, M.; Tricoire, H.; Chemineau, P. Biology of Mammalian Photoperiodism and the Critical Role of the Pineal Gland and Melatonin. J. Biol. Rhythm. 2001, 16, 336–347. [Google Scholar] [CrossRef]
- Thiéry, J.-C.; Lomet, D.; Bougoin, S.; Malpaux, B. Turnover Rate of Cerebrospinal Fluid in Female Sheep: Changes Related to Different Light-Dark Cycles. Fluids Barriers CNS 2009, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Viguie, C.; Caraty, A.; Locatelli, A.; Malpaux, B. Regulation of Luteinizing Hormone-Releasing Hormone (LHRH) Secretion by Melatonin in the Ewe.I. Simultaneous Delayed Increase in LHRH and Luteinizing Hormone Pulsatile Secretion1. Biol. Reprod. 1995, 52, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Thiéry, J.C.; Chemineau, P.; Hernandez, X.; Migaud, M.; Malpaux, B. Neuroendocrine Interactions and Seasonality. Domest. Anim. Endocrinol. 2002, 23, 87–100. [Google Scholar] [CrossRef]
- Simonneaux, V.; Bahougne, T. A Multi-Oscillatory Circadian System Times Female Reproduction. Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, M.M.; Sisk, C.; Ross, H.E.; Smale, L. Circadian Regulation of Gonadotropin-Releasing Hormone Neurons and the Preovulatory Surge in Luteinizing Hormone in the Diurnal Rodent, Arvicanthis Niloticus, and in a Nocturnal Rodent, Rattus Norvegicus1. Biol. Reprod. 2004, 70, 1049–1054. [Google Scholar] [CrossRef] [Green Version]
- Reuss, S. Components and Connections of the Circadian Timing System in Mammals. Cell Tissue Res. 1996, 285, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.G.; Swanson, L.W. Efferent Projections of the Suprachiasmatic Nucleus: II. Studies Using Retrograde Transport of Fluorescent Dyes and Simultaneous Peptide Immunohistochemistry in the Rat. J. Comp. Neurol. 1987, 258, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Currie, W.D.; Medhamurthy, R.J.; Cook, S.J.; Rawlings, N.C. Seasonal Fluctuation in Diurnal Rhythms of Luteinizing Hormone Secretion in Ewes during the Mid-Luteal Phase of the Oestrous Cycle. Reproduction 1993, 97, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A.; Almeida, O.F.X.; Klandorf, H.; Cunningham, R.A. Hourly Fluktuation in the Blood Levels of Melatonin, Prolactin, Luteinizing Hormone, Follicle-Stimulating Hormone, Testosterone, Tri-Iodothyronine, Thyroxine and Cortisol in Rams under Artificial Photoperiods, and the Effects of Cranial Sympathectomy. J. Endocrinol. 1982, 92, 237–250. [Google Scholar] [CrossRef]
- Misztal, T.; Romanowicz, K.; Barcikowski, B. Melatonin—A Modulator of the GnRH/LH Axis in Sheep. Reprod. Biol. 2002, 2, 267–275. [Google Scholar]
- Russel, A. Body Condition Scoring of Sheep. In Pract. 1984, 6, 91–93. [Google Scholar] [CrossRef]
- Strzetelski, J. Standards for Ruminant Feeding; Instytut Zootechniki PIB: Kraków, Poland, 2009; ISBN 978-83-7607-072-8. [Google Scholar]
- Herman, A.P.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Antushevich, H.; Herman, A.; Paczesna, K.; Romanowicz, K.; Tomaszewska-Zaremba, D. Peripheral Inhibitor of AChE, Neostigmine, Prevents the Inflammatory Dependent Suppression of GnRH/LH Secretion during the Follicular Phase of the Estrous Cycle. BioMed Res. Int. 2017, 2017, 6823209. [Google Scholar] [CrossRef] [Green Version]
- Krawczyńska, A.; Antushevich, H.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Herman, A.; Zięba, D. Photoperiodic Conditions as a Factor Modulating Leptin Influenceon Pro-Inflammatory Cytokinesand Their Receptors Gene Expression in Ewe’s Aorta. J. Anim. Feed Sci. 2019, 28, 128–137. [Google Scholar] [CrossRef]
- Stupnicki, R.; Madej, A. Radioimmunoassay of LH in Blood Plasma of Farm Animals. Endokrinologie 1976, 68, 6–13. [Google Scholar]
- Fraser, S.; Cowen, P.; Franklin, M.; Franey, C.; Arendt, J. Direct Radioimmunoassay for Melatonin in Plasma. Clin. Chem. 1983, 29, 396–397. [Google Scholar] [CrossRef]
- Król, K.; Tomaszewska-Zaremba, D.; Herman, A. Photoperiod-Dependent Effect of Inflammation on Nocturnal Gene Expression of Proinflammatory Cytokines and Their Receptors in Pars Tuberalis of Ewe. J. Anim. Feed Sci. 2016, 25, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, R. Quantification on the LightCycler. In Rapid Cycle Real-Time PCR; Meuer, S., Wittwer, C., Nakagawara, K.-I., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 21–34. ISBN 978-3-540-66736-0. [Google Scholar]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Haziak, K.; Romanowicz, K.; Misztal, T.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. The Effect of Rivastigmine on the LPS-Induced Suppression of GnRH/LH Secretion during the Follicular Phase of the Estrous Cycle in Ewes. Anim. Reprod. Sci. 2013, 138, 203–212. [Google Scholar] [CrossRef] [PubMed]
- McElhinny, T.L.; Sisk, C.L.; Holekamp, K.E.; Smale, L. A Morning Surge in Plasma Luteinizing Hormone Coincides with Elevated Fos Expression in Gonadotropin-Releasing Hormone-Immunoreactive Neurons in the Diurnal Rodent, Arvicanthis Niloticus1. Biol. Reprod. 1999, 61, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Kerdelhué, B.; Brown, S.; Lenoir, V.; Queenan, J.T., Jr.; Jones, G.S.; Scholler, R.; Jones, H.W., Jr. Timing of Initiation of the Preovulatory Luteinizing Hormone Surge and Its Relationship with the Circadian Cortisol Rhythm in the Human. Neuroendocrinology 2002, 75, 158–163. [Google Scholar] [CrossRef]
- Cahill, D.J.; Wardle, P.G.; Harlow, C.R.; Hull, M.G.R. Onset of the Preovulatory Luteinizing Hormone Surge: Diurnal Timing and Critical Follicular Prerequisites. Fertil. Steril. 1998, 70, 56–59. [Google Scholar] [CrossRef]
- Warren, W.S.; Champney, T.H.; Cassone, V.M. The Suprachiasmatic Nucleus Controls the Circadian Rhythm of Heart Rate via the Sympathetic Nervous System. Physiol. Behav. 1994, 55, 1091–1099. [Google Scholar] [CrossRef]
- Gray, G.D.; Södersten, P.; Tallentire, D.; Davidson, J.M. Effects of Lesions in Various Structures of the Suprachiasmatic-Preoptic Region on LH Regulation and Sexual Behavior in Female Rats. Neuroendocrinology 1978, 25, 174–191. [Google Scholar] [CrossRef]
- Wiegand, S.J.; Terasawa, E.; Bridson, W.E.; Goy, R.W. Effects of Discrete Lesions of Preoptic and Suprachiasmatic Structures in the Female Rat. Neuroendocrinology 1980, 31, 147–157. [Google Scholar] [CrossRef]
- de la lglesia, H.O.; Blaustein, J.D.; Bittman, E.L. The Suprachiasmatic Area in the Female Hamster Projects to Neurons Containing Estrogen Receptors and GnRH. NeuroReport 1995, 6, 1715–1722. [Google Scholar] [CrossRef]
- van der Beek, E.M.; Wiegant, V.M.; van Oudheusden, H.J.C.; van der Donk, H.A.; van den Hurk, R.; Buijs, R.M. Synaptic Contacts between Gonadotropin-Releasing Hormone-Containing Fibers and Neurons in the Suprachiasmatic Nucleus and Perichiasmatic Area: An Anatomical Substrate for Feedback Regulation? Brain Res. 1997, 755, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Watts, A.G.; Swanson, L.W.; Sanchez-Watts, G. Efferent Projections of the Suprachiasmatic Nucleus: I. Studies Using Anterograde Transport OfPhaseolus Vulgaris Leucoagglutinin in the Rat. J. Comp. Neurol. 1987, 258, 204–229. [Google Scholar] [CrossRef]
- Card, J.; Brecha, N.; Karten, H.; Moore, R. Immunocytochemical Localization of Vasoactive Intestinal Polypeptide- Containing Cells and Processes in the Suprachiasmatic Nucleus of the Rat: Light and Electron Microscopic Analysis. J. Neurosci. 1981, 1, 1289–1303. [Google Scholar] [CrossRef] [Green Version]
- Swaab, D.F.; Pool, C.W.; Nijveldt, F. Immunofluorescence of Vasopressin and Oxytocin in the Rat Hypothalamo-Neurohypophyseal System. J. Neural Transm. 1975, 36, 195–215. [Google Scholar] [CrossRef] [Green Version]
- Thind, K.K.; Boggan, J.E.; Goldsmith, P.C. Interactions between Vasopressin- and Gonadotropin-Releasing-Hormone-Containing Neuroendocrine Neurons in the Monkey Supraoptic Nucleus. Neuroendocrinology 1991, 53, 287–297. [Google Scholar] [CrossRef]
- Ghuman, S.; Morris, R.; Spiller, D.; Smith, R.; Dobson, H. Integration Between Different Hypothalamic Nuclei Involved in Stress and GnRH Secretion in the Ewe: Hypothalamic Nuclei Involved in Stress and GnRH Secretion. Reprod. Domest. Anim. 2010, 45, 1065–1073. [Google Scholar] [CrossRef]
- Reuss, S.; Hermes, B.; Fuchs, E.; Hiemke, C. Day- and Night-Time Contents of Monoamines and Their Metabolites in the Medial Preoptic Area of the Rat Hypothalamus. Neurosci. Lett. 1999, 266, 29–32. [Google Scholar] [CrossRef]
- Scott, C.J.; Cumminst, J.T.; Clarke, I.J. Effects on Plasma Luteinizing Hormone Levels of Microinjection of Noradrenaline and Adrenaline into the Septo-Preoptic Area of the Brain of the Ovariectomized Ewe: Changes with Season and Chronic Oestrogen Treatment. J. Neuroendocrinol. 1992, 4, 131–141. [Google Scholar] [CrossRef]
- Kochman, K.; Przekop, F.; Okrasa, S. Neural regulation of the reproductive processes at the central level. In Physiological Regulation of Reproduction in Female; Krzymowski, T., Ed.; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2007; ISBN 978-83-7299-509-4. [Google Scholar]
- Hermes, B.; Hiemke, C.; Reuss, S. Day- and Nighttime Content of Monoamines and Their Metabolites in the Pineal Gland of Rat and Hamster. Neurosci. Lett. 1994, 179, 119–122. [Google Scholar] [CrossRef]
- Schomerus, C. Mechanisms Regulating Melatonin Synthesis in the Mammalian Pineal Organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383. [Google Scholar] [CrossRef]
- Tricoire, H.; Locatelli, A.; Chemineau, P.; Malpaux, B. Melatonin Enters the Cerebrospinal Fluid through the Pineal Recess. Endocrinology 2002, 143, 84–90. [Google Scholar] [CrossRef]
- Skipor, J.; Thiery, J.-C. The Choroid Plexus-Cerebrospinal Fluid System: Undervaluated Pathway of Neuroendocrine Signaling into the Brain. Acta Neurobiol. Exp. 2008, 68, 414–428. [Google Scholar]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and Reproduction Revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, P.; Williams, L.M. The Pars Tuberalis of the Pituitary: A Gateway for Neuroendocrine Output. Rev. Reprod. 1996, 1, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Herman, A.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Tomaszewska-Zaremba, D. Caffeine Stimulates in Vitro Pituitary LH Secretion in Lipopolysaccharide-Treated Ewes. Reprod. Biol. 2015, 15, 20–26. [Google Scholar] [CrossRef]
- Wojtulewicz, K.; Tomaszewska-Zaremba, D.; Herman, A. Endotoxin-Induced Inflammation Suppresses the Effect of Melatonin on the Release of LH from the Ovine Pars Tuberalis Explants—Ex Vivo Study. Molecules 2017, 22, 1933. [Google Scholar] [CrossRef] [Green Version]
- Mignot, M.; Skinner, D.C. Colocalization of GH, TSH and Prolactin, but Not ACTH, with ?LH-Immunoreactivity: Evidence for Pluripotential Cells in the Ovine Pituitary. Cell Tissue Res. 2005, 319, 413–421. [Google Scholar] [CrossRef]
- Perez-Castro, C.; Renner, U.; Haedo, M.R.; Stalla, G.K.; Arzt, E. Cellular and Molecular Specificity of Pituitary Gland Physiology. Physiol. Rev. 2012, 92, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Lafarque, M.M.; Ezquer, M.; Aguado, L.I.; Oliveros, L.B. Bovine Pars Tuberalis Secretions Release Growth Hormone from Rat Pars Distalis of Pituitary Gland. Neuroendocrinol. Lett. 2004, 25, 273–277. [Google Scholar]
- Malpaux, B.; Daveau, A.; Maurice, F.; Gayrard, V.; Thiery, J.-C. Short-Day Effects of Melatonin on Luteinizing Hormone Secretion in the Ewe: Evidence for Central Sites of Action in the Mediobasal Hypothalamus1. Biol. Reprod. 1993, 48, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, G.A. Effects of Placing Micro-Implants of Melatonin in the Pars Tuberalis, Pars Distalis and the Lateral Septum of the Forebrain on the Secretion of FSH and Prolactin, and Testicular Size in Rams. J. Endocrinol. 1994, 142, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A.; Maeda, K.-I. Reproductive Effects of Placing Micro-Implants of Melatonin in the Mediobasal Hypothalamus and Preoptic Area in Rams. J. Endocrinol. 1992, 132, 201–215. [Google Scholar] [CrossRef]
- Malpaux, B.; Daveau, A.; Maurice-Mandon, F.; Duarte, G.; Chemineau, P. Evidence That Melatonin Acts in the Premammillary Hypothalamic Area to Control Reproduction in the Ewe: Presence of Binding Sites and Stimulation of Luteinizing Hormone Secretion by in Situ Microimplant Delivery. Endocrinology 1998, 139, 1508–1516. [Google Scholar] [CrossRef]
- Romanowicz, K.; Misztal, T.; Gajewska, A.; Barcikowski, B. Daily GnRH and LH Secretion in Ewes Is Not Modified by Exogenous Melatonin during Seasonal Anestrus. Acta Neurobiol. Exp. 2001, 61, 289–297. [Google Scholar]
- Misztal, T.; Romanowicz, K.; Barcikowski, B. Effect of Melatonin on Daily LH Secretion in Intact and Ovariectomized Ewes during the Breeding Season. Anim. Reprod. Sci. 2002, 69, 187–198. [Google Scholar] [CrossRef]
- Herman, A.; Misztal, T.; Romanowicz, K.; Tomaszewska-Zaremba, D. Central Injection of Exogenous IL-1β in the Control Activities of Hypothalamic-Pituitary-Gonadal Axis in Anestrous Ewes: IL-1β Modulates the HPG Axis in Ewes. Reprod. Domest. Anim. 2012, 47, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.P.; Tomaszewska-Zaremba, D.; Kowalewska, M.; Szczepkowska, A.; Oleszkiewicz, M.; Krawczyńska, A.; Wójcik, M.; Antushevich, H.; Skipor, J. Neostigmine Attenuates Proinflammatory Cytokine Expression in Preoptic Area but Not Choroid Plexus during Lipopolysaccharide-Induced Systemic Inflammation. Mediat. Inflamm. 2018, 2018, 9150207. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle. Int. J. Mol. Sci. 2019, 20, 4598. [Google Scholar] [CrossRef] [Green Version]
- Taishi, P.; Bredow, S.; Guha-Thakurta, N.; Obál, F.; Krueger, J.M. Diurnal Variations of Interleukin-1β MRNA and β-Actin MRNA in Rat Brain. J. Neuroimmunol. 1997, 75, 69–74. [Google Scholar] [CrossRef]
- Ciechanowska, M.; Łapot, M.; Mateusiak, K.; Przekop, F. Neuroendocrine Regulation of GnRH Release and Expression of GnRH and GnRH Receptor Genes in the Hypothalamus-Pituitary Unit in Different Physiological States. Reprod. Biol. 2010, 10, 85–124. [Google Scholar] [CrossRef]


| GenBank Acc. No. | Gene | Amplicon Size (bp) | Forward/Reverse | Sequence 5′ → 3′ | Reference |
|---|---|---|---|---|---|
| NM_001034034 | GAPDH glyceraldehyde-3-phosphate dehydrogenase | 134 | forward | AGAAGGCTGGGGCTCACT | Herman et al. [24] |
| reverse | GGCATTGCTGACAATCTTGA | ||||
| U39357 | ACTB beta actin | 168 | forward | CTTCCTTCCTGGGCATGG | Herman et al. [24] |
| reverse | GGGCAGTGATCTCTTTCTGC | ||||
| NM_001076910 | PPIC cyclophilin C | 131 | forward | ACGGCCAAGGTCTTCTTTG | Herman et al. [24] |
| reverse | TATCCTTTCTCTCCCGTTGC | ||||
| X52488 | LHβ luteinizing hormone beta-subunit | 184 | forward | AGATGCTCCAGGGACTGCT | Herman et al. [24] |
| reverse | TGCTTCATGCTGAGGCAGTA | ||||
| NM-001009397 | GnRHR gonadotropin-releasing hormone receptor | 150 | forward | TCTTTGCTGGACCACAGTTAT | Herman et al. [24] |
| reverse | GGCAGCTGAAGGTGAAAAAG | ||||
| U02517 | GnRH gonadotropin-releasing hormone | 123 | forward | GCCCTGGAGGAAAGAGAAAT | Herman et al. [24] |
| reverse | GAGGAGAATGGGACTGGTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopycińska, K.; Wojtulewicz, K.; Herman, A.P.; Tomaszewska-Zaremba, D. The Effect of Photoperiodic Conditions on GnRH/LH Secretion in Ewes. Animals 2022, 12, 283. https://doi.org/10.3390/ani12030283
Kopycińska K, Wojtulewicz K, Herman AP, Tomaszewska-Zaremba D. The Effect of Photoperiodic Conditions on GnRH/LH Secretion in Ewes. Animals. 2022; 12(3):283. https://doi.org/10.3390/ani12030283
Chicago/Turabian StyleKopycińska, Kamila, Karolina Wojtulewicz, Andrzej Przemysław Herman, and Dorota Tomaszewska-Zaremba. 2022. "The Effect of Photoperiodic Conditions on GnRH/LH Secretion in Ewes" Animals 12, no. 3: 283. https://doi.org/10.3390/ani12030283






