Metabolite Comparison between Serum and Follicular Fluid of Dairy Cows with Inactive Ovaries Postpartum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Clinical Information
2.2. Blood Biochemical Index Detection
2.3. Sample Pre-Treatment
2.4. Detection of Serum and FF by LC/MS
2.5. Differential Screening and Statistical Analysis of Data
2.6. Correlation Analysis between DMs and Metabolic Pathway Analysis
3. Results
3.1. Clinical Information
3.2. Follicle Development Information
3.3. Serum Biochemical Indicator Levels
3.4. Analysis of Serum and FF Metabolomics
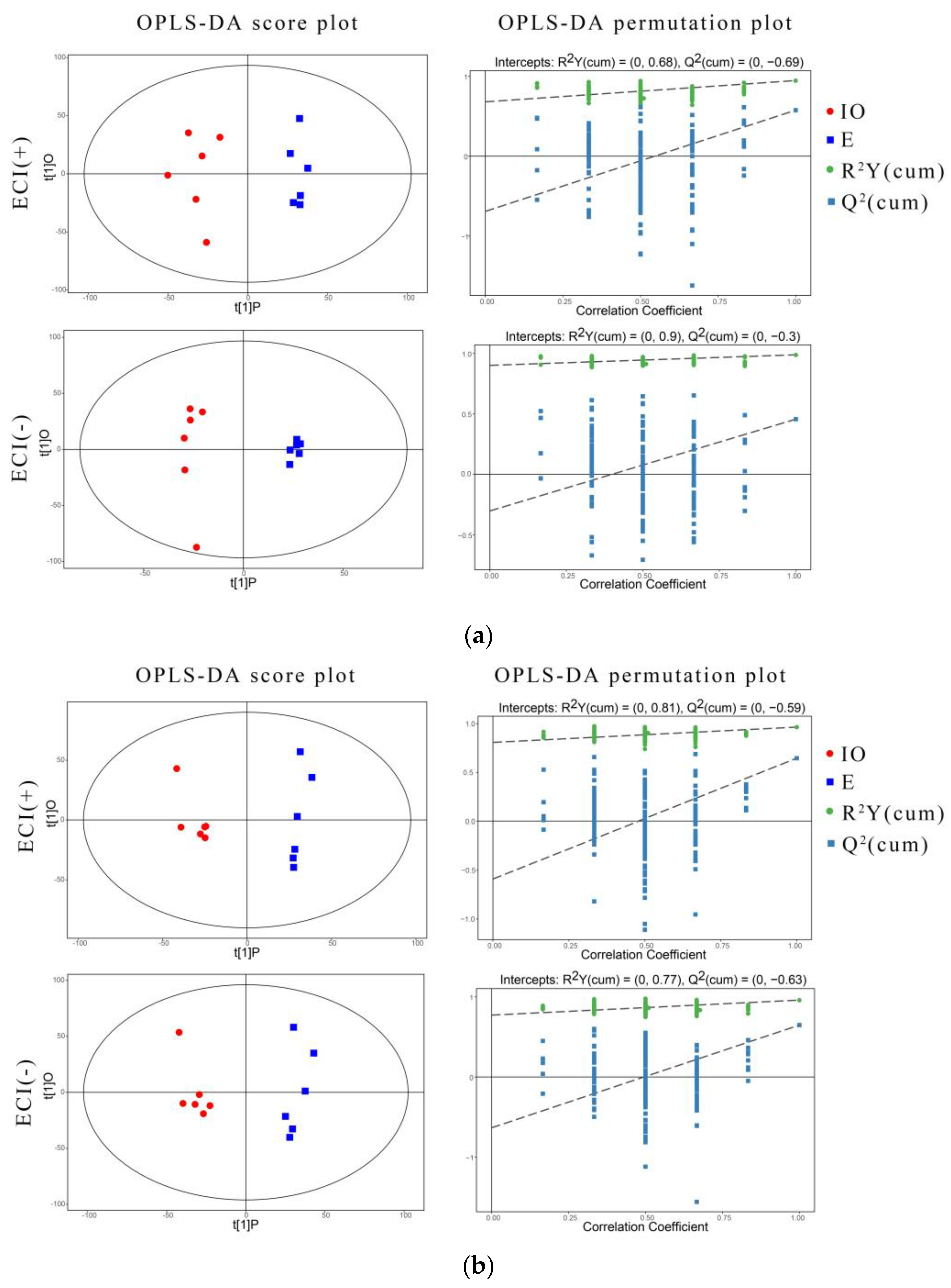
3.4.1. Orthogonal Partial Least Squares Discriminant Analysis and Permutation Test
3.4.2. DM Screening
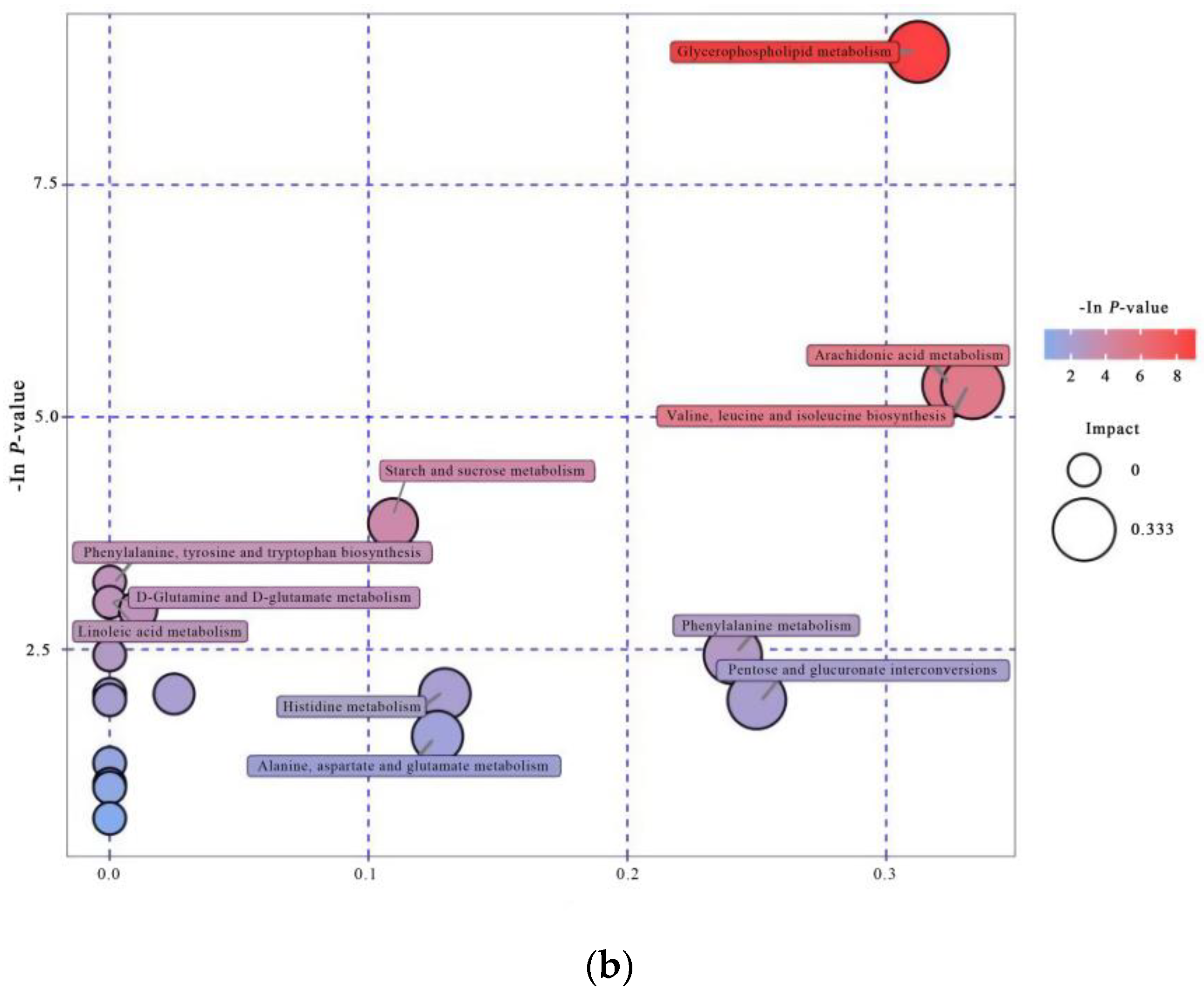
3.4.3. Pathway Analysis
4. Discussion
4.1. Changes in Serum Metabolites in IO Cows
4.2. Changes in FF Metabolites in IO Cows
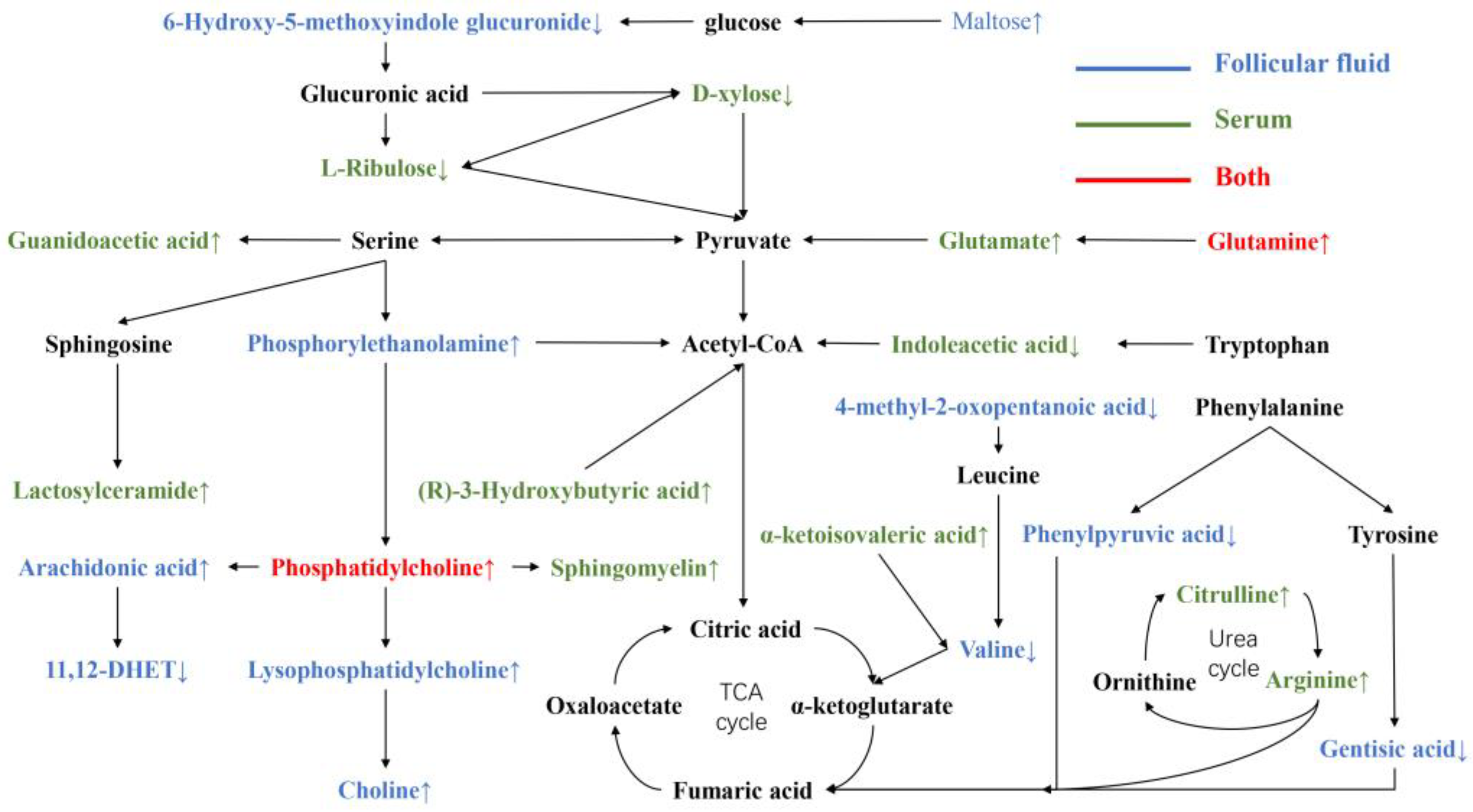
4.3. Comparison of Metabolites in Serum and FF in IO Cows
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhu, H.; Wang, Y.; Shengli, L.I.; Zhang, S.; Cao, Z. Knowledge Mapping Analysis on Global Dairy Reproduction Research. Chin. J. Anim. Vet. Sci. 2014, 45, 494–501. [Google Scholar]
- Roche, J.R.; Burke, C.R.; Meier, S.; Walker, C.G. Nutrition × reproduction interaction in pasture-based systems: Is nutrition a factor in reproductive failure? Anim. Prod. Ence. 2011, 51, 1045. [Google Scholar] [CrossRef]
- Roth, Z.; Inbar, G.; Arav, A. Comparison of oocyte developmental competence and follicular steroid content of nulliparous heifers and cows at different stages of lactation. Theriogenology 2008, 69, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.R. Energy balance relationships with follicular development, ovulation, and fertility in postpartum dairy cows. Livest. Prod. Sci. 2003, 83, 211–218. [Google Scholar] [CrossRef]
- Revelli, A.; Delle Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzer, D.; Murach, K.F.; Lottspeich, F.; Staudach, A.; Illmensee, K. Different protein patterns derived from follicular fluid of mature and immature human follicles. Hum. Reprod. 1996, 11, 798–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrazza, R.A.; Garcia, H.; Schmidt, E.; Mihm Carmichael, M.; Souza, F.F.; Burchmore, R.; Sartori, R.; Eckersall, P.D.; Ferreira, J. Quantitative proteomic profiling of bovine follicular fluid during follicle development. Biol. Reprod. 2017, 97, 835–849. [Google Scholar] [CrossRef] [Green Version]
- Schweigert, F.J.; Gericke, B.; Wolfram, W.; Kaisers, U.; Dudenhausen, J.W. Peptide and protein profiles in serum and follicular fluid of women undergoing IVF. Hum. Reprod. 2006, 21, 2960–2968. [Google Scholar] [CrossRef] [Green Version]
- Hennet, M.L.; Combelles, C.M. The antral follicle: A microenvironment for oocyte differentiation. Int. J. Dev. Biol. 2012, 56, 819–831. [Google Scholar] [CrossRef]
- Leroy, J.L.; Rizos, D.; Sturmey, R.; Bossaert, P.; Gutierrez-Adan, A.; Van Hoeck, V.; Valckx, S.; Bols, P.E. Intrafollicular conditions as a major link between maternal metabolism and oocyte quality: A focus on dairy cow fertility. Reprod. Fertil. Dev. 2011, 24, 1–12. [Google Scholar] [CrossRef]
- Van Hoeck, V.; Leroy, J.L.; Arias Alvarez, M.; Rizos, D.; Gutierrez-Adan, A.; Schnorbusch, K.; Bols, P.E.; Leese, H.J.; Sturmey, R.G. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: Mechanistic insights. Reproduction 2013, 145, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrenzycki, C.; Stinshoff, H. Maturation environment and impact on subsequent developmental competence of bovine oocytes. Reprod. Domest. Anim. 2013, 48 (Suppl. S1), 38–43. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef] [Green Version]
- Ametaj, B.N.; Wishart, D.S.; Dunn, S.M.; Saleem, F.; Mandal, R.; Hailemariam, D. Metabolomics approach reveals altered plasma amino acid and sphingolipid profiles associated with patholological state in transition dairy cows. Curr. Metab. 2014, 2, 184–195. [Google Scholar]
- NRC Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001.
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Nelson, S.T.; Haadem, C.S.; Nødtvedt, A.; Hessle, A.; Martin, A.D. Automated activity monitoring and visual observation of estrus in a herd of loose housed Hereford cattle: Diagnostic accuracy and time to ovulation. Theriogenology 2017, 87, 205–211. [Google Scholar] [CrossRef]
- Pancarcı, S.M.; Güngör, O.; Atakişi, O.; Ciğremiş, Y.; Arı, U.Ç.; Bollwein, H. Changes in follicular blood flow and nitric oxide levels in follicular fluid during follicular deviation in cows. Anim. Reprod. Sci. 2011, 123, 149–156. [Google Scholar] [CrossRef]
- Moore, S.G.; O’Gorman, A.; Brennan, L.; Fair, T.; Butler, S.T. Follicular fluid and serum metabolites in Holstein cows are predictive of genetic merit for fertility. Reprod. Fertil. Dev. 2017, 29, 658–669. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, F.; Zhang, H.; Xu, C.; Wu, L.; Xia, C. Follicular Fluid Metabolite Changes in Dairy Cows with Inactive Ovary Identified Using Untargeted Metabolomics. Biomed. Res. Int. 2020, 2020, 9837543. [Google Scholar] [CrossRef]
- Ginther, O.J. The theory of follicle selection in cattle. Domest. Anim. Endocrinol. 2016, 57, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Petersson, K.J.; Gustafsson, H.; Strandberg, E.; Berglund, B. Atypical progesterone profiles and fertility in Swedish dairy cows. J. Dairy Sci. 2006, 89, 2529–2538. [Google Scholar] [CrossRef]
- Butler, W.R.; Everett, R.W.; Coppock, C.E. The relationships between energy balance, milk production and ovulation in postpartum Holstein cows. J. Anim Sci. 1981, 53, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihsanullah; Qureshi, M.S.; Suhail, S.M.; Akhtar, S.; Khan, R.U. Postpartum endocrine activities, metabolic attributes and milk yield are influenced by thermal stress in crossbred dairy cows. Int. J. Biometeorol. 2017, 61, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Mann, S.; Nydam, D.V.; Overton, T.R.; Behling-Kelly, E. Impact of dietary plane of energy during the dry period on lipoprotein parameters in the transition period in dairy cattle. J. Anim. Physiol. Anim. Nutr. 2016, 100, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shu, S.; Bai, Y.; Wang, D.; Xia, C.; Xu, C. Plasma Protein Comparison between Dairy Cows with Inactive Ovaries and Estrus. Sci. Rep. 2019, 9, 13709. [Google Scholar] [CrossRef] [Green Version]
- Nandi, S.; Tripathi, S.K.; Gupta, P.; Mondal, S. Effect of metabolic stressors on survival and growth of in vitro cultured ovine preantral follicles and enclosed oocytes. Theriogenology 2017, 104, 80–86. [Google Scholar] [CrossRef]
- Ferreira, F.A.; Gomez, R.G.; Joaquim, D.C.; Watanabe, Y.F.; de Castro e Paula, L.A.; Binelli, M.; Rodrigues, P.H. Short-term urea feeding decreases in vitro hatching of bovine blastocysts. Theriogenology 2011, 76, 312–319.e1. [Google Scholar] [CrossRef]
- Forde, N.; O’Gorman, A.; Whelan, H.; Duffy, P.; O’Hara, L.; Kelly, A.K.; Havlicek, V.; Besenfelder, U.; Brennan, L.; Lonergan, P. Lactation-induced changes in metabolic status and follicular-fluid metabolomic profile in postpartum dairy cows. Reprod. Fertil. 2016, 28, 1882–1892. [Google Scholar] [CrossRef]
- Giallongo, F.; Harper, M.T.; Oh, J.; Lopes, J.C.; Lapierre, H.; Patton, R.A.; Parys, C.; Shinzato, I.; Hristov, A.N. Effects of rumen-protected methionine, lysine, and histidine on lactation performance of dairy cows. J. Dairy Sci. 2016, 99, 4437–4452. [Google Scholar] [CrossRef] [Green Version]
- Ardawi, M.S.; Newsholme, E.A. Glutamine, the immune system, and the intestine. J. Lab. Clin. Med. 1990, 115, 654–655. [Google Scholar] [PubMed]
- Ostojic, S.M. Benefits and drawbacks of guanidinoacetic acid as a possible treatment to replenish cerebral creatine in AGAT deficiency. Nutr. Neurosci. 2019, 22, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bai, K.; He, J.; Su, W.; Dong, L.; Zhang, L.; Wang, T. Leucine improves growth performance of intrauterine growth retardation piglets by modifying gene and protein expression related to protein synthesis. Nutrition 2016, 32, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, N.; Futamura, T.; Kakumoto, K.; Salehi, A.M.; Sellgren, C.M.; Holmén-Larsson, J.; Jakobsson, J.; Pålsson, E.; Landén, M.; Hashimoto, K. Blood metabolomics analysis identifies abnormalities in the citric acid cycle, urea cycle, and amino acid metabolism in bipolar disorder. BBA Clin. 2016, 5, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef]
- Zhao, F.F.; Wu, T.Y.; Wang, H.R.; Ding, L.Y.; Ahmed, G.; Li, H.W.; Tian, W.; Shen, Y.Z. Jugular arginine infusion relieves lipopolysaccharide-triggered inflammatory stress and improves immunity status of lactating dairy cows. J. Dairy Sci. 2018, 101, 5961–5970. [Google Scholar] [CrossRef] [Green Version]
- Luo, B.; Groenke, K.; Takors, R.; Wandrey, C.; Oldiges, M. Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. J. Chromatogr. A 2007, 1147, 153–164. [Google Scholar] [CrossRef]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef] [Green Version]
- Leng, X.; Kinnun, J.J.; Cavazos, A.T.; Canner, S.W.; Shaikh, S.R.; Feller, S.E.; Wassall, S.R. All n-3 PUFA are not the same: MD simulations reveal differences in membrane organization for EPA, DHA and DPA. Biochim. Biophys Acta Biomembr. 2018, 1860, 1125–1134. [Google Scholar] [CrossRef]
- Seeley, S.K.; Poposki, J.A.; Maksimchuk, J.; Tebbe, J.; Gaudreau, J.; Mannervik, B.; Bull, A.W. Metabolism of oxidized linoleic acid by glutathione transferases: Peroxidase activity toward 13-hydroperoxyoctadecadienoic acid. Biochim. Biophys Acta. 2006, 1760, 1064–1070. [Google Scholar] [CrossRef]
- Hui, R.; Kameda, H.; Risinger, J.I.; Angerman-Stewart, J.; Han, B.; Barrett, J.C.; Eling, T.E.; Glasgow, W.C. The linoleic acid metabolite, 13-HpODE augments the phosphorylation of EGF receptor and SHP-2 leading to their increased association. Prostaglandins Leukot. Essent. Fat. Acids. 1999, 61, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hage Hassan, R.; Bourron, O.; Hajduch, E. Defect of insulin signal in peripheral tissues: Important role of ceramide. World J. Diabetes. 2014, 5, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.C.; McGinnis, L.K.; Lawitts, J.A.; Biggers, J.D. Mouse embryo development following IVF in media containing either L-glutamine or glycyl-L-glutamine. Hum. Reprod. 2005, 20, 1364–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y.; et al. L-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016, 60, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, R.; Sørensen, P.; Zwierzchowski, L.; Snochowski, M.; Løvendahl, P. Genetic variation in stimulated GH release and in IGF-I of young dairy cattle and their associations with the leucine/valine polymorphism in the GH gene. J. Anim. Sci. 2001, 79, 470–476. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [Green Version]
- Cetica, P.; Pintos, L.; Dalvit, G.; Beconi, M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 2002, 124, 675–681. [Google Scholar] [CrossRef]
- Chatterjee, I.B. Ascorbic acid metabolism. World Rev. Nutr. Diet. 1978, 30, 69–87. [Google Scholar] [CrossRef]
- De Almeida, R.F.; Fedorov, A.; Prieto, M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: Boundaries and composition of lipid rafts. Biophys J. 2003, 85, 2406–2416. [Google Scholar] [CrossRef] [Green Version]
- Jope, R.S. High affinity choline transport and acetylCoA production in brain and their roles in the regulation of acetylcholine synthesis. Brain Res. 1979, 180, 313–344. [Google Scholar] [CrossRef]
- Conti, M. Specificity of the cyclic adenosine 3’,5’-monophosphate signal in granulosa cell function. Biol. Reprod. 2002, 67, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, K.M.; Menon, B. Structure, function and regulation of gonadotropin receptors—A perspective. Mol. Cell Endocrinol. 2012, 356, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Louhio, H.; Tuuri, T.; Sjöberg, J.; Hreinsson, J.; Telfer, E.E.; Hovatta, O. In vitro effect of cyclic adenosine 3’, 5’-monophosphate (cAMP) on early human ovarian follicles. J. Assist. Reprod. Genet. 2004, 21, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Eppig, J.J. Prostaglandin E2 stimulates cumulus expansion and hyaluronic acid synthesis by cumuli oophori isolated from mice. Biol. Reprod. 1981, 25, 191–195. [Google Scholar] [CrossRef]
- Wang, X.J.; Dyson, M.T.; Jo, Y.; Eubank, D.W.; Stocco, D.M. Involvement of 5-lipoxygenase metabolites of arachidonic acid in cyclic AMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J. Steroid. Biochem. Mol. Biol. 2003, 85, 159–166. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Luo, G.; Tang, X.; Ma, L.; Zheng, Y.; Liu, S.; Price, C.A.; Jiang, Z. Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells. Animals 2019, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, T.M.; Gonçalves, R.F.; Melo, C.; de Oliveira, D.N.; Lima, E.O.; Visintin, J.A.; de Achilles, M.A.; Catharino, R.R. A Metabolomic Overview of Follicular Fluid in Cows. Front. Vet. Sci. 2018, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Bender, K.; Walsh, S.; Evans, A.C.; Fair, T.; Brennan, L. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction 2010, 139, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, R.J.; Irving-Rodgers, H.F. Morphological classification of bovine ovarian follicles. Reproduction 2010, 139, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senbon, S.; Hirao, Y.; Miyano, T. Interactions between the oocyte and surrounding somatic cells in follicular development: Lessons from in vitro culture. J. Reprod. Dev. 2003, 49, 259–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Project | Estrus Cows (n = 6) | Inactive Ovary Cows (n = 6) |
|---|---|---|
| Age (years) | 3.37 ± 0.54 | 3.13 ± 0.92 |
| Parity | 2.33 ± 0.52 | 2.00 ± 0.89 |
| BCS 1 | 2.92 ± 0.49 | 2.67 ± 0.20 |
| Milk yield (kg/d) | 38.48 ± 3.47 | 44.00 ± 4.17 * |
| Follicular Diameter | Estrus Cows (n = 6) | Inactive Ovary Cows (n = 6) |
|---|---|---|
| 50 d postpartum (mm) | 6.67 ± 1.70 | 5.33 ± 0.56 |
| 55 d postpartum (mm) | 13.67 ± 0.71 | 7.33 ± 0.42 ** |
| Growth rate (mm/d) | 1.40 ± 0.21 | 0.40 ± 0.17 ** |
| Project | Estrus Cows (n = 6) | Inactive Ovary Cows (n = 6) |
|---|---|---|
| BHB (mmol/L) | 0.78 ± 0.37 | 1.37 ± 0.49 * |
| NEFA (mmol/L) | 0.44 ± 0.09 | 0.73 ± 0.25 * |
| Glucose (mmol/L) | 3.63 ± 0.35 | 2.99 ± 0.20 * |
| Calcium (mmol/L) | 2.07 ± 0.28 | 2.12 ± 0.23 |
| Phosphorus (mmol/L) | 1.85 ± 0.32 | 1.63 ± 0.22 |
| Magnesium (mmol/L) | 1.22 ± 0.11 | 1.20 ± 0.09 |
| ALT (U/L) | 16.83 ± 7.78 | 10.31 ± 1.21 |
| AST (U/L) | 52.67 ± 21.59 | 41.00 ± 9.70 |
| TP (g/L) | 55.05 ± 17.71 | 44.07 ± 9.41 |
| Category | ID | Metabolites | FC | p-Value b | RT (min) | VIP a | FD c | Mode |
|---|---|---|---|---|---|---|---|---|
| Serum | 1 | l-glutamine | 0.534 | 0.022 | 393.81 | 1.48 | ↑ | ESI+ |
| 2 | Guanidoacetic acid | 0.606 | 0.015 | 367.36 | 1.31 | ↑ | ESI+ | |
| 3 | Citrulline | 0.470 | 0.006 | 429.12 | 1.63 | ↑ | ESI+ | |
| 4 | l-arginine | 0.674 | 0.041 | 548.22 | 1.40 | ↑ | ESI+ | |
| 5 | l-glutamic acid | 0.412 | 0.002 | 393.74 | 1.81 | ↑ | ESI+ | |
| 6 | 4-Pyridoxic acid | 1.493 | 0.049 | 34.89 | 1.73 | ↓ | ESI− | |
| 7 | Indoleacetic acid | 5.658 | 0.040 | 149.56 | 2.47 | ↓ | ESI− | |
| 8 | Alpha-ketoisovaleric acid | 0.460 | 0.030 | 64.89 | 1.83 | ↑ | ESI− | |
| 9 | (R)-3-hydroxybutyric acid | 0.611 | 0.007 | 247.68 | 2.10 | ↑ | ESI− | |
| 10 | Lactosylceramide (d18:1/16:0) | 0.551 | 0.018 | 207.64 | 1.41 | ↑ | ESI+ | |
| 11 | Phosphatidylcholine (16:1(9Z)/16:0) | 0.539 | 0.011 | 201.46 | 1.68 | ↑ | ESI+ | |
| 12 | Sphingomyelin (d18:1/18:1(9Z)) | 0.670 | 0.039 | 200.91 | 1.34 | ↑ | ESI+ | |
| 13 | d-xylose | 1.943 | 0.025 | 154.16 | 2.00 | ↓ | ESI− | |
| 14 | l-ribulose | 2.090 | 0.009 | 315.92 | 2.52 | ↓ | ESI− | |
| FF | 1 | l-valine | 2.965 | 0.000 | 302.69 | 1.92 | ↓ | ESI− |
| 2 | l-glutamine | 0.449 | 0.034 | 406.31 | 1.47 | ↑ | ESI+ | |
| 3 | Ketoleucine | 2.364 | 0.002 | 57.88 | 1.83 | ↓ | ESI− | |
| 4 | Phenylpyruvic acid | 1.618 | 0.033 | 92.35 | 1.41 | ↓ | ESI− | |
| 5 | Gentisic acid | 2.684 | 0.023 | 61.34 | 1.44 | ↓ | ESI− | |
| 6 | Choline | 0.568 | 0.010 | 280.86 | 1.70 | ↑ | ESI+ | |
| 7 | LysoPC (14:1(9Z)) | 0.379 | 0.0070 | 211.14 | 1.85 | ↑ | ESI+ | |
| 8 | Phosphatidylcholine (22:4(7Z,10Z,13Z,16Z)/14:0) | 0.538 | 0.040 | 36.10 | 1.13 | ↑ | ESI+ | |
| 9 | PE (18:4 (6Z,9Z,12Z,15Z)/P-18:1(11Z)) | 0.303 | 0.015 | 58.74 | 1.68 | ↑ | ESI+ | |
| 10 | Arachidonic acid | 0.309 | 0.044 | 46.47 | 1.45 | ↑ | ESI− | |
| 11 | 11,12-DiHETrE | 1.824 | 0.010 | 78.28 | 1.66 | ↓ | ESI− | |
| 12 | d-maltose | 0.380 | 0.028 | 416.44 | 1.33 | ↑ | ESI− | |
| 13 | 6-Hydroxy-5-methoxyindole glucuronide | 2.920 | 0.004 | 313.81 | 1.59 | ↓ | ESI+ | |
| 14 | Urocanic acid | 0.468 | 0.020 | 322.52 | 1.73 | ↑ | ESI+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Song, Y.; Sun, S.; Zhao, C.; Fu, S.; Xia, C.; Bai, Y. Metabolite Comparison between Serum and Follicular Fluid of Dairy Cows with Inactive Ovaries Postpartum. Animals 2022, 12, 285. https://doi.org/10.3390/ani12030285
Wang Z, Song Y, Sun S, Zhao C, Fu S, Xia C, Bai Y. Metabolite Comparison between Serum and Follicular Fluid of Dairy Cows with Inactive Ovaries Postpartum. Animals. 2022; 12(3):285. https://doi.org/10.3390/ani12030285
Chicago/Turabian StyleWang, Zhijie, Yuxi Song, Shuhan Sun, Chang Zhao, Shixin Fu, Cheng Xia, and Yunlong Bai. 2022. "Metabolite Comparison between Serum and Follicular Fluid of Dairy Cows with Inactive Ovaries Postpartum" Animals 12, no. 3: 285. https://doi.org/10.3390/ani12030285






