Effects of LED Light Illumination on the Growth, Digestive Enzymes, and Photoacclimation of Goniopora columna in Captivity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Experimental Conditions
2.3. Determination of Polyp Count, Growth, and Survival Rate
2.4. Photoacclimation of G. columna
2.5. Analysis of Coral Body Composition
2.6. Analysis of Digestive Enzymes
2.7. Analysis of Zooxanthellae Density and Chlorophyll a
2.8. Statistical Analysis
3. Results
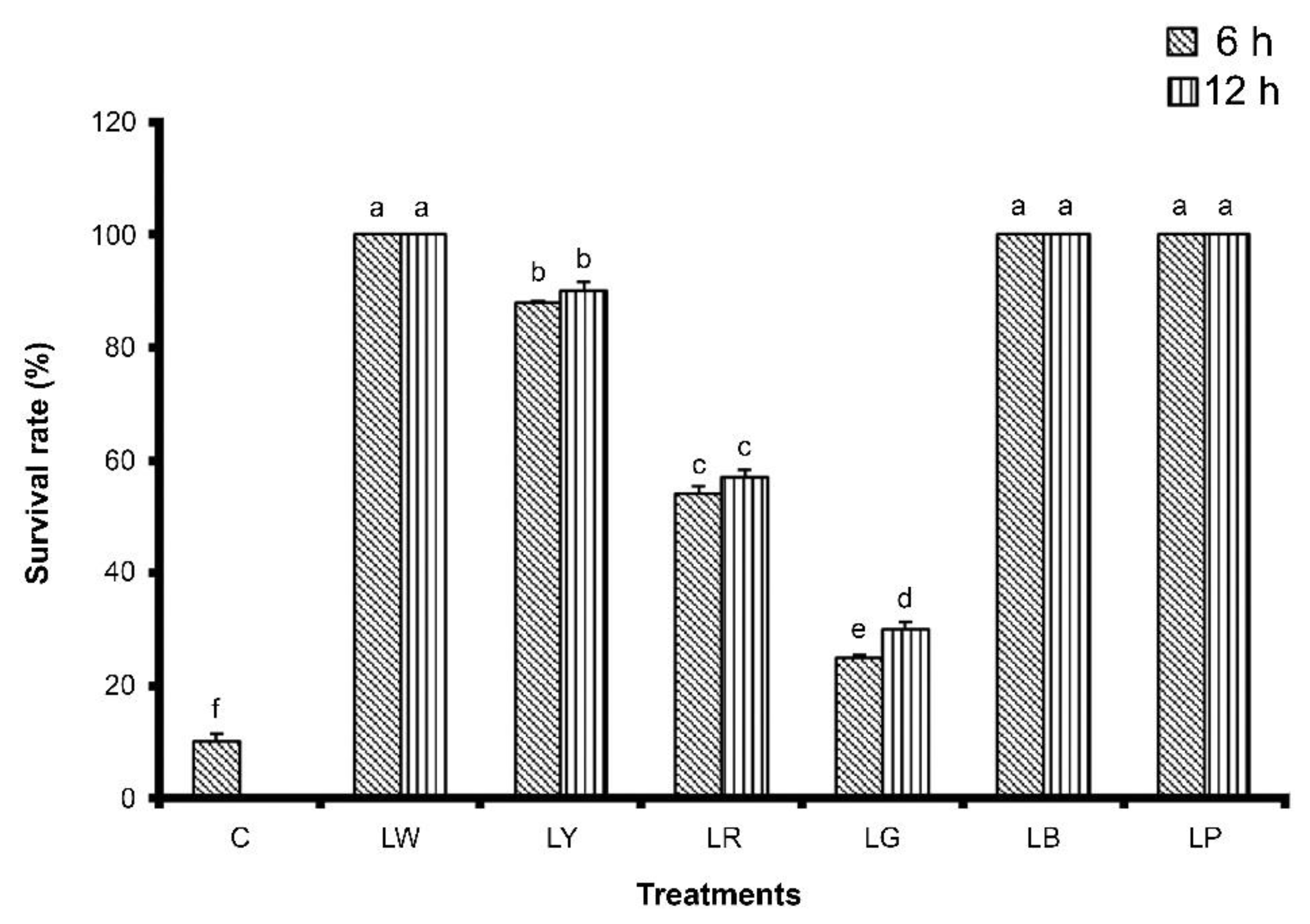
3.1. Effects of Different Types of Illumination on G. columna Growth and Survival
3.2. Effects of Different Types of Illumination on Coral Body Composition
3.3. Effects of Different Types of Illumination on Coral Digestive Enzymes
3.4. Effects of Light Wavelength on Coral Photoacclimation
3.5. Effects of Light Wavelength on PAR, Zooxanthellae Density, and Chlorophyll a Concentration
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkov, I.; Banavar, J.R.; Hubbell, S.P.; Maritan, A. Patterns of relative species abundance in rainforests and coral reefs. Nature 2007, 450, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-S.; Sun, W.-T.; Pan, C.-H. Feeding of a Scleractinian Coral, Goniopora columna, on Microalgae, Yeast, and Artificial Feed in Captivity. Animals 2021, 11, 3009. [Google Scholar] [CrossRef] [PubMed]
- Dustan, P. Depth-dependent photoadaptation by zooxanthellae of the reef coral Montastrea annularis. Mar. Biol. 1982, 68, 253–264. [Google Scholar] [CrossRef]
- Dubinsky, Z.; Falkowski, P.G.; Porter, J.W.; Muscatine, L. Absorption and Utilization of Radiant Energy by Light- and Shade-Adapted Colonies of the Hermatypic Coral Stylophora pistillata. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1984, 222, 203–214. [Google Scholar]
- Porter, J.W.; Muscatine, L.; Dubinsky, Z.; Falkowski, P.G. Primary production and photoadaptation in light- and shade-adapted colonies of the symbiotic coral, stylophora pistillata. Proc. R. Soc. Lond. B. 1984, 222, 161–180. [Google Scholar]
- Osinga, R.; Janssen, M.; Janse, M. The role of light in coral physiology and its implications for coral husbandry. In Advances in Coral Husbandry in Public Aquariums; Burgers’ Zoo: Arnhem, The Netherlands, 2008; pp. 173–183. [Google Scholar]
- Muscatine, L. The role of symbiotic algae in carbon and energy flux in reef corals. Coral Reefs 1990, 25, 75–87. [Google Scholar]
- Wijgerde, T.; van Melis, A.; Silva, C.I.; Leal, M.C.; Vogels, L.; Mutter, C.; Osinga, R. Red light represses the photophysiology of the scleractinian coral Stylophora pistillata. PLoS ONE 2014, 9, e92781. [Google Scholar] [CrossRef]
- Roth, A.A.; Clausen, C.D.; Yahiku, P.Y.; Clausen, V.E.; Cox, W.W. Some Effects of Light on Coral Growth. Pac. Sci. 1982, 36, 65–81. [Google Scholar]
- Levy, O.; Dubinsky, Z.; Achituv, Y. Photobehavior of stony corals: Responses to light spectra and intensity. J. Exp. Biol. 2003, 206, 4041–4049. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.J.; Shaw, C.; Turner, J.R. Factors controlling the tentacle and polyp expansion behaviour of selected temperate Anthozoa. J. Mar. Biol. Assoc. UK 2006, 86, 977–992. [Google Scholar] [CrossRef]
- Sebens, K.P.; DeRiemer, K. Diel cycles of expansion and contraction in coral reef anthozoans. Mar. Biol. 1997, 43, 247–256. [Google Scholar] [CrossRef]
- Lewis, J.B.; Price, W.S. Feeding mechanisms and feeding strategies of Atlantic reef corals. J. Zool. 1975, 176, 527–544. [Google Scholar] [CrossRef]
- Levy, O.; Mizrahi, L.; Chadwick, N. Factors Controlling the Expansion Behavior of Favia favus (Cnidaria: Scleractinia): Effects of Light, Flow, and Planktonic Prey. Biol. Bull. 2001, 200, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Lasker, H.R. Sediment rejection by reef corals: The roles of behavior and morphology in Montastrea cavernosa (Linnaeus). J. Exp. Mar. Biol. Ecol. 1980, 47, 77–87. [Google Scholar] [CrossRef]
- Stafford-Smith, M.G.; Ormond, R.F.G. Sediment-rejection mechanisms of 42 species of Australian scleractinian corals. Aust. J. Mar. Freshw. Res. 1992, 43, 638–705. [Google Scholar] [CrossRef]
- Lesser, M.P.; Shick, J.M. Effects of irradiance and ultraviolet radiation on photoadaptation in the zooxanthellae of Aiptasia pallida: Primary production, photoinhibition, and enzymic defenses against oxygen toxicity. Mar. Biol. 1989, 102, 243–255. [Google Scholar] [CrossRef]
- Shick, J.M. Diffusion Limitation and Hyperoxic Enhancement of Oxygen Consumption in Zooxanthellate Sea Anemones, Zoanthids, and Corals. Biol. Bull. 1990, 179, 148–158. [Google Scholar] [CrossRef]
- Enríquez, S.; Méndez, E.R.; -Prieto, R.I. Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol. Oceanogr. 2005, 50, 1025–1032. [Google Scholar] [CrossRef]
- Enríquez, S.; Méndez, E.R.; Hoegh-Guldberg, O.; Iglesias-Prieto, R. Key functional role of the optical properties of coral skeletons in coral ecology and evolution. Proc. R. Soc. B Biol. Sci. 2017, 284, 20161667. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, P.G.; Dubinsky, Z.; Muscatine, L.; Porter, J.W. Light and the bioenergetics of a symbiotic coral. Bioscience 1984, 34, 705–709. [Google Scholar] [CrossRef]
- Anthony, K.R.; Connolly, S.R.; Hoegh-Guldberg, O. Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnol. Oceanogr. 2007, 52, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Lundstedt, L.; Melo, J.; Moraes, G. Digestive enzymes and metabolic profile of Pseudoplatystoma corruscans (Teleostei: Siluriformes) in response to diet composition. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 137, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Raz-Bahat, M.; Douek, J.; Moiseeva, E.; Peters, E.C.; Rinkevich, B. The digestive system of the stony coral Stylophora pistillata. Cell Tissue Res. 2017, 368, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Xie, S.; Lei, W.; Zhu, X.; Yang, Y. Effect of light intensity on growth, survival and skin color of juvenile Chinese longsnout catfish (Leiocassis longirostris Gunther). Aquaculture 2005, 248, 299–306. [Google Scholar] [CrossRef]
- Hancz, C.; Romvári, R.; Szabó, A.; Molnár, T.; Magyary, I.; Horn, P. Measurement of total body composition changes of common carp by computer tomography. Aquac. Res. 2003, 34, 991–997. [Google Scholar] [CrossRef]
- Zeng, N.N.; Jiang, M.; Wen, H.; Liu, W.; Wu, F.; Tian, J.; Guo, Z.B. Effects of water temperatures and dietary protein levels on growth, body composition and blood biochemistry of juvenile GIFT tilapia (Oreochromis niloticus). Aquac. Nutr. 2020, 27, 240–251. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Stark, J.; Fischer, A.B. Evaluation of artificial light regimes and substrate types for aquaria propagation of the staghorn coral Acropora solitaryensis. Aquaculture 2007, 269, 278–289. [Google Scholar] [CrossRef]
- Bhandari, R.R.; Sharma, P.K. UV-B radiation and high light induced oxidative damage in Phormidium corium may cause bleaching to associated coral reefs. Indian J. Mar. Sci. 2010, 39, 423–428. [Google Scholar]
- Tilstra, A.; Wijgerde, T.; Dini-Andreote, F.; Eriksson, B.K.; Salles, J.F.; Pen, I.; Osinga, R.; Wild, C. Light induced intraspecific variability in response to thermal stress in the hard coral Stylophora pistillata. Peer J. 2017, 5, e3802. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.; Pimentel, T.; Serôdio, J.; Rosa, R.; Calado, R. Comparative performance of light emitting plasma (LEP) and light emitting diode (LED) in ex situ aquaculture of scleractinian corals. Aquaculture 2013, S402–S403, 38–45. [Google Scholar] [CrossRef]
- Schutter, M.; van Velthoven, B.; Janse, M.; Osinga, R.; Janssen, M.; Wijffels, R.; Verreth, J. The effect of irradiance on long-term skeletal growth and net photosynthesis in Galaxea fascicularis under four light conditions. J. Exp. Mar. Biol. Ecol. 2008, 367, 75–80. [Google Scholar] [CrossRef]
- Sujirachato, P.; Thamrongnawasawat, T.; Thongtham, N.; Jantrarotai, P.; Worachananant, S. Survival rate of coral fragments transplanted by different methods. Galaxea J. Coral Reef Stud. 2013, 15, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Agricultural Chemists. Official Methods for the Analysis, 14th ed.; AOAC: Arlington, VA, USA, 1984. [Google Scholar]
- Bishop, M. Clinical Chemistry: Principles, Techniques, and Correlations; Jones & Bartlett Publishers: Burlington, MA, USA, 2020. [Google Scholar]
- Tietz, N.W.; Berger, S. Fundamentals of Clinical Chemistry; Saunders: Philadelphia, PA, USA, 1976. [Google Scholar]
- Sun, J.-Y.; Du, J.; Qian, L.-C.; Jing, M.-Y.; Weng, X.-Y. Distribution and characteristics of endogenous digestive enzymes in the red-eared slider turtle, Trachemys scripta elegans. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 147, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, I.G. Studies on the digestive lipases of milkfish, Chanos chanos. Aquaculture 1990, 89, 315–325. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylases, α and β. Methods Enzymol. 1955, 1, 149–158. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Yamazato, K.; van Woesik, R. Photo-acclimation dynamics of the coral Stylophora pistillata to low and extremely low light. J. Exp. Mar. Biol. Ecol. 2001, 263, 211–225. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1957, 167, 191–194. [Google Scholar] [CrossRef]
- Maragos, J.E. A Study of the Ecology of Hawaiian Reef Corals. Ph.D. Thesis, University of Hawaii, Honolulu, HI, USA, 1974. [Google Scholar]
- Fabricius, K.E.; Mieog, J.C.; Colin, P.L.; Idip, D.; Van Oppen, M.J.H. Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol. Ecol. 2004, 13, 2445–2458. [Google Scholar] [CrossRef]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Jones, R. Impacts of light limitation on corals and crustose coralline algae. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Guest, J.; Todd, P.; Goh, B.; Chou, L. The effect of transplantation on reproduction in clonal ramets of Goniopora columna on Singapore’s coral reefs. Invertebr. Reprod. Dev. 2007, 50, 133–138. [Google Scholar] [CrossRef]
- Robbins, R.; Shick, J. Expansion-contraction behavior in the sea anemone Metridium senile: Environmental cues and energetic consequences. In Nutrition in the Lower Metazoa; Elsevier: Amsterdam, The Netherlands, 1980; pp. 101–116. [Google Scholar]
- Furla, P.; Galgani, I.; Durand, I.; Allemand, D. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 2000, 203, 3445–3457. [Google Scholar] [CrossRef] [PubMed]
- Ferrier-Pages, C.; Gattuso, J.-P.; Jaubert, J. Effect of small variations in salinity on the rates of photosynthesis and respiration of the zooxanthellate coral Stylophora pistillata. Mar. Ecol. Prog. Ser. 1999, 181, 309–314. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Allemand, D.; Gattuso, J.-P.; Jaubert, J.; Rassoulzadegan, F. Microheterotrophy in the zooxanthellate coral Stylophora pistillata: Effect of light and prey density. Limnol. Oceanogr. 1998, 43, 1639–1648. [Google Scholar] [CrossRef]
- Clayton, W.S.; Lasker, H.R. Effects of light and dark treatments on feeding by the reef coral Pocillopora damicornis (Linnaeus). J. Exp. Mar. Biol. Ecol. 1982, 63, 269–279. [Google Scholar] [CrossRef]
- Hii, Y.-S.; Soo, C.-L.; Liew, H.-C. Feeding of scleractinian coral, Galaxea fascicularis, on Artemia salina nauplii in captivity. Aquac. Int. Vol. 2009, 17, 363–376. [Google Scholar] [CrossRef]
- Boschma, H. On the feeding reactions and digestion in the coral polyp Astrangia danae, with notes on its symbiosis with zooxanthellae. Biol. Bull. 1925, 49, 407–439. [Google Scholar] [CrossRef]
- Bumann, D. Localization of digestion activities in the sea anemone Haliplanella luciae. Biol. Bull. 1995, 189, 236–237. [Google Scholar] [CrossRef]
- Tsai, C.-H. Effects of Light Intensity on the Morphology and Physiology of the Soft Coral (Pachyclavularia Violacea); NSYSU: Kaohsiung, Taiwan, 2005. [Google Scholar]
- Caruso, G.; Denaro, M.G.; Genovese, L. Digestive Enzymes in Some Teleost Species of Interest for Mediterranean Aquaculture. Open Fish Sci. J. 2009, 2, 74–86. [Google Scholar] [CrossRef]
- Yang, S.; Du, J.; Duan, Y.; Xiao, Q.; Li, N.; Lin, Q.; Zhao, L.; Du, Z.; Zhou, J.; Du, J. Differences in the digestive enzyme activity, intestinal mucosa and microbial community in loach cultivated in two separate environments. BMC Microbiol. 2018, 18, 113. [Google Scholar] [CrossRef] [Green Version]
- Levy, O.; Appelbaum, L.; Leggat, W.; Gothlif, Y.; Hayward, D.C.; Miller, D.J.; Hoegh-Guldberg, O. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science 2007, 318, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Exposure Times | Light | Wavelength of Light (nm) | Photosynthetically Active Radiation (μmol m−2s−1) |
|---|---|---|---|
| 0 h | C | Dark | 0 |
| 6 h | LW | 444–696 | 102.23 ± 1.01 |
| LY | 570–590 | 53.63 ± 0.32 | |
| LR | 620–650 | 44.05 ± 1.01 | |
| LG | 500–540 | 46.35 ± 0.75 | |
| LB | 440–470 | 66.67 ± 0.38 | |
| LP | 400–430 | 71.03 ± 0.21 | |
| 12 h | LW | 444–696 | 100.58 ± 0.73 |
| LY | 570–590 | 54.02 ± 0.57 | |
| LR | 620–650 | 43.98 ± 1.31 | |
| LG | 500–540 | 48.49 ± 1.54 | |
| LB | 440–470 | 68.03 ± 0.42 | |
| LP | 400–430 | 70.49 ± 0.44 |
| 6 h | 12 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WaterQuality Conditions | C | LW | LY | LG | LR | LB | LP | LW | LY | LG | LR | LB | LP |
| Temperature (°C) | 26.23 ± 0.73 | 26.15 ± 0.23 | 26.23 ± 0.71 | 26.41 ± 0.52 | 26.22 ± 0.25 | 26.41 ± 0.32 | 26.04 ± 0.43 | 26.91 ± 0.33 | 26.48 ± 0.64 | 26.32 ± 0.31 | 26.93 ± 0.34 | 26.63 ± 0.30 | 26.42 ± 0.21 |
| Salinity (PSU) | 35.12 ± 0.42 | 35.50 ± 0.31 | 35.24 ± 0.12 | 35.42 ± 0.38 | 34.82 ± 0.92 | 35.41 ± 0.91 | 35.73 ± 0.52 | 35.25 ± 0.49 | 35.33 ± 0.23 | 34.41 ± 0.52 | 35.23 ± 0.21 | 35.02 ± 0.38 | 34.94 ± 0.32 |
| pH | 8.01 ± 0.41 | 8.05 ± 0.29 | 8.28 ± 0.32 | 8.03 ± 0.31 | 8.32 ± 0.91 | 8.21 ± 0.42 | 8.01 ± 0.21 | 8.26 ± 0.31 | 8.03 ± 0.33 | 8.02 ± 0.48 | 8.14 ± 0.93 | 8.03 ± 0.31 | 8.32 ± 0.53 |
| Ammonia nitrogen (mg/L) | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.05 | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 |
| Nitrous acid (mg/L) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| Nitric acid (PPM) | 0.15 ± 0.04 | 0.15 ± 0.04 | 0.21 ± 0.02 | 0.19 ± 0.02 | 0.09 ± 0.03 | 0.20 ± 0.03 | 0.21 ± 0.02 | 0.22 ± 0.05 | 0.21 ± 0.02 | 0.18 ± 0.02 | 0.20 ± 0.04 | 0.19 ± 0.03 | 0.19 ± 0.02 |
| Calcium (PPM) | 400 ± 43.21 | 410 ± 43.21 | 410 ± 15.27 | 408 ± 18.21 | 403 ± 21.32 | 415 ± 17.62 | 412 ± 9.01 | 432 ± 32.53 | 421 ± 14.42 | 410 ± 31.21 | 408 ± 31.31 | 414 ± 3.21 | 422 ± 17.21 |
| Magnesium (PPM) | 1280 ± 54.42 | 1280 ± 54.42 | 1289 ± 32.12 | 1300 ± 21.32 | 1281 ± 15.21 | 1321 ± 21.21 | 1332 ± 21.37 | 1321 ± 22.01 | 1298 ± 31.26 | 1320 ± 11.23 | 1320 ± 10.32 | 1318 ± 11.35 | 1299 ± 9.32 |
| Phosphate (PPM) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Stretching and Contractile | Stretching and Contractile Behaviors | Score |
|---|---|---|
 | Complete contraction | 0 |
 | When the polyps were elongated slightly | 1 |
 | When some polyps, but less than half in total (50%), were extended | 2 |
 | When most polyps, more than half in total (75%), were extended | 3 |
 | When all polyps were fully extended (100%) | 4 |
| Nutritional Indicators | Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | LW | LY | LR | LG | LB | LP | |||||||
| 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | ||
| Protein (µg) | 226.44 ± 7.08 f | 366.95 ± 7.00 c | 372.13 ± 1.21 c | 357.21 ± 6.70 d | 343.25 ± 3.40 d | 224.12 ± 4.42 f | 234.10 ± 3.74 f | 263.99 ± 7.32 e | 276.32 ± 6.24 e | 390.06 ± 3.38 b | 395.06 ± 3.09 b | 426.61 ± 6.42 a | 421.11 ± 2.32 a |
| Lipid (µg) | 1.59 ± 0.21 | 1.39 ± 0.47 | 1.35 ± 0.16 | 1.51 ± 0.12 | 1.32 ± 0.35 | 0.98 ± 0.34 | 1.03 ± 0.32 | 1.29 ± 0.18 | 1.45 ± 0.12 | 1.64 ± 0.27 | 1.35 ± 0.15 | 1.66 ± 0.66 | 1.43 ± 0.25 |
| Glucose (µg) | 1.30 ± 0.21 | 1.37 ± 0.58 | 1.28 ± 0.24 | 1.32 ± 0.41 | 1.28 ± 0.34 | 1.33 ± 0.37 | 1.22 ± 0.26 | 1.39 ± 0.22 | 1.28 ± 0.13 | 1.34 ± 0.17 | 1.48 ± 0.14 | 1.58 ± 0.19 | 1.59 ± 0.12 |
| Treatments | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test Items | C | LW | LY | LR | LG | LB | LP | ||||||
| 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | 6 h | 12 h | ||
| Protease (U/mg protein) | 58.55 ± 5.83 g | 169.04 ± 4.11 c | 197.32 ± 3.42 b | 155.20 ± 8.45 d | 159.12 ± 5.24 d | 97.27 ± 2.49 f | 98.21 ± 1.89 f | 114.01 ± 2.56 e | 109.01 ± 3.54 e | 191.65 ± 3.91 b | 184.02 ± 5.38 b | 231.37 ± 9.00 a | 237.21 ± 5.40 a |
| Lipase (U/mg protein) | 7.53 ± 0.85 d | 9.77 ± 0.58 c | 9.85 ± 0.67 c | 9.05 ± 0.37 c | 8.37 ± 0.45 d | 6.73 ± 0.56 e | 7.21 ± 0.49 e | 6.04 ± 1.36 e | 5.03 ± 1.42 e | 11.90 ± 1.00 b | 9.53 ± 0.87 c | 12.05 ± 1.74 ab | 14.24 ± 1.31 a |
| Amylase (U/mg protein) | 1.77 ± 0.12 | 1.95 ± 0.32 | 1.89 ± 0.17 | 1.87 ± 0.12 | 1.96 ± 0.43 | 1.73 ± 0.14 | 1.84 ± 0.43 | 1.54 ± 0.16 | 1.65 ± 0.12 | 1.93 ± 0.38 | 1.57 ± 0.24 | 1.90 ± 0.26 | 2.03 ± 0.19 |
| Exposure Times | Treatments | Zooxanthellae (Cells × 107 m−2) | Chlorophyll a (µg cm−2) |
|---|---|---|---|
| 0 h | C | 0.3 ± 0.13 b | 14 ± 1.33 b |
| 6 h | LW | 3.9 ± 1.74 a | 52 ± 3.72 a |
| LY | 3.9 ± 0.73 a | 51 ± 2.53 a | |
| LR | 3.9 ± 1.18 a | 51 ± 4.52 a | |
| LG | 3.9 ± 1.02 a | 50 ± 2.34 a | |
| LB | 4.0 ± 1.32 a | 52 ± 4.21 a | |
| LP | 4.0 ± 0.81 a | 52 ± 3.42 a | |
| 12 h | LW | 4.0 ± 2.14 a | 53 ± 5.21 a |
| LY | 3.9 ± 2.13 a | 51 ± 3.04 a | |
| LR | 3.9 ± 2.51 a | 51 ± 4.01 a | |
| LG | 3.9 ± 3.23 a | 50 ± 5.02 a | |
| LB | 4.0 ± 2.64 a | 53 ± 3.84 a | |
| LP | 4.0 ± 1.92 a | 53 ± 3.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-M.; Cheng, Y.-R.; Lin, H.-Y.; Sun, W.-T.; Pan, C.-H.; Ding, D.-S. Effects of LED Light Illumination on the Growth, Digestive Enzymes, and Photoacclimation of Goniopora columna in Captivity. Animals 2022, 12, 306. https://doi.org/10.3390/ani12030306
Cheng C-M, Cheng Y-R, Lin H-Y, Sun W-T, Pan C-H, Ding D-S. Effects of LED Light Illumination on the Growth, Digestive Enzymes, and Photoacclimation of Goniopora columna in Captivity. Animals. 2022; 12(3):306. https://doi.org/10.3390/ani12030306
Chicago/Turabian StyleCheng, Chiu-Min, Yu-Rong Cheng, Hsuan-Yu Lin, Wei-Ting Sun, Chih-Hung Pan, and De-Sing Ding. 2022. "Effects of LED Light Illumination on the Growth, Digestive Enzymes, and Photoacclimation of Goniopora columna in Captivity" Animals 12, no. 3: 306. https://doi.org/10.3390/ani12030306
APA StyleCheng, C.-M., Cheng, Y.-R., Lin, H.-Y., Sun, W.-T., Pan, C.-H., & Ding, D.-S. (2022). Effects of LED Light Illumination on the Growth, Digestive Enzymes, and Photoacclimation of Goniopora columna in Captivity. Animals, 12(3), 306. https://doi.org/10.3390/ani12030306






