Rumen Fermentation of Feed Mixtures Supplemented with Clay Minerals in a Semicontinuous In Vitro System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Chemical and Microbiological Analyses
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angulo, E.; Brufau, J.; Esteve-Garcia, E. Effect of sepiolite product on pellet durability in pig diets differing in particle size and in broiler starter and finisher diets. Anim. Feed Sci. Technol. 1996, 63, 25–34. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxic. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadziakiewicza, M.; Kehoe, S.; Micek, P. Physico-chemical properties of clay minerals and their use as a health promoting feed additive. Animals 2019, 9, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, L.J.; Mackay, V.G. The investigation of sodium bicarbonate or bentonite as supplements in silages fed to lactating cows. Can. J. Anim. Sci. 1983, 63, 939–947. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Eun, J.S.; Young, A.J.; Stott, R.D.; Peterson, S. Effects of supplementation of natural zeolite on intake, digestion, ruminal fermentation, and lactational performance of dairy cows. Prof. Anim. Sci. 2010, 26, 647–654. [Google Scholar] [CrossRef]
- Sulzberger, S.; Kalebich, C.C.; Melnichenko, S.; Cardoso, F.C. Effects of clay after a grain challenge on milk composition, and on ruminal, blood and faecal pH in Holstein cows. J. Dairy Sci. 2016, 99, 8028–8040. [Google Scholar] [CrossRef] [Green Version]
- Karatzia, M.A.; Pourliotis, K.; Katsoulos, P.D.; Karatzias, H. Effects of in-feed inclusion of clinoptilolite on blood serum concentrations of aluminum and inorganic phosphorus and on ruminal pH and volatile fatty acid concentrations in dairy cows. Biol. Trace Elem. Res. 2011, 42, 159–166. [Google Scholar] [CrossRef]
- Ural, D.A.; Cengiz, O.; Ural, K.; Ozaydinl, S. Dietary clinoptilolite addition as a factor for the improvement of milk yield in dairy cows. J. Anim. Vet. Adv. 2013, 12, 140–145. [Google Scholar]
- Khachlouf, K.; Hamed, H.; Gdoura, R.; Gargouri, A. Effects of zeolite supplementation on dairy cow production and ruminal parameters—A review. Ann. Anim. Sci. 2018, 18, 857–877. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.A.; Sweeney, T.F.; Muller, L.D. Effects of feeding synthetic zeolite A and sodium bicarbonate on milk production nutrient digestion, and rate of digesta passage in dairy cows. J. Dairy Sci. 1988, 71, 946–953. [Google Scholar] [CrossRef]
- Bosi, P.; Creston, D.; Casin, L. Production performance of dairy cows after the dietary addition of clinoptilolite. Ital. J. Anim. Sci. 2002, 1, 187–195. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Scientific Opinion on the safety and efficacy of a preparation of bentonite-and sepiolite (Toxfin ® Dry) as feed additive for all species. EFSA J. 2013, 11, 3179. [Google Scholar] [CrossRef]
- Ivan, M.; Dayrell, M.D.; Mahadevan, S.; Hidiroglou, M. Effects of bentonite on wool growth and nitrogen metabolism in fauna-free and faunated sheep. J. Anim. Sci. 1992, 70, 3194–3202. [Google Scholar] [CrossRef]
- Schlattl, M.; Buffler, M.; Windisch, W. Clay minerals affect the solubility of Zn and other bivalent cations in the digestive tract of ruminants in vitro. Animals 2021, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Rindsig, R.B.; Schultz, L.H. Effect of bentonite on nitrogen and mineral balances and ration digestibility of high-grain dairy rations fed to lactating dairy cows. J. Dairy Sci. 1970, 53, 888–892. [Google Scholar] [CrossRef]
- Burçak, E.; Yalçin, S. Effects of dietary sepiolite usage on performance, carcass characteristics, blood parameters and rumen fluid metabolites in Merino cross breed lambs. Appl. Clay Sci. 2018, 163, 291–298. [Google Scholar] [CrossRef]
- Hamilton, B.A.; Carmichael, A.W.; Kempton, T.J. Effect on milk production of adding bentonite and reactive limestone to maize grain supplements for grazing cows. Aust. J. Exp. Agric. 1988, 28, 25–28. [Google Scholar] [CrossRef]
- Tucker, W.B.; Shin, I.S.; Hogue, J.F.; Aslam, M.; Adams, G.D.; Van Koevering, M.T.; Vernon, R.K.; Cummings, K.R. Natural sodium sesquicarbonate fed for an entire lactation: Influence on performance and acid-base status of dairy cows. J. Dairy Sci. 1994, 77, 3111–3117. [Google Scholar] [CrossRef]
- Clark, J.H.; Christensen, R.A.; Bateman, H.G.; Cummings, K.R. Effects of sodium sesquicarbonate on dry matter intake and production of milk and milk components by Holstein cows. J. Dairy Sci. 2009, 92, 3354–3363. [Google Scholar] [CrossRef] [Green Version]
- Wolter, R.; Dunoyer, C.; Henry, N.; Seegmuller, N. Les argiles en alimentation animale: Interet general. Recl. Med. Vet. 1990, 166, 21–27. [Google Scholar]
- Rodriguez-Beltrán, J.; Rodriguez-Rojas, A.; Yubero, E.; Blázquez, J. The animal food supplement sepiolite promotes a direct horizontal transfer of antibiotic resistance plasmids between bacterial species. Antimicrob. Agents Chemother. 2013, 57, 2651–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonty, G.; Jouany, J.P.; Forano, E.; Gouet, P.H. Nutrition Des Ruminants Domestiques: L’écosystème Microbien du Réticulo Rumen; INRA, Ed.; Route de Saint Cyr: Paris, France, 1995. [Google Scholar]
- Elitok, B.; Guvlu, S. Investigation on effects of orally given sepiolite on ruminal protozoa in cattle. Res. Med. Health Sci. 2017, 4, 163–173. [Google Scholar]
- Ivan, M.; Dayrell, d.S.; Hidiroglou, M. Effects of bentonite and monensin on selected elements in the stomach and liver of fauna-free and faunated sheep. J. Dairy Sci. 1992, 75, 201–208. [Google Scholar] [CrossRef]
- Jouany, J.P.; Morgavi, D.P. Use of natural products as alternatives to antibiotic feed additives in ruminant production. Animal 2007, 1, 1443–1466. [Google Scholar] [CrossRef] [Green Version]
- Fondevila, M.; Pérez-Espés, B. A new in vitro system to study the effect of liquid phase turnover and pH on microbial fermentation of concentrate diets for ruminants. Anim. Feed Sci. Technol. 2008, 144, 196–211. [Google Scholar] [CrossRef]
- Prates, A.; de Oliveira, J.A.; Abecia, L.; Fondevila, M. Effects of preservation procedures of rumen inoculum on in vitro microbial diversity and fermentation. Anim. Feed Sci. Technol. 2010, 155, 186–193. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Amanzougarene, Z.; Fondevila, M. Fitting of pH conditions for the study of concentrate feeds fermentation by the in vitro gas production technique. Anim. Prod. Sci. 2018, 58, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Qian, D.; Tang, Y.; Qian, Y.; Wu, D.; Su, S.; Shang, E. Rapid determination of amino acid in fruits of Ziziphus jujuba by hydrophilic interaction Ultra-High Performance Liquid Chromatography coupled with Triple-Quadruple Mass Spectrometry. J. Agric. Food Chem. 2013, 61, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orue, S.M.; Balcells, J.; Zakraoui, F.; Castrillo, C. Quantification and chemical composition of mixed bacteria harvested from solid fractions of rumen digesta: Effect of detachment procedure. Anim. Feed Sci. Technol. 1998, 71, 269–282. [Google Scholar] [CrossRef]
- Analytical Software; Statistix 10 for Windows; Analytical Software: Tallahasee, FL, USA, 2010.
- Meschy, F.; Bravo, D.; Sauvant, D. Analyse quantitative des réponses des vaches laitières à l’apport de substances tampon. INRA Prod. Anim. 2004, 17, 11–18. [Google Scholar] [CrossRef]
- Ouhida, I.; Pérez, J.F.; Piedrafita, J.; Gasa, J. The effect of sepiolite in broiler chicken diets of high, medium and low viscosity. Productive performance and nutritive value. Anim. Feed Sci. Technol. 2000, 85, 183–194. [Google Scholar] [CrossRef]
- Xia, M.S.; Hu, C.H.; Xu, Z.R. Effects of copper bearing montmorillonite on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed Sci. Technol. 2005, 118, 307–317. [Google Scholar] [CrossRef]
- Yong, R.N.; Warkentin, D.P.; Phadungchewit, Y.; Galve, R. Buffer capacity and lead retention in some clay materials. Water Air Soil Pollut. 1990, 53, 53–67. [Google Scholar] [CrossRef]
- Giger-Reverdin, S.; Duvaux-Ponter, C.; Sauvant, D.; Martin, O.; Nunes, I.; Müller, R. Intrinsic buffering capacity of feeds. Anim. Feed Sci. Technol. 2002, 96, 83–102. [Google Scholar] [CrossRef]
- Bath, D.L.; Bishop, S.E.; Peterson, N.G.; Hight, W.B.; de Peters, E.J. Response in two commercial Holstein herds to addition of sodium bicarbonate to alfalfa hay-based diets. J. Dairy Sci. 1985, 68, 1835–1840. [Google Scholar] [CrossRef]
- Eng, K.S.; Bechtel, R.; Hutcheson, D. The use of Biolite (a calcium clinoptilolite zeolite) in diets for natural beef production. In Zeolite 06; Bowman, R.S., Delap, S.E., Eds.; New Mexico Institute of Mining and Technology: Socorro, NM, USA, 2006; p. 29. [Google Scholar]
- Grabherr, H.; Spolders, M.; Lebzien, P.; Huther, L.; Flachowsky, G.; Fürll, M.; Grun, M. Effect of zeolite A on rumen fermentation and phosphorus metabolism in dairy cows. Arch. Anim. Nutr. 2009, 63, 321–336. [Google Scholar] [CrossRef]
- McCollum, E.T.; Galyean, M.I. Effects of clinoptilolite on rumen fermentation, digestion and feedlot performance in beef steers fed high concentrate diets. J. Dairy Sci. 1983, 56, 517–524. [Google Scholar]
- Kihal, A.; Rodríguez-Prado, M.; Godoy, C.; Cristofol, C.; Calsamiglia, S. In vitro assessment of the capacity of certain micotoxin binders to absorb some aminoacids and water-soluble proteins. J. Dairy Sci. 2020, 103, 3125–3132. [Google Scholar] [CrossRef] [PubMed]
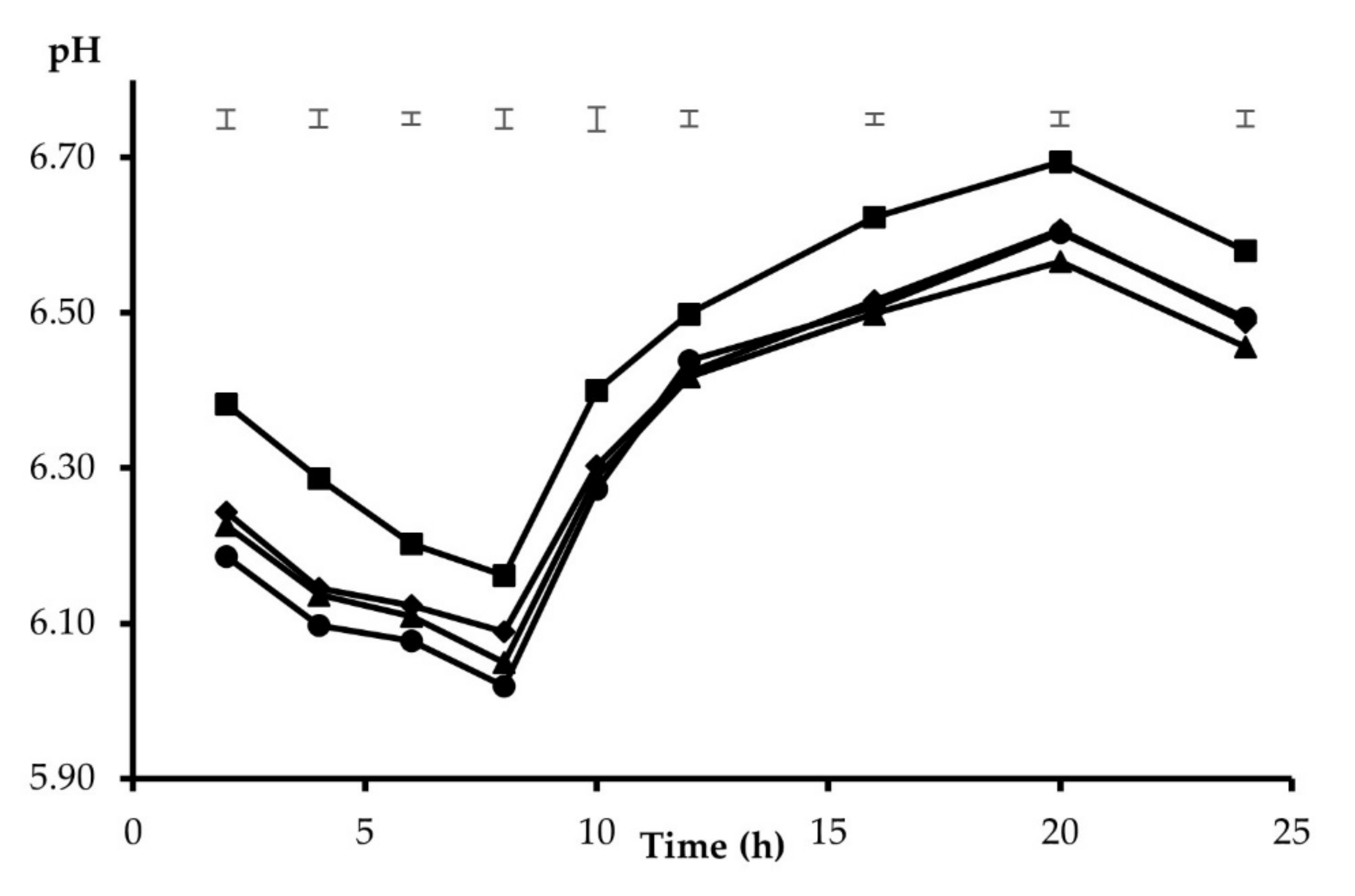
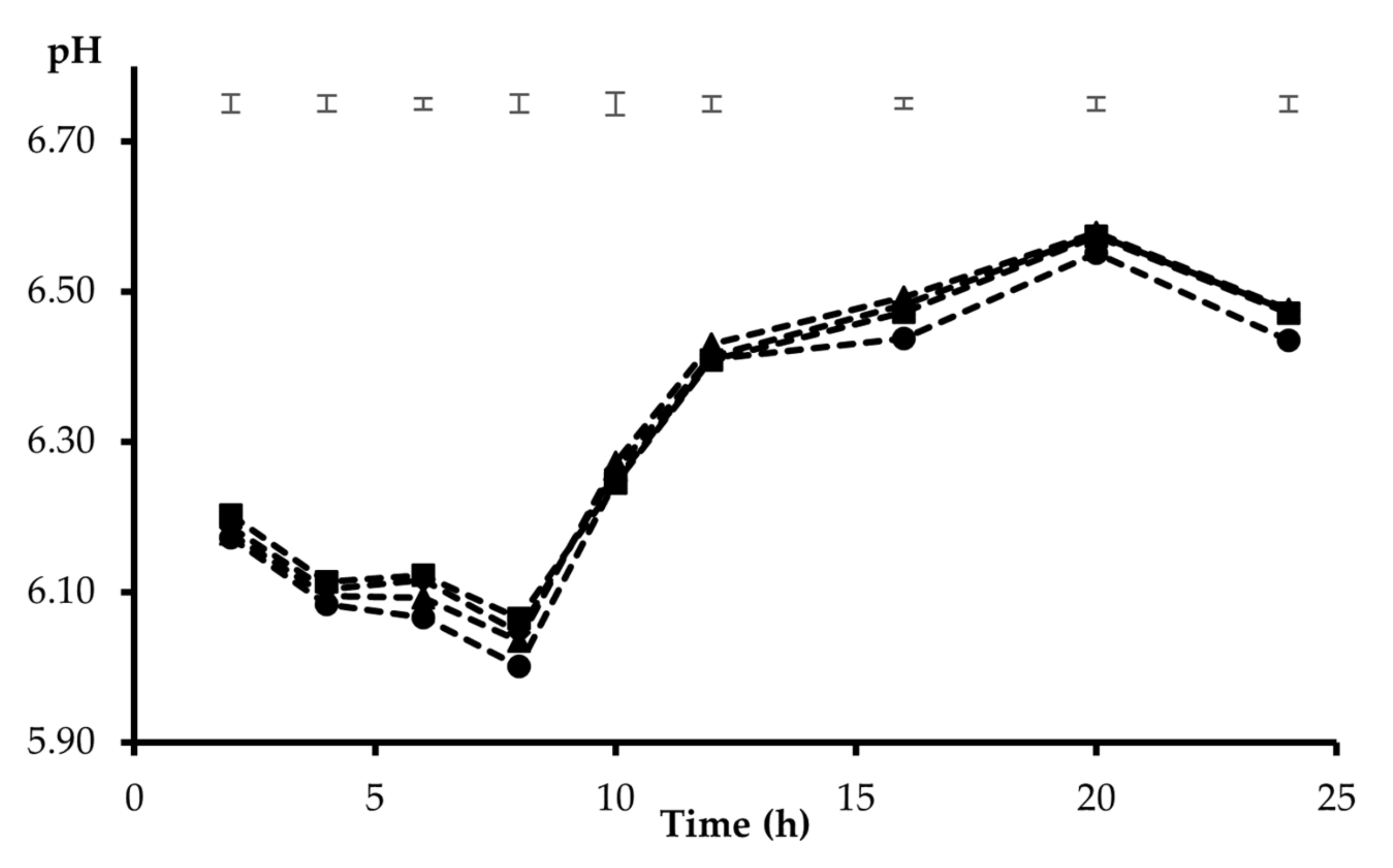
| Ingredients | DM | OM | CP | NDF |
|---|---|---|---|---|
| Maize grain | 859 | 987 | 85 | 103 |
| Barley grain | 899 | 977 | 89 | 178 |
| Soybean meal | 889 | 929 | 507 | 92 |
| Wheat bran | 861 | 942 | 195 | 484 |
| Alfalfa hay | 914 | 887 | 191 | 376 |
| Estimated composition: | ||||
| HC | 887 | 936 | 212 | 236 |
| HF | 899 | 913 | 202 | 301 |
| 2 h | 4 h | 6 h | 8 h | 10 h | 12 h | 16 h | 20 h | 24 h | |
|---|---|---|---|---|---|---|---|---|---|
| HC C | 20.8 a | 37.6 a | 50.4 a | 61.4 a | 74.4 a | 86.9 a | 105.6 a | 124.4 a,b | 141.4 a |
| Z | 19.4 a | 33.2 a | 45.3 a | 56.0 a | 68.3 a | 81.7 a,b | 99.1 a | 116.9 b | 133.2 b |
| B | 19.8 a | 36.4 a | 49.3 a | 59.9 a | 73.2 a | 86.7 a | 105.5 a | 125.7 a | 141.6 a |
| S | 14.9 b,c | 28.3 b | 39.3 b | 47.9 b | 56.7 b | 65.6 c | 83.0 b | 100.7 c | 115.4 c |
| HF F | 17.6 a,b | 29.5 a,b | 39.1 b | 47.4 b | 58.0 b | 67.9 bc | 81.3 b | 95.6 c | 107.2 b,c |
| Z | 11.8 c | 23.1 b | 33.3 b,c | 41.8 b | 51.4 b | 60.8 c | 74.6 b | 88.4 c | 100.2 c |
| B | 10.9 c | 19.0 b | 27.7 c | 35.7 c | 44.4 c | 52.5 d | 64.7 c | 76.3 d | 86.2 d |
| S | 14.1 b,c | 26.2 b | 37.0 b | 45.7 b | 54.7 b | 64.1 c | 76.3 b | 89.5 c | 102.5 c |
| sem | 0.81 | 1.14 | 1.18 | 1.20 | 1.33 | 1.51 | 1.51 | 1.61 | 1.52 |
| p-value | |||||||||
| Substrate | 0.005 | 0.008 | 0.008 | 0.007 | 0.007 | 0.004 | 0.002 | 0.002 | 0.004 |
| Additive | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Subs.–Add. | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| DMd | LAM | SAM | |
|---|---|---|---|
| HC C | 0.506 | 330 | 290 |
| Z | 0.530 | 379 | 307 |
| B | 0.545 | 345 | 279 |
| S | 0.490 | 392 | 291 |
| HF F | 0.458 | 354 | 251 |
| Z | 0.471 | 365 | 272 |
| B | 0.460 | 374 | 264 |
| S | 0.474 | 421 | 300 |
| sem | 0.0175 | 35.68 | 26.60 |
| p-value: | |||
| Substrate | 0.21 | 0.47 | 0.42 |
| Additive | 0.51 | 0.35 | 0.72 |
| Subs.–Add. | 0.30 | 0.93 | 0.79 |
| VFA | Acetate | Propionate | Butyrate | Valerate | BCFA | Ammonia | |
|---|---|---|---|---|---|---|---|
| HC C | 26.73 | 0.609 | 0.224 | 0.130 | 0.013 | 0.018 | 5.52 a |
| Z | 26.53 | 0.611 | 0.227 | 0.127 | 0.013 | 0.011 | 5.27 a,b |
| B | 25.14 | 0.614 | 0.225 | 0.128 | 0.017 | 0.011 | 4.90 b |
| S | 23.21 | 0.587 | 0.236 | 0.139 | 0.013 | 0.012 | 5.07 a,b |
| HF F | 25.03 | 0.615 | 0.219 | 0.128 | 0.013 | 0.013 | 5.88 a |
| Z | 26.93 | 0.618 | 0.219 | 0.124 | 0.013 | 0.013 | 5.82 a |
| B | 24.55 | 0.598 | 0.229 | 0.133 | 0.013 | 0.013 | 5.84 a |
| S | 27.13 | 0.618 | 0.219 | 0.125 | 0.013 | 0.013 | 6.16 a |
| sem | 1.200 | 0.0116 | 0.0062 | 0.0044 | 0.0005 | 0.0004 | 0.098 |
| p-value | |||||||
| Substrate | 0.48 | 0.44 | 0.31 | 0.43 | 0.11 | 0.022 | 0.017 |
| Additive | 0.43 | 0.72 | 0.74 | 0.54 | 0.70 | 0.67 | 0.023 |
| Subs.–Add. | 0.14 | 0.29 | 0.42 | 0.22 | 0.20 | 0.076 | 0.005 |
| VFA | Acetate | Propionate | Butyrate | Valerate | BCFA | Ammonia | |
|---|---|---|---|---|---|---|---|
| HC C | 25.37 | 0.562 | 0.245 | 0.142 | 0.024 | 0.013 | 8.03 a |
| Z | 22.26 | 0.546 | 0.260 | 0.145 | 0.024 | 0.012 | 6.86 b |
| B | 23.72 | 0.555 | 0.252 | 0.144 | 0.024 | 0.013 | 6.78 b |
| S | 23.10 | 0.545 | 0.259 | 0.147 | 0.024 | 0.013 | 7.34 a,b |
| HF F | 25.65 | 0.579 | 0.238 | 0.132 | 0.021 | 0.015 | 7.94 a,b |
| Z | 21.34 | 0.564 | 0.243 | 0.139 | 0.023 | 0.016 | 8.02 a,b |
| B | 21.98 | 0.562 | 0.247 | 0.139 | 0.023 | 0.015 | 8.00 a,b |
| S | 25.06 | 0.580 | 0.238 | 0.132 | 0.022 | 0.014 | 7.76 a,b |
| sem | 1.780 | 0.0136 | 0.0067 | 0.0052 | 0.0005 | 0.0007 | 0.217 |
| p-value | |||||||
| Substrate | 0.55 | 0.032 | 0.13 | 0.028 | 0.16 | 0.013 | 0.050 |
| Additive | 0.24 | 0.68 | 0.54 | 0.76 | 0.79 | 0.62 | 0.054 |
| Subs.–Add. | 0.87 | 0.76 | 0.59 | 0.79 | 0.80 | 0.40 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanzougarene, Z.; Fondevila, M. Rumen Fermentation of Feed Mixtures Supplemented with Clay Minerals in a Semicontinuous In Vitro System. Animals 2022, 12, 345. https://doi.org/10.3390/ani12030345
Amanzougarene Z, Fondevila M. Rumen Fermentation of Feed Mixtures Supplemented with Clay Minerals in a Semicontinuous In Vitro System. Animals. 2022; 12(3):345. https://doi.org/10.3390/ani12030345
Chicago/Turabian StyleAmanzougarene, Zahia, and Manuel Fondevila. 2022. "Rumen Fermentation of Feed Mixtures Supplemented with Clay Minerals in a Semicontinuous In Vitro System" Animals 12, no. 3: 345. https://doi.org/10.3390/ani12030345
APA StyleAmanzougarene, Z., & Fondevila, M. (2022). Rumen Fermentation of Feed Mixtures Supplemented with Clay Minerals in a Semicontinuous In Vitro System. Animals, 12(3), 345. https://doi.org/10.3390/ani12030345






