Elephant Endotheliotropic Herpesvirus 4 and Clostridium perfringens Type C Fatal Co-Infection in an Adult Asian Elephant (Elephas maximus)
Abstract
:Simple Summary
Abstract
1. Introduction
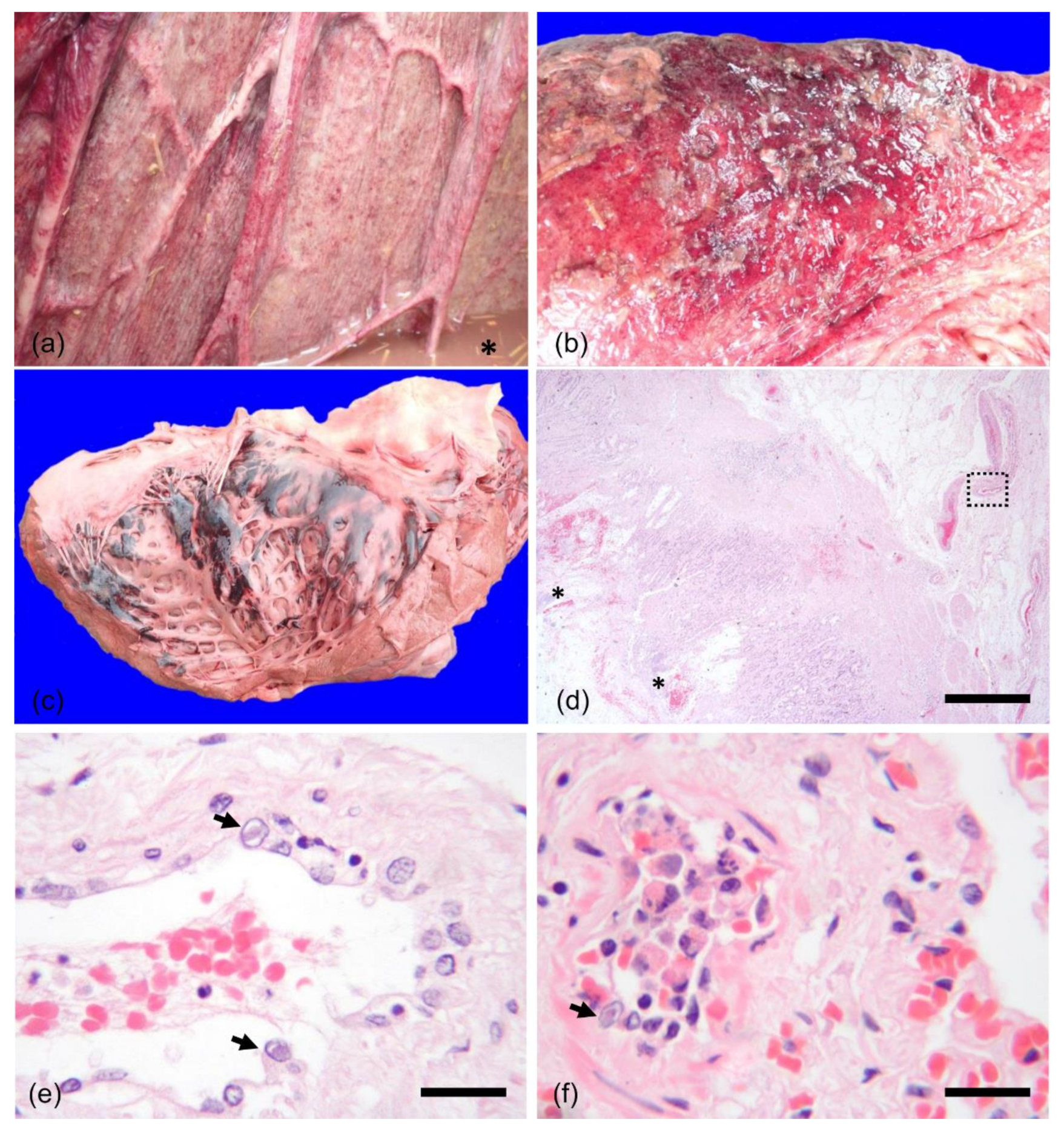
2. History and Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garner, M.M.; Helmick, K.; Ochsenreiter, J.; Richman, L.K.; Latimer, E.; Wise, A.G.; Maes, R.K.; Kiupel, M.; Nordhausen, R.W.; Zong, J.-C.; et al. Clinico-pathologic features of fatal disease attributed to new variants of endotheliotropic herpesviruses in two Asian elephants (Elephas maximus). Vet. Pathol. 2009, 46, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, K.L.; Kristensen, A.T.; Bertelsen, M.F.; Denk, D. Retrospective review of 27 European cases of fatal elephant endotheliotropic herpesvirus-haemorrhagic disease reveals evidence of disseminated intravascular coagulation. Sci. Rep. 2021, 11, 14173. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.L.; Nielsen, S.S.; Martinussen, T.; Bertelsen, M.F. Quantification and risk factor analysis of elephant endotheliotropic herpesvirus-haemorrhagic disease fatalities in Asian elephants (Elephas maximus) in Europe (1985–2017). J. Zoo Aquar. Res. 2021, 9, 8–13. [Google Scholar] [CrossRef]
- Jeffrey, A.; Evans, T.S.; Molter, C.; Howard, L.L.; Ling, P.; Goldstein, T.; Gilardi, K. Noninvasive sampling for detection of elephant endotheliotropic herpesvirus and genomic DNA in Asian (Elephas maximus) and African (Loxodonta africana) elephants. J. Zoo Wildl. Med. 2020, 51, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Long, S.Y.; Latimer, E.M.; Hayward, G.S. Review of elephant endotheliotropic herpesviruses and acute hemorrhagic disease. ILAR J. 2016, 56, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachariah, A.; Sajesh, P.K.; Santhosh, S.; Bathrachalam, C.; Megha, M.; Pandiyan, J.; Jishnu, M.; Kobragade, R.S.; Long, S.Y.; Zong, J.-C.; et al. Extended genotypic evaluation and comparison of twenty-two cases of lethal EEHV1 hemorrhagic disease in wild and captive Asian elephants in India. PLoS ONE 2018, 13, e0202438. [Google Scholar] [CrossRef] [Green Version]
- Zachariah, A.; Zong, J.-C.; Long, S.Y.; Latimer, E.M.; Heaggans, S.Y.; Richman, L.K.; Hayward, G.S. Fatal herpesvirus hemorrhagic disease in wild and orphan Asian elephants in southern India. J. Wildl. Dis. 2013, 49, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Bronson, E.; McClure, M.; Sohl, J.; Wiedner, E.; Cox, S.; Latimer, E.M.; Pearson, V.R.; Hayward, G.S.; Fuery, A.; Ling, P.D. Epidemiologic evaluation of elephant endotheliotropic herpesvirus 3B infection in an African elephant (Loxodonta africana). J. Zoo Wildl. Med. 2017, 48, 335–343. [Google Scholar] [CrossRef]
- Pursell, T.; Clinton, J.L.S.; Tan, J.; Peng, R.; Qin, X.; Doddapaneni, H.; Menon, V.; Momin, Z.; Kottapalli, K.; Howard, L.; et al. Primary infection may be an underlying factor contributing to lethal hemorrhagic disease caused by elephant endotheliotropic herpesvirus 3 in African elephants (Loxodonta africana). Microbiol. Spectr. 2021, 9, e0098321. [Google Scholar] [CrossRef] [PubMed]
- Perrin, K.L.; Bertelsen, M.F.; Denk, D.; Kristensen, A.T. Current understanding of the pathogenesis of elephant endotheliotropic herpesvirus-hemorrahgic disease. In Proceedings of the Joint AAZV/EAZWV Conference, Virtual, 4 October–5 November 2021; p. 144. [Google Scholar]
- Burgdorf-Moisuk, A.B.; Connolly, M.; Raines, J. Multi-institutional collaboration for the successful treatment of EEHV 3A in a juvenile African elephant (Loxodonta africana). In Proceedings of the Joint AAZV/EAZWV Conference, Virtual, 4 October–5 November 2021; p. 143. [Google Scholar]
- Hoornweg, T.E.; Schaftenaar, W.; Maurer, G.; Van den Doel, P.B.; Molenaar, F.M.; Chamouard-Galante, A.; Vercammen, F.; Rutten, V.P.M.G.; De Haan, C.A.M. Elephant endotheliotropic herpesvirus is omnipresent in elephants in European zoos and an Asian elephant range country. Viruses 2021, 13, 283. [Google Scholar] [CrossRef]
- Schaftenaar, W.; Reid, C.; Martina, B.; Fickel, J.; Osterhaus, A.D. Nonfatal clinical presentation of elephant endotheliotropic herpes virus discovered in a group of captive Asian elephants (Elephas maximus). J. Zoo Wildl. Med. 2010, 41, 626–632. [Google Scholar] [CrossRef]
- Fuery, A.; Pursell, T.; Tan, J.; Peng, R.; Burbelo, P.D.; Hayward, G.S.; Ling, P.D. Lethal hemorrhagic disease and clinical illness associated with elephant endotheliotropic herpesvirus 1 are caused by primary infection: Implications for the detection of diagnostic proteins. J. Virol. 2020, 94, e01528-19. [Google Scholar] [CrossRef] [Green Version]
- Richman, L.K.; Montali, R.J.; Garber, R.L.; Kennedy, M.A.; Lehnhardt, J.; Hilderbrandt, T.; Schmitt, D.; Hardy, D.; Alcendor, D.J.; Hayward, G.S. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science 1999, 283, 1171. [Google Scholar] [CrossRef] [PubMed]
- Boonprasert, K.; Punyapornwithaya, V.; Tankaew, P.; Angkawanish, T.; Sriphiboon, S.; Titharam, C.; Brown, J.L.; Somgird, C. Survival analysis of confirmed elephant endotheliotropic herpes virus cases in Thailand from 2006–2018. PLoS ONE 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, F.M.; Silvestre, P. Clinical approach to colic and collapse in an Asian elephant (Elephas maximus) with Salmonella saintpaul septicaemia and subsequent ileus. Vet. Rec. Case Rep. 2021, e214. [Google Scholar] [CrossRef]
- Wissink-Argilaga, N.; Dastjerdi, A.; Molenaar, F.M. Using in-house hematology to direct decision-making in the successful treatment and monitoring of a clinical and subsequently subclinical case of elephant endotheliotropic herpesvirus 1B. J. Zoo Wildl. Med. 2019, 50, 498–502. [Google Scholar] [CrossRef]
- Dastjerdi, A.; Seilern-Moy, K.; Darpel, K.; Steinbach, F.; Molenaar, F. Surviving and fatal elephant endotheliotropic herpesvirus-1A infections in juvenile Asian elephants—Lessons learned and recommendations on anti-herpesviral therapy. BMC Vet. Res. 2016, 12, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.; Sripiboon, S.; Pringproa, K.; Chuammitri, P.; Punyapornwithaya, V.; Boonprasert, K.; Tankaew, P.; Angkawanish, T.; Namwongprom, K.; Arjkumpa, O.; et al. Clinical characteristics of elephant endotheliotropic herpesvirus (EEHV) cases in Asian elephants (Elephas maximus) in Thailand during 2006–2019. Vet. Q. 2021, 41, 268–279. [Google Scholar] [CrossRef]
- Richman, L.K.; Montali, R.J.; Hayward, G.S. Review of a newly recognized disease of elephants caused by endotheliotropic herpesviruses. Zoo Biol. 2000, 19, 383. [Google Scholar] [CrossRef]
- Sripiboon, S.; Angkawanish, T.; Boonprasert, K.; Sombutputorn, P.; Langkaphin, W.; Ditcham, W.; Warren, K. Succesful treatment of a clinical elephant endotheliotropic herpesvirus infection: The dynamics of viral load, genotype analysis, and treatment with acyclovir. J. Zoo Wildl. Med. 2017, 48, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.L.; Hardy, D.A.; Montali, R.J.; Richman, L.K.; Lindsay, W.A.; Isaza, R.; West, G. Use of famciclovir for the treatment of endotheliotrophic herpesvirus infections in Asian elephants (Elephas maximus). J. Zoo Wildl. Med. 2000, 31, 518–522. [Google Scholar] [CrossRef]
- Drake, G.J.; Haycock, J.; Dastjerdi, A.; Davies, H.; Lopez, F.J. Use of immunostimulants in the successful treatment of a clinical EEHV1A infection in an Asian elephant (Elephas maximus). Vet. Rec. Case Rep. 2020, 8, e001158. [Google Scholar] [CrossRef]
- Richman, L.K.; Montali, R.J.; Cambre, R.C.; Schmitt, D.; Hardy, D.; Hildbrandt, T.; Bengis, R.G.; Hamzeh, F.M.; Shahkolahi, A.; Hayward, G.S. Clinical and pathological findings of a newly recognized disease of elephants caused by endotheliotropic herpesviruses. J. Wildl. Dis. 2000, 36, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cracknell, J. Elephant Endotheliotrophic Herpes Virus (EEHV) Protocol, Version 1.3; BIAZA/United Kingdom Elephant Health Programme: London, UK, 2008; pp. 1–46. [Google Scholar]
- Kochagul, V.; Srivorakul, S.; Boonsri, K.; Somgird, C.; Sthitmatee, N.; Thitaram, C.; Pringproa, K. Production of antibody against elephant endotheliotropic herpesvirus (EEHV) unveils tissue tropisms and routes of viral transmission in EEHV-infected Asian elephants. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sripiboon, S.; Tankaew, P.; Lungka, G.; Thitaram, C. The occurrence of elephant endotheliotropic herpesvirus in captive Asian elephants (Elephas maximus): First case of EEHV4 in Asia. J. Zoo Wildl. Med. 2013, 44, 100. [Google Scholar] [CrossRef]
- Das, A.; Mazumder, Y.; Dutta, B.K.; Shome, B.R.; Bujarbaruah, K.M.; Sharma, G.D. Clostridium perfringens type A beta2 toxin in elephant (Elephas maximus indicus) and pygmy hog (Sus salvanius) with haemorrhagic enteritis in Assam, India. Afr. J. Microbiol. Res. 2008, 2, 196–201. [Google Scholar]
- Bacciarini, L.N.; Pagan, O.; Frey, J.; Gröne, A. Clostridium perfringens β2-toxin in an African elephant (Loxodonta africana) with ulcerative enteritis. Vet. Rec. 2001, 149, 618–620. [Google Scholar] [CrossRef]
- Bojesen, A.M.; Olsen, K.E.P.; Bertelsen, M.F. Fatal enterocolitis in Asian elephants (Elephas maximus) caused by Clostridium difficile. Vet. Microbiol. 2006, 116, 329–335. [Google Scholar] [CrossRef]
- Boonsri, K.; Somgird, C.; Noinafai, P.; Pringproa, K.; Janyamethakul, T.; Angkawanish, T.; Brown, J.L.; Tankaew, P.; Srivorakul, S.; Thitaram, C. Elephant endotheliotropic herpesvirus associated with Clostridium perfringens infection in two asian elephant (Elephas maximus) calves. J. Zoo Wildl. Med. 2018, 49, 178–182. [Google Scholar] [CrossRef]
- Stanton, J.J.; Zong, J.-C.; Latimer, E.; Tan, J.; Herron, A.; Hayward, G.S.; Ling, P.D. Detection of pathogenic elephant endotheliotropic herpesvirus in routine trunk washes from healthy adult Asian elephants (Elephas maximus) by use of a real-time quantitative polymerase chain reaction assay. Am. J. Vet. Res. 2010, 71, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Haycock, J.; Seilern-Moy, K.; Molenaar, F.; Dastjerdi, A. Refining a multiplex qPCR assay to simultaneously detect elephant endotheliotropic herpesvirus infections in Asian elephants (Elephas maximus). In Proceedings of the 10th International Elephant Endotheliotropic Herpesvirus (EEHV) Workshop, Houston, TX, USA, 17–18 February 2015; p. 22. [Google Scholar]
- Heikinheimo, A.; Korkeala, H. Multiplex PCR assay for toxinotyping Clostridium perfringens isolates obtained from Finnish broiler chickens. Lett. Appl. Microbiol. 2005, 40, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.; Bueschel, D. Multiplex PCR Procedure for Genotyping Clostridium Perfringens; University of Arizona: Tucson, AZ, USA, 1999. [Google Scholar]
- Tsai, M.-S.; Newman, C.; Macdonald, D.W.; Buesching, C.D. Stress-related herpesvirus reactivation in badgers can result in clostridium proliferation. EcoHealth 2021, 18, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.S.; Baldwin, C.A.; Pence, M.; West, J.; Bernard, J.; Liggett, A.; Miller, D.; Hines II, M.E. Seroprevalence and comparison of isolates of endometriotropic bovine herpesvirus-4. J. Vet. Diagn. Investig. 2002, 14, 457–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flach, E.J.; Reid, H.; Pow, I.; Klemt, A. Gamma herpesvirus carrier status of captive artiodactyls. Res. Vet. Sci. 2002, 73, 93–99. [Google Scholar] [CrossRef]
- Neto, R.T.; Uzal, F.A.; Hodzic, E.; Persiani, M.; Jolissaint, S.; Alcaraz, A.; Carvallo, F.R. Coinfection with Clostridium piliforme and felid herpesvirus 1 in a kitten. J. Vet. Diagn. Investig. 2015, 27, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.J.; Robinson, I.M.; Dougherty, R.W.; Bucklin, J.A. Grain overload in cattle and sheep: Changes in microbial populations in the cecum and rumen. Am. J. Vet. Res. 1975, 36, 181–185. [Google Scholar]
- Butler, E.A.; Jensen, W.F.; Johnson, R.E.; Scott, J.M. Grain overload and secondary effects as potential mortality factors of moose in North Dakota. Alces 2008, 44, 73–79. [Google Scholar]
- Zentek, J.; Marquart, B.; Pietrzak, T.; Ballevre, O.; Rochat, F. Dietary effects on bifidobacteria and Clostridium perfringens in the canine intestinal tract. J. Anim. Physiol. Anim. Nutr. 2003, 87, 397–407. [Google Scholar] [CrossRef]
- Ramos, C.P.; Diniz, A.N.; Ribeiro, M.G.; De Paula, C.L.; Costa, E.A.; Sonne, L.; Pereira, S.T.; Lopes, C.E.B.; Renno, M.C.; Silva, R.O.S. Enteric organisms detected in feces of dogs with bloody diarrhea: 45 cases. Top. Companion Anim. Med. 2021, 45, 100549. [Google Scholar] [CrossRef]
- Delmée, M.; Van Broeck, J.; Simon, A.; Janssens, M.; Avesani, V. Laboratory diagnosis of Clostridium difficile-associated diarrhoea: A plea for culture. J. Med. Microbiol. 2005, 54, 187–191. [Google Scholar] [CrossRef]
- Scharling, F.S.; Bertelsen, M.F.; Sós, E.; Bojesen, A.M. Prevalence of Salmonella species, Clostridium perfringens, and Clostridium difficile in the feces of healthy elephants (Loxodonta species and Elaphas maximus) in Europe. J. Zoo Wildl. Med. 2021, 51, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Carter, M.E.; Markey, B.K.; Carter, G.R. Clinical Veterinary Microbiology; Wolfe: London, UK, 1994; pp. 1–648. [Google Scholar]
- Zaragoza, N.E.; Orellana, C.A.; Moonen, G.A.; Moutafis, G.; Marcellin, E. Vaccine production to protect animals against pathogenic clostridia. Toxins 2019, 11, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimoldi, G.; Uzal, F.; Chin, R.P.; Palombo, E.A.; Awad, M.; Lyras, D.; Shivaprasad, H.L. Necrotic enteritis in chickens associated with Clostridium sordellii. Avian Dis. 2015, 59, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.L.; Wilson, W.D. Clostridium septicum septicemia in a neonatal foal with hemorrhagic enteritis. Cornell Vet. 1993, 83, 143–151. [Google Scholar]
- Uzal, F.A.; Songer, J.G. Infections by Clostridium perfringens type B. In Clostridial Diseases of Animals; Uzal, F., Songer, J., Prescott, J., Popoff, M., Eds.; Willey and Blackwell: Ames, IA, USA, 2016; pp. 139–142. [Google Scholar]
- D’Agostino, J.J.; Latimer, E.M.; McCrae, E.A.; Elliott, S.N.; Romanoski, M.C.; Payton, M.E. Influence of herd size and animal tranfers on shedding frequency and quantity of elephant endotheliotropic herpesvirus in Asian elephants (Elephas maximus). In Proceedings of the Joint AAZV/EAZWV Conference, Virtual, 4 October–5 November 2021; p. 149. [Google Scholar]
- Bennett, L.; Dunham, S.; Yon, L.; Chapman, S.; Kenaghan, M.; Purdie, L.; Tarlinton, R. Longitudinal study of Asian elephants, Elephas maximus, indicates intermittent shedding of elephant endotheliotropic herpesvirus 1 during pregnancy. Vet. Rec. Open 2015, 2, e000088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richman, L.K.; Montali, R.J. Elephant herpesvirus infections. In Infectious Diseases of Wild Mammals, 3rd ed.; Williams, E.S., Barker, I.K., Eds.; Iowa State University Press: Ames, IA, USA, 2001; pp. 170–173. [Google Scholar]
- Chooi, K.F.; Zahari, Z.Z. Salmonellosis in a captive Asian elephant. J. Zoo Anim. Med. 1988, 19, 48–50. [Google Scholar] [CrossRef]
- Ortega, J.; Corpa, J.M.; Orden, J.A.; Blanco, J.; Carbonell, M.D.; Gerique, A.C.; Latimer, E.; Hayward, G.S.; Roemmelt, A.; Kraemer, T.; et al. Acute death associated with Citrobacter freundii infection in an African elephant (Loxodonta africana). J. Vet. Diagn. Investig. 2015, 27, 632–636. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, T.; Rocchigiani, G.; Zendri, F.; Drake, G.; Lopez, J.; Chantrey, J.; Ricci, E. Elephant Endotheliotropic Herpesvirus 4 and Clostridium perfringens Type C Fatal Co-Infection in an Adult Asian Elephant (Elephas maximus). Animals 2022, 12, 349. https://doi.org/10.3390/ani12030349
Costa T, Rocchigiani G, Zendri F, Drake G, Lopez J, Chantrey J, Ricci E. Elephant Endotheliotropic Herpesvirus 4 and Clostridium perfringens Type C Fatal Co-Infection in an Adult Asian Elephant (Elephas maximus). Animals. 2022; 12(3):349. https://doi.org/10.3390/ani12030349
Chicago/Turabian StyleCosta, Taiana, Guido Rocchigiani, Flavia Zendri, Gabby Drake, Javier Lopez, Julian Chantrey, and Emanuele Ricci. 2022. "Elephant Endotheliotropic Herpesvirus 4 and Clostridium perfringens Type C Fatal Co-Infection in an Adult Asian Elephant (Elephas maximus)" Animals 12, no. 3: 349. https://doi.org/10.3390/ani12030349
APA StyleCosta, T., Rocchigiani, G., Zendri, F., Drake, G., Lopez, J., Chantrey, J., & Ricci, E. (2022). Elephant Endotheliotropic Herpesvirus 4 and Clostridium perfringens Type C Fatal Co-Infection in an Adult Asian Elephant (Elephas maximus). Animals, 12(3), 349. https://doi.org/10.3390/ani12030349






