Animal Welfare and Parasite Infections in Organic and Conventional Dairy Farms: A Comparative Pilot Study in Central Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Selection
2.2. Welfare Assessment
2.3. Milk Yield and Quality
2.4. Parasitological Sampling and Analysis
2.5. Statistical Analysis
3. Results
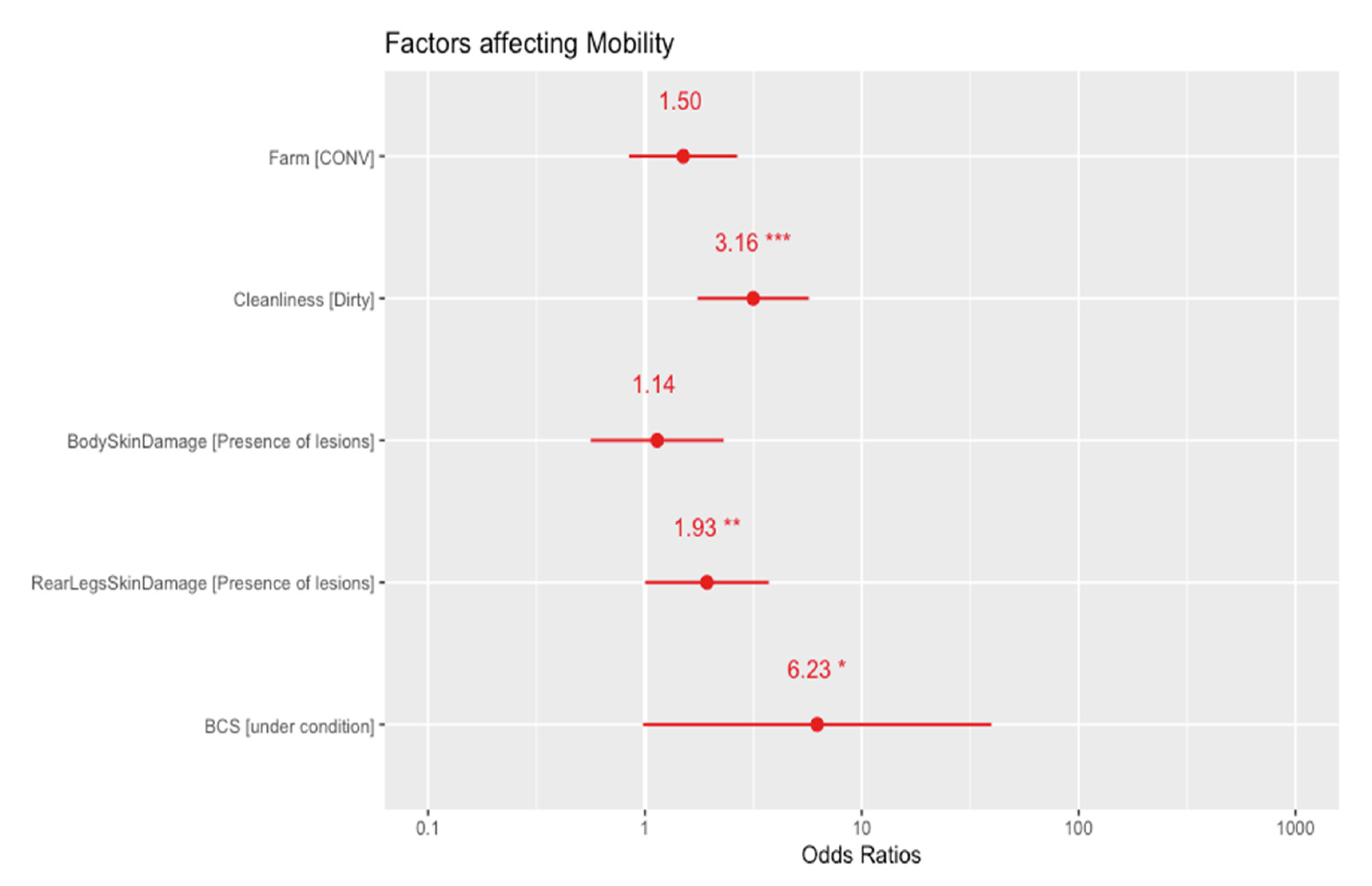
3.1. Welfare Assessment
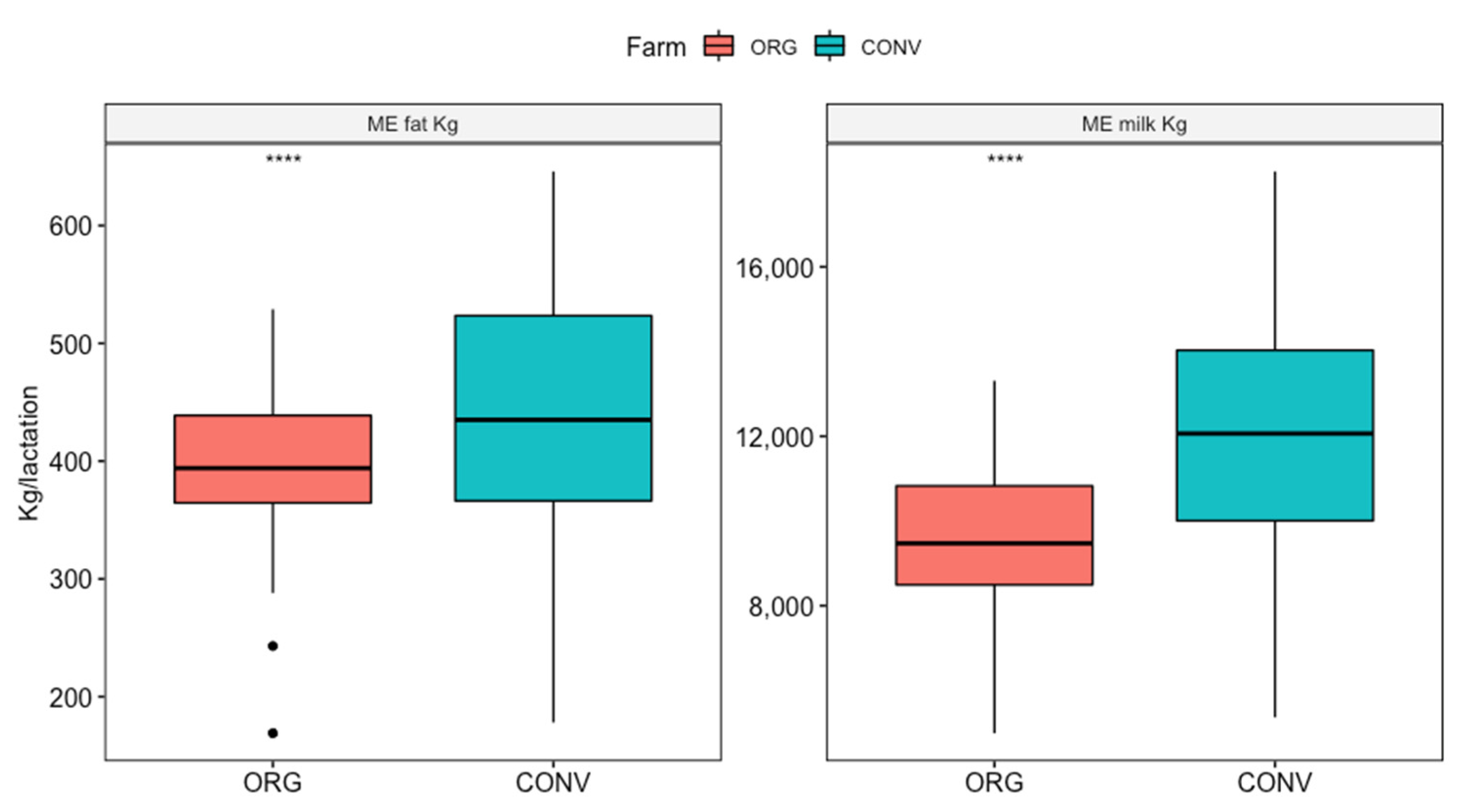
3.2. Milk Yield and Quality
3.3. Parasitological Assessment
4. Discussion
4.1. Welfare Assessment
4.2. Milk Yield and Quality
4.3. Parasitological Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions: The European Green Deal. Communication No. COM/2019/640. Brussels. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 25 November 2021).
- European Commission. Communication from The European Commission to The European Parliament, the European Economic and Social Committee and The Committee of The Regions: A Farm to Fork Strategy for Fair, Healthy and Environmentally-Friendly Food System. Communication No. COM/2020/381. Brussels. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0381 (accessed on 25 November 2021).
- SINAB Bio in Cifre 2020. Available online: http://www.sinab.it/sites/default/files/share/BIO%20IN%20CIFRE%202020.pdf (accessed on 1 December 2021).
- European Parliament; The Council of the European Union. Regulation (Eu) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. J. Eur. Union 2018, 150, 1–92. [Google Scholar]
- Blanco-Penedo, I.; Fall, N.; Emanuelson, U. Effects of Turning to 100% Organic Feed on Metabolic Status of Swedish Organic Dairy Cows. Livest. Sci. 2012, 143, 242–248. [Google Scholar] [CrossRef]
- Special Eurobarometer 504. In Europeans, Agriculture and the CAP; European Commission: Brussels, Belgium, 2020; Available online: https://europa.eu/eurobarometer/surveys/detail/2229 (accessed on 10 December 2021).
- Wallenbeck, A.; Rousing, T.; Sørensen, J.T.; Bieber, A.; Spengler Neff, A.; Fuerst-Waltl, B.; Winckler, C.; Peiffer, C.; Steininger, F.; Simantke, C.; et al. Characteristics of Organic Dairy Major Farm Types in Seven European Countries. Org. Agric. 2019, 9, 275–291. [Google Scholar] [CrossRef] [Green Version]
- Reijs, J.W.; Daatselaar, C.H.G.; Helming, J.F.M.; Jager, J.H.; Beldman, A.C.G. Grazing Dairy Cows in North-West Europe: Economic Farm Performance and Future Developments with Emphasis on the Dutch Situation; LEI Wageningen UR: Wageningen, The Netherlands, 2013; ISBN 978-90-8615-637-5. [Google Scholar]
- IFOAM Principles of Organic Agriculture. Available online: https://wwwifoambio/sites/default/files/poa_english_webpdf (accessed on 1 December 2021).
- Åkerfeldt, M.P.; Gunnarsson, S.; Bernes, G.; Blanco-Penedo, I. Health and Welfare in Organic Livestock Production Systems—A Systematic Mapping of Current Knowledge. Org. Agric. 2021, 11, 105–132. [Google Scholar] [CrossRef]
- Wagner, K.; Brinkmann, J.; Bergschmidt, A.; Renziehausen, C.; March, S. The Effects of Farming Systems (Organic vs. Conventional) on Dairy Cow Welfare, Based on the Welfare Quality® Protocol. Animal 2021, 15, 100301. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial Assessment of the Economic Burden of Major Parasitic Helminth Infections to the Ruminant Livestock Industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Rose Vineer, H.; Morgan, E.R.; Hertzberg, H.; Bartley, D.J.; Bosco, A.; Charlier, J.; Chartier, C.; Claerebout, E.; De Waal, T.; Hendrickx, G.; et al. Increasing Importance of Anthelmintic Resistance in European Livestock: Creation and Meta-Analysis of an Open Database. Parasite 2020, 27, 69. [Google Scholar] [CrossRef]
- Vercruysse, J.; Dijk, V.; Morgan, J.; Geary, E.R.; Samson-Himmelstjerna, V.; Claerebout, E. Control of Helminth Ruminant Infections by 2030. Parasitology 2018, 145, 1655–1664. [Google Scholar] [CrossRef] [Green Version]
- ANAFIBJ. Statistiche Nazionali. Available online: http://www.anafi.it/it/pubblicazioni-statistiche/statistiche-nazionali-2019 (accessed on 1 December 2021).
- Italian Statistic Institute: Istituto Nazionale di Statistica (ISTAT); Caratteristiche Strutturali delle Aziende Agricole. 6° Censimento Generale Dell’agricoltura. 2010. Available online: https://www.istat.it/it/files/2011/03/1425-12_Vol_VI_Cens_Agricoltura_INT_CD_1_Trimboxes_ipp.pdf (accessed on 10 December 2020).
- AssureWel Dairy Cows. Available online: http://www.assurewel.org/dairycows.html (accessed on 1 December 2021).
- EFSA. Scientific Opinion on Welfare of Dairy Cows in Relation to Leg and Locomotion Problems Based on a Risk Assessment with Special Reference to the Impact of Housing, Feeding, Management and Genetic Selection. EFSA J. 2009, 7, 1142. [Google Scholar] [CrossRef]
- Rutherford, K.M.D.; Langford, F.M.; Jack, M.C.; Sherwood, L.; Lawrence, A.B.; Haskell, M.J. Lameness Prevalence and Risk Factors in Organic and Non-Organic Dairy Herds in the United Kingdom. Vet. J. 2009, 180, 95–105. [Google Scholar] [CrossRef]
- MAAF. Manual of Veterinary Parasitological Laboratory Techniques; Ministry of Agriculture, Fisheries and Food (MAFF), Her Majesty’s Stationary Office: London, UK, 1986.
- Sloss, M.; Kemp, R.; Zajac, A.; American Association of Veterinary Parasitologists. Veterinary Clinical Parasitology; Iowa State University Press: Ames, IA, USA, 1994; ISBN 978-0813817330. [Google Scholar]
- Verocai, G.; Chaudhry, U.; Lejeune, M. Diagnostic Methods for Detecting Internal Parasites of Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Conboy, G.A. Review of Veterinary Clinical Parasitology, 8th ed.; Wiley Blackwell: Danvers, MA, USA, 2012; ISBN 978-0-8138-2053-8. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Gelman, A.; Su, Y.-S. Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models; R Package Version 1.11-2; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Sjöström, K.; Fall, N.; Blanco-Penedo, I.; Duval, J.E.; Krieger, M.; Emanuelson, U. Lameness Prevalence and Risk Factors in Organic Dairy Herds in Four European Countries. Livest. Sci. 2018, 208, 44–50. [Google Scholar] [CrossRef]
- Blanco-Penedo, I.; Ouweltjes, W.; Ofner-Schröck, E.; Brügemann, K.; Emanuelson, U. Symposium Review: Animal Welfare in Free-Walk Systems in Europe. J. Dairy Sci. 2020, 103, 5773–5782. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.A.; Richert, R.M.; Cicconi-Hogan, K.M.; Gamroth, M.J.; Schukken, Y.H.; Stiglbauer, K.E.; Ruegg, P.L. Comparison of Selected Animal Observations and Management Practices Used to Assess Welfare of Calves and Adult Dairy Cows on Organic and Conventional Dairy Farms. J. Dairy Sci. 2014, 97, 4269–4280. [Google Scholar] [CrossRef] [PubMed]
- Barker, Z.E.; Leach, K.A.; Whay, H.R.; Bell, N.J.; Main, D.C.J. Assessment of Lameness Prevalence and Associated Risk Factors in Dairy Herds in England and Wales. J. Dairy Sci. 2010, 93, 932–941. [Google Scholar] [CrossRef]
- Dippel, S.; Dolezal, M.; Brenninkmeyer, C.; Brinkmann, J.; March, S.; Knierim, U.; Winckler, C. Risk Factors for Lameness in Freestall-Housed Dairy Cows across Two Breeds, Farming Systems, and Countries. J. Dairy Sci. 2009, 92, 5476–5486. [Google Scholar] [CrossRef] [Green Version]
- Richert, R.; Cicconi, K.; Gamroth, M.; Schukken, Y.; Stiglbauer, K.; Ruegg, P. Perceptions and Risk Factors for Lameness on Organic and Small Conventional Dairy Farms. J. Dairy Sci. 2013, 96, 5018–5026. [Google Scholar] [CrossRef]
- Haskell, M.J.; Rennie, L.J.; Bowell, V.A.; Bell, M.J.; Lawrence, A.B. Housing System, Milk Production, and Zero-Grazing Effects on Lameness and Leg Injury in Dairy Cows. J. Dairy Sci. 2006, 89, 4259–4266. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Mendo, O.; Von Keyserlingk, M.A.G.; Veira, D.M.; Weary, D.M. Effects of Pasture on Lameness in Dairy Cows. J. Dairy Sci. 2007, 90, 1209–1214. [Google Scholar] [CrossRef]
- Duval, E.; von Keyserlingk, M.A.G.; Lecorps, B. Organic Dairy Cattle: Do European Union Regulations Promote Animal Welfare? Animals 2020, 10, 1786. [Google Scholar] [CrossRef]
- Silva, J.B.; Fagundes, G.M.; Soares, J.P.G.; Fonseca, A.H.; Muir, J.P. A Comparative Study of Production Performance and Animal Health Practices in Organic and Conventional Dairy Systems. Trop. Anim. Health Prod. 2014, 46, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randall, L.V.; Green, M.J.; Chagunda, M.G.G.; Mason, C.; Archer, S.C.; Green, L.E.; Huxley, J.N. Low Body Condition Predisposes Cattle to Lameness: An 8-Year Study of One Dairy Herd. J. Dairy Sci. 2015, 98, 3766–3777. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.V.; Green, M.J.; Green, L.E.; Chagunda, M.G.G.; Mason, C.; Archer, S.C.; Huxley, J.N. The Contribution of Previous Lameness Events and Body Condition Score to the Occurrence of Lameness in Dairy Herds: A Study of 2 Herds. J. Dairy Sci. 2018, 101, 1311–1324. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, M.B.; Ramanoon, S.Z.; Mossadeq, W.M.S.; Mansor, R.; Syed-Hussain, S.S. Association between Lameness and Indicators of Dairy Cow Welfare Based on Locomotion Scoring, Body and Hock Condition, Leg Hygiene and Lying Behavior. Animals 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicalho, R.C.; Machado, V.S.; Caixeta, L.S. Lameness in Dairy Cattle: A Debilitating Disease or a Disease of Debilitated Cattle? A Cross-Sectional Study of Lameness Prevalence and Thickness of the Digital Cushion. J. Dairy Sci. 2009, 92, 3175–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oehm, A.W.; Knubben-Schweizer, G.; Rieger, A.; Stoll, A.; Hartnack, S. A Systematic Review and Meta-Analyses of Risk Factors Associated with Lameness in Dairy Cows. BMC Vet. Res. 2019, 15, 346. [Google Scholar] [CrossRef] [PubMed]
- Beer, G.; Alsaaod, M.; Starke, A.; Schuepbach-Regula, G.; Müller, H.; Kohler, P.; Steiner, A. Use of Extended Characteristics of Locomotion and Feeding Behavior for Automated Identification of Lame Dairy Cows. PLoS ONE 2016, 11, e0155796. [Google Scholar] [CrossRef] [Green Version]
- Kathambi, E.K.; Vanleeuwen, J.A.; Gitau, G.K.; Kamunde, C. Risk Factors Associated with Cows’ Lying Time, Stall and Cows’ Own Cleanliness in Smallholder Dairy Farms in Kenya. Vet. World 2019, 12, 1085–1092. [Google Scholar] [CrossRef] [Green Version]
- Solano, L.; Barkema, H.W.; Pickel, C.; Orsel, K. Effectiveness of a Standardized Footbath Protocol for Prevention of Digital Dermatitis. J. Dairy Sci. 2017, 100, 1295–1307. [Google Scholar] [CrossRef]
- De Pinho Manzi, M.; Nóbrega, D.B.; Faccioli, P.Y.; Troncarelli, M.Z.; Menozzi, B.D.; Langoni, H. Relationship between Teat-End Condition, Udder Cleanliness and Bovine Subclinical Mastitis. Res. Vet. Sci. 2012, 93, 430–434. [Google Scholar] [CrossRef]
- Tongel, P.; Brouček, J. Influence of Hygienic Condition on Prevalence of Mastitis and Lameness in Dairy Cows. Slovak J. Anim. Sci. 2010, 2, 95–99. [Google Scholar]
- Hultgren, J.; Bergsten, C. Effects of a Rubber-Slatted Flooring System on Cleanliness and Foot Health in Tied Dairy Cows. Prev. Vet. Med. 2001, 52, 75–89. [Google Scholar] [CrossRef]
- Norring, M.; Manninen, E.; De Passillé, A.M.; Rushen, J.; Munksgaard, L.; Saloniemi, H. Effects of Sand and Straw Bedding on the Lying Behavior, Cleanliness, and Hoof and Hock Injuries of Dairy Cows. J. Dairy Sci. 2008, 91, 570–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruud, L.E.; Bøe, K.E.; Østerås, O. Risk Factors for Dirty Dairy Cows in Norwegian Freestall Systems. J. Dairy Sci. 2010, 93, 5216–5224. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.H.; Thomsen, P.T.; Sørensen, J.T. Identifying Risk Factors for Poor Hind Limb Cleanliness in Danish Loose-Housed Dairy Cows. Animal 2011, 5, 1613–1619. [Google Scholar] [CrossRef] [Green Version]
- Schuppli, C.A.; von Keyserlingk, M.A.G.; Weary, D.M. Access to Pasture for Dairy Cows: Responses from an Online Engagement. J. Anim. Sci. 2014, 92, 5185–5192. [Google Scholar] [CrossRef]
- Ellis, K.A.; Innocent, G.T.; Mihm, M.; Cripps, P.; McLean, W.G.; Howard, C.V.; Grove-White, D. Dairy Cow Cleanliness and Milk Quality on Organic and Conventional Farms in the UK. J. Dairy Res. 2007, 74, 302–310. [Google Scholar] [CrossRef]
- Adams, A.; Lombard, J.; Fossler, C.; Román-Muñiz, I.; Kopral, C. Associations between Housing and Management Practices and the Prevalence of Lameness, Hock Lesions, and Thin Cows on US Dairy Operations. J. Dairy Sci. 2017, 100, 2119–2136. [Google Scholar] [CrossRef]
- Kielland, C.; Ruud, L.E.; Zanella, A.J.; Østerås, O. Prevalence and Risk Factors for Skin Lesions on Legs of Dairy Cattle Housed in Freestalls in Norway. J. Dairy Sci. 2009, 92, 5487–5496. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, K.M.D.; Langford, F.M.; Jack, M.C.; Sherwood, L.; Lawrence, A.B.; Haskell, M.J. Hock Injury Prevalence and Associated Risk Factors on Organic and Nonorganic Dairy Farms in the United Kingdom. J. Dairy Sci. 2008, 91, 2265–2274. [Google Scholar] [CrossRef]
- Burow, E.; Thomsen, P.T.; Rousing, T.; Sørensen, J.T. Daily Grazing Time as a Risk Factor for Alterations at the Hock Joint Integument in Dairy Cows. Animal 2013, 7, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Jewell, M.T.; Cameron, M.; Spears, J.; McKenna, S.L.; Cockram, M.S.; Sanchez, J.; Keefe, G.P. Prevalence of Hock, Knee, and Neck Skin Lesions and Associated Risk Factors in Dairy Herds in the Maritime Provinces of Canada. J. Dairy Sci. 2019, 102, 3376–3391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potterton, S.L.; Green, M.J.; Harris, J.; Millar, K.M.; Whay, H.R.; Huxley, J.N. Risk Factors Associated with Hair Loss, Ulceration, and Swelling at the Hock in Freestall-Housed UK Dairy Herds. J. Dairy Sci. 2011, 94, 2952–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Body Condition Score and Its Association with Dairy Cow Productivity, Health, and Welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fall, N.; Grohn, Y.T.; Forslund, K.; Essen-Gustafsson, B.; Niskanen, R.; Emanuelson, U. An Observational Study on Early-Lactation Metabolic Profiles in Swedish Organically and Conventionally Managed Dairy Cows. J. Dairy Sci. 2008, 91, 3983–3992. [Google Scholar] [CrossRef] [PubMed]
- Roesch, M.; Doherr, M.G.; Blum, J.W. Performance of Dairy Cows on Swiss Farms with Organic and Integrated Production. J. Dairy Sci. 2005, 88, 2462–2475. [Google Scholar] [CrossRef]
- Trachsel, P.; Busato, A.; Blum, J.W. Body Conditions Scores of Dairy Cattle in Organic Farms. J. Anim. Physiol. Anim. Nutr. 2000, 84, 112–124. [Google Scholar] [CrossRef]
- Veissier, I.; Aubert, A.; Boissy, A. Animal Welfare: A Result of Animal Background and Perception of Its Environment. Anim. Front. 2012, 2, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Mullen, K.A.E.; Dings, E.H.A.; Kearns, R.R.; Washburn, S.P. A Comparison of Production, Reproduction, and Animal Health for Pastured Dairy Cows Managed Either Conventionally or with Use of Organic Principles. Prof. Anim. Sci. 2015, 31, 167–174. [Google Scholar] [CrossRef]
- Garmo, R.T.; Waage, S.; Sviland, S.; Henriksen, B.I.F.; Østerås, O.; Reksen, O. Reproductive Performance, Udder Health, and Antibiotic Resistance in Mastitis Bacteria Isolated from Norwegian Red Cows in Conventional and Organic Farming. Acta Vet. Scand. 2010, 52, 11. [Google Scholar] [CrossRef] [Green Version]
- Ahlman, T. Organic Dairy Production. Herd Characteristics and Genotype by Environment Interactions; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2010; ISBN 978-91-576-7472-2. [Google Scholar]
- Nauta, W.J.; Baars, T.; Bovenhuis, H. Converting to Organic Dairy Farming: Consequences for Production, Somatic Cell Scores and Calving Interval of First Parity Holstein Cows. Livest. Sci. 2006, 99, 185–195. [Google Scholar] [CrossRef]
- Sundberg, T.; Berglund, B.; Rydhmer, L.; Strandberg, E. Fertility, Somatic Cell Count and Milk Production in Swedish Organic and Conventional Dairy Herds. Livest. Sci. 2009, 126, 176–182. [Google Scholar] [CrossRef]
- Van Wagenberg, C.P.A.; De Haas, Y.; Hogeveen, H.; Van Krimpen, M.M.; Meuwissen, M.P.M.; Van Middelaar, C.E.; Rodenburg, T.B. Animal Board Invited Review: Comparing Conventional and Organic Livestock Production Systems on Different Aspects of Sustainability. Animal 2017, 11, 1839–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.H.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Invited Review: Organic and Conventionally Produced Milk-An Evaluation of Factors Influencing Milk Composition. J. Dairy Sci. 2015, 98, 721–746. [Google Scholar] [CrossRef] [Green Version]
- Adler, S.A.; Jensen, S.K.; Govasmark, E.; Steinshamn, H. Effect of Short-Term versus Long-Term Grassland Management and Seasonal Variation in Organic and Conventional Dairy Farming on the Composition of Bulk Tank Milk. J. Dairy Sci. 2013, 96, 5793–5810. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Bermúdez, R.; Miranda, M.; Orjales, I.; Rey-Crespo, F.; Muñoz, N.; López-Alonso, M. Holstein-Friesian Milk Performance in Organic Farming in North Spain: Comparison with Other Systems and Breeds. Span. J. Agric. Res. 2017, 15, 20. [Google Scholar] [CrossRef] [Green Version]
- Vicini, J.; Etherton, T.; Kris-Etherton, P.; Ballam, J.; Denham, S.; Staub, R.; Goldstein, D.; Cady, R.; McGrath, M.; Lucy, M. Survey of Retail Milk Composition as Affected by Label Claims Regarding Farm-Management Practices. J. Am. Diet. Assoc. 2008, 108, 1198–1203. [Google Scholar] [CrossRef]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat Composition of Organic and Conventional Retail Milk in Northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef]
- Kouřimská, L.; Legarová, V.; Panovská, Z.; Pánek, J. Quality of Cows’ Milk from Organic and Conventional Farming. Czech J. Food Sci. 2014, 32, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Butler, G.; Collomb, M.; Rehberger, B.; Sanderson, R.; Eyre, M.; Leifert, C. Conjugated Linoleic Acid Isomer Concentrations in Milk from High- and Low-Input Management Dairy Systems. J. Sci. Food Agric. 2009, 89, 697–705. [Google Scholar] [CrossRef]
- Ellis, K.A.; Jackson, A.; Bexiga, R.; Matthews, J.; McGoldrick, J.; Gilleard, J.; Forbes, A.B. Use of Diagnostic Markers to Monitor Fasciolosis and Gastrointestinal Nematodes on an Organic Dairy Farm. Vet. Rec. 2011, 169, 524. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Dahlström, F.; Engström, A.; Hessle, A. Antibodies to Major Pasture Borne Helminth Infections in Bulk-Tank Milk Samples from Organic and Nearby Conventional Dairy Herds in South-Central Sweden. Vet. Parasitol. 2010, 171, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Forstmaier, T.; Knubben-Schweizer, G.; Strube, C.; Zablotski, Y.; Wenzel, C.; Manfredi, M.T.; Gazzonis, A.L.; Mortarino, M.; Villa, L.; Zanzani, S. Rumen (Calicophoron/Paramphistomum spp.) and Liver Flukes (Fasciola hepatica) in Cattle—Prevalence, Distribution, and Impact of Management Factors in Germany. Animals 2021, 9, 2727. [Google Scholar] [CrossRef]
- Orjales, I.; Mezo, M.; Miranda, M.; González-Warleta, M.; Rey-Crespo, F.; Vaarst, M.; Thamsborg, S.; Diéguez, F.J.; Castro-Hermida, J.A.; López-Alonso, M. Helminth Infections on Organic Dairy Farms in Spain. Vet. Parasitol. 2017, 243, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Sorge, U.S.; Moon, R.D.; Stromberg, B.E.; Schroth, S.L.; Michels, L.; Wolff, L.J.; Kelton, D.F.; Heins, B.J. Parasites and Parasite Management Practices of Organic and Conventional Dairy Herds in Minnesota. J. Dairy Sci. 2015, 98, 3143–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapsch, C.; Schweizer, G.; Grimm, F.; Kohler, L.; Bauer, C.; Deplazes, P.; Braun, U.; Torgerson, P. Estimating the True Prevalence of Fasciola Hepatica in Cattle Slaughtered in Switzerland in the Absence of an Absolute Diagnostic Test. Int. J. Parasitol. 2006, 36, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
| Measures | Description | Score |
|---|---|---|
| Mobility | Good/Imperfect | 0/1 |
| Impaired | 2 | |
| Severely Impaired | 3 | |
| Body Condition Score | Thin | 1 |
| Moderate | 2–3 | |
| Fat | 4–5 | |
| Cleanliness | No dirt/only minor splashing | 0 |
| Very dirty: plaques > forearm length | 2 | |
| Hair Loss, Lesions and Swellings * | No/slight skin damage/swelling | 0 |
| Hairless patch (1 or more ≥ 2 cm) | H | |
| Lesion (1 or more ≥ 2 cm) | L | |
| Mild swelling | 1S | |
| Substantial swelling (≥5 cm in diameter) | 2S | |
| Response of cattle to stockperson | Sociable | 0 |
| Relaxed | 1 | |
| Nervous | 2 | |
| Broken tails | Number of broken tails observed | |
| Mastitis | Number of recorded cases of mastitis per 100 cows for the previous 12 months. | |
| ORG | CONV | |||
|---|---|---|---|---|
| Welfare Measures | n/tot (%) | Mean ± s.d./Farm | n/tot (%) | mean ± s.d./Farm |
| Lame animals | 29/150 (19.3) | 5.8 ± 2.6 | 41/150 (27.3) | 9 ± 2.4 |
| Unclean animals | 44/150 (29.3) | 8.8 ± 2.2 | 29/150 (19.3) | 5.8 ± 1.1 |
| Animals with rear leg skin damage | 15/150 (10.0) B | 3 ± 0.7 | 40/150 (26.7) A | 8 ± 2.0 |
| Animals with head and body damage | ||||
| Total | 27/150 (18.0) | 5.4 ± 1.1 | 31/150 (20.7) | 6.2 ± 1.9 |
| Animals with head and neck skin damage | 5/150 (3.3) | 1 ± 0.7 | 5/150 (3.3) | 1.2 ± 1.1 |
| Animals with body skin damage | 7/150 (4.7) | 1.4 ± 1.1 | 11/150 (7.3) | 2 ± 1.2 |
| Animals with front legs skin damage | 15/150 (10.0) | 3 ± 0.7 | 14/150 (9.3) | 3 ± 0.7 |
| BCS | ||||
| 1 | 1/150 (0.7) | 0.2 ± 0.4 | 4/150 (2.7) | 0.8 ± 0.4 |
| 2 | 34/150 (22.7) | 6.8 ± 1.9 | 35/150 (23.3) | 7 ± 1.6 |
| 3 | 85/150 (56.7) | 17 ± 3.2 | 86/150 (57.3) | 17.2 ± 4.1 |
| 4 | 30/150 (20.0) | 6 ± 1.6 | 25/150 (16.7) | 6 ± 2.3 |
| Milk Yield and Quality | CONV | ORG |
|---|---|---|
| ME milk (kg) | 12047.2 ± 2635.3 A | 9656.9 ± 1620.7 B |
| ME fat (kg) | 450.3 ± 102.8 A | 396.6 ± 66.8 B |
| Fat (%) * | 3.7 ± 0.6 B | 4.1 ± 0.6 A |
| Protein (%) * | 3.1 ± 0.4 | 3.1 ± 0.2 |
| ORG | CONV | TOTAL | |||
|---|---|---|---|---|---|
| Flotation | McMaster | Flotation | McMaster | Flotation | |
| n/tot (%) | OPG/EPG | n/tot (%) | OPG/EPG | n/tot (%) | |
| Coccidia | 10/150 (6.7) | <50–150 | 8/150 (5.3) | <50–150 | 18/300 (6) |
| Strongilidae | 6/150 (4) | <50–50 | 8/150 (5.3) | <50 | 14/300 (4.7) |
| Trichuris spp. | 0 | - | 1/150 (0.7) | <50 | 1/300 (0.3) |
| Positive * cows | 16/150 (10.7) | - | 12/150 (8) | - | 28/300 (9.3) |
| Negative cows | 134/150 (89.3) | - | 138 (150/192) | - | 272/300 (90.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chincarini, M.; Lanzoni, L.; Di Pasquale, J.; Morelli, S.; Vignola, G.; Paoletti, B.; Di Cesare, A. Animal Welfare and Parasite Infections in Organic and Conventional Dairy Farms: A Comparative Pilot Study in Central Italy. Animals 2022, 12, 351. https://doi.org/10.3390/ani12030351
Chincarini M, Lanzoni L, Di Pasquale J, Morelli S, Vignola G, Paoletti B, Di Cesare A. Animal Welfare and Parasite Infections in Organic and Conventional Dairy Farms: A Comparative Pilot Study in Central Italy. Animals. 2022; 12(3):351. https://doi.org/10.3390/ani12030351
Chicago/Turabian StyleChincarini, Matteo, Lydia Lanzoni, Jorgelina Di Pasquale, Simone Morelli, Giorgio Vignola, Barbara Paoletti, and Angela Di Cesare. 2022. "Animal Welfare and Parasite Infections in Organic and Conventional Dairy Farms: A Comparative Pilot Study in Central Italy" Animals 12, no. 3: 351. https://doi.org/10.3390/ani12030351
APA StyleChincarini, M., Lanzoni, L., Di Pasquale, J., Morelli, S., Vignola, G., Paoletti, B., & Di Cesare, A. (2022). Animal Welfare and Parasite Infections in Organic and Conventional Dairy Farms: A Comparative Pilot Study in Central Italy. Animals, 12(3), 351. https://doi.org/10.3390/ani12030351









