Effect of 15° Reverse Trendelenburg Position on Arterial Oxygen Tension during Isoflurane Anesthesia in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Anesthesia
2.3. Body Positioning Protocols during Anesthesia
2.4. Monitoring and Support during Anesthesia
2.5. Recovery from Anesthesia
2.6. Calculations
2.7. Statistical Analysis
3. Results
3.1. Respiratory Variables
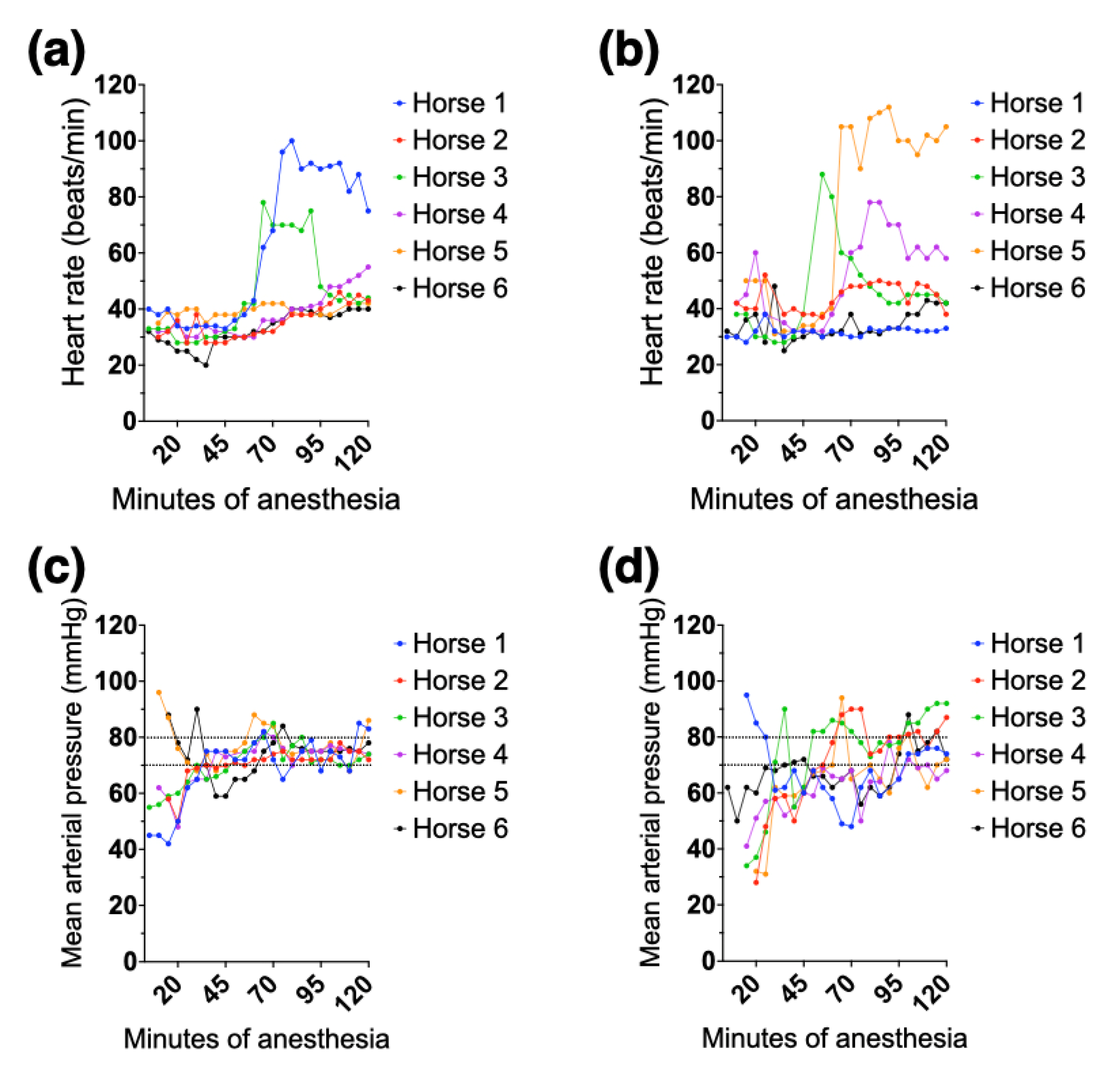
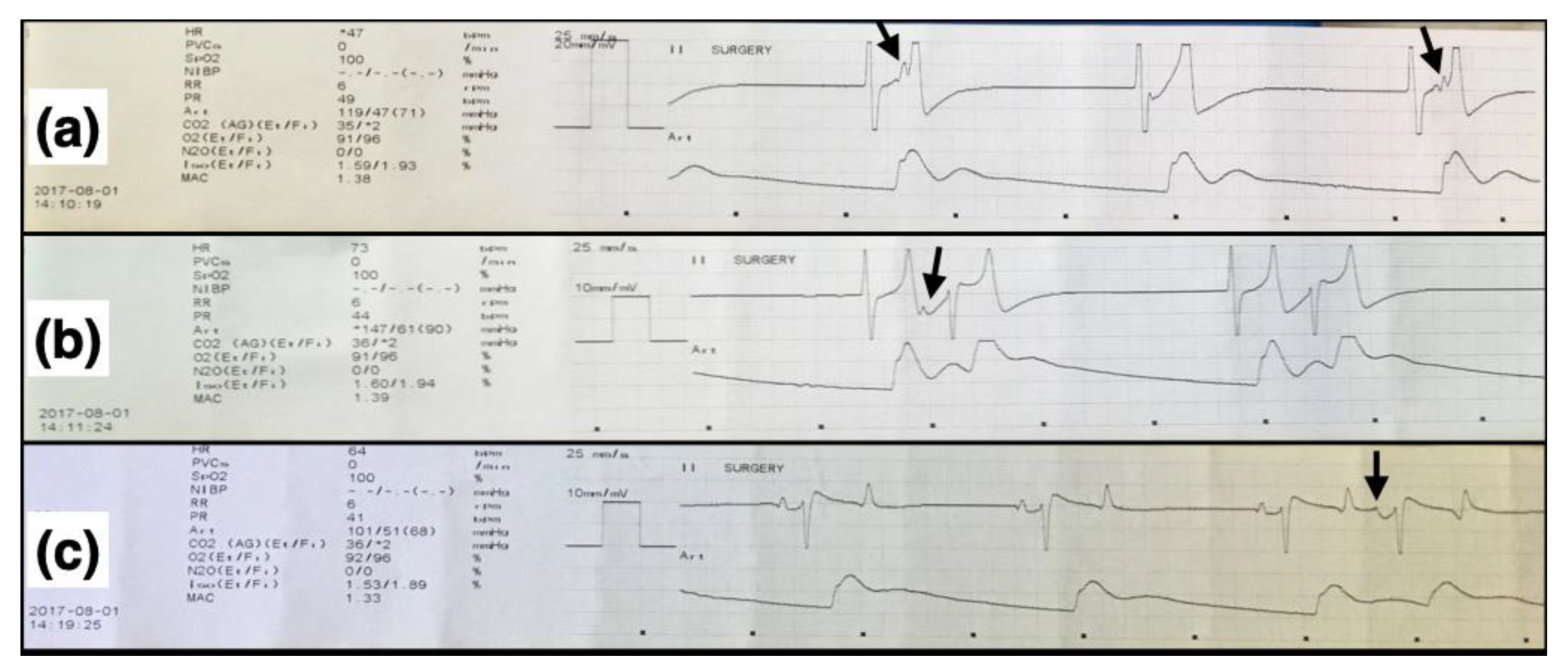
3.2. Clinical Cardiovascular and Other Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, L.W.; Gillespie, J.R.; Tyler, W.S. Alveolar-arterial oxygen tension differences in anaesthetized horses. Br. J. Anaesth 1968, 40, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.R.; Tyler, W.S.; Hall, L.W. Cardiopulmonary dysfunction in anesthetized, laterally recumbent horses. Am. J. Vet. Res. 1969, 30, 61–72. [Google Scholar] [PubMed]
- Hall, L.W. Disturbances of cardiopulmonary function in anaesthetised horses. Equine Vet. J. 1971, 3, 95–98. [Google Scholar] [CrossRef] [PubMed]
- McDonell, W.N.; Hall, L.W.; Jeffcott, L.B. Radiographic evidence of impaired pulmonary function in laterally recumbent anaesthetised horses. Equine Vet. J. 1979, 11, 24–32. [Google Scholar] [CrossRef]
- Nyman, G.; Hedenstierna, G. Ventilation-perfusion relationhsips in the anaesthetised horse. Equine Vet. J. 1989, 21, 274–281. [Google Scholar] [CrossRef]
- Marntell, S.; Nyman, G.; Hedenstierna, G. High inspired oxygen concentrations increase intrapulmonary shunt in anaesthetized horses. Vet. Anaesth. Analg. 2005, 32, 338–347. [Google Scholar] [CrossRef]
- Marntell, S.; Nyman, G.; Funkquist, P.; Hedenstierna, G. Effects of acepromazine on pulmonary gas exchange and circulation during sedation and dissociative anaesthesia in horses. Vet. Anaesth. Analg. 2005, 32, 83–93. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Muir, W.W. Oxygenation, oxygen delivery and anaesthesia in the horse. Equine Vet. J. 2015, 47, 25–35. [Google Scholar] [CrossRef]
- Steffey, E.P.; Wheat, J.D.; Meagher, D.M.; Norrie, R.D.; McKee, J.; Brown, M.; Arnold, J. Body position and mode of ventilation influences arterial pH, oxygen, and carbon dioxide tensions in halothane-anesthetized horses. Am. J. Vet. Res. 1977, 38, 379–382. [Google Scholar]
- Young, L.E.; Marlin, D.J.; McMurphy, R.M.; Walsh, K.; Dixon, P.M. Effects of inhaled nitric oxide 10 ppm in spontaneously breathing horses anaesthetized with halothane. Br. J. Anaesth 1999, 83, 321–324. [Google Scholar] [CrossRef]
- Moens, Y.; Lagerweij, E.; Gootjes, P.; Poortman, J. Distribution of inspired gas to each lung in the anaesthetised horse and influence of body shape. Equine Vet. J. 1995, 27, 110–116. [Google Scholar] [CrossRef]
- Mansel, J.C.; Clutton, R.E. The influence of body mass and thoracic dimensions on arterial oxygenation in anaesthetized horses and ponies. Vet. Anaesth. Analg. 2008, 35, 392–399. [Google Scholar] [CrossRef]
- Trenholme, H.N.; Barletta, M.; Quandt, J.E.; Reed, R.A.; Kleine, S.A.; Hofmeister, E.H. Arterial oxygenation in anesthetized horses placed in a 5-degree reverse trendelenburg position. Res. Vet. Sci. 2021, 135, 304–309. [Google Scholar] [CrossRef]
- Schauvliege, S.; Binetti, A.; Duchateau, L.; van Dijk, J.J.; Gasthuys, F. Cardiorespiratory effects of a 7 degrees reverse trendelenburg position in anaesthetized horses: A randomized clinical trial. Vet. Anaesth. Analg. 2018, 45, 648–657. [Google Scholar] [CrossRef]
- Hopster, K.; Rohn, K.; Ohnesorge, B.; Kastner, S.B.R. Controlled mechanical ventilation with constant positive end-expiratory pressure and alveolar recruitment manoeuvres during anaesthesia in laterally or dorsally recumbent horses. Vet. Anaesth. Analg. 2017, 44, 121–126. [Google Scholar] [CrossRef]
- Hopster, K.; Kastner, S.B.; Rohn, K.; Ohnesorge, B. Intermittent positive pressure ventilation with constant positive end-expiratory pressure and alveolar recruitment manoeuvre during inhalation anaesthesia in horses undergoing surgery for colic, and its influence on the early recovery period. Vet. Anaesth. Analg. 2011, 38, 169–177. [Google Scholar] [CrossRef]
- Mosing, M.; Rysnik, M.; Bardell, D.; Cripps, P.J.; MacFarlane, P. Use of continuous positive airway pressure (CPAP) to optimise oxygenation in anaesthetised horses--a clinical study. Equine. Vet. J. 2013, 45, 414–418. [Google Scholar] [CrossRef]
- Lee, Y.H.; Clarke, K.W.; Alibhai, H.I. The cardiopulmonary effects of clenbuterol when administered to dorsally recumbent halothane-anaesthetised ponies--failure to increase arterial oxygenation. Res. Vet. Sci. 1998, 65, 227–232. [Google Scholar] [CrossRef]
- Robertson, S.A.; Bailey, J.E. Aerosolized salbutamol (albuterol) improves PaO2 in hypoxaemic anaesthetized horses, a prospective clinical trial in 81 horses. Vet. Anaesth. Analg. 2002, 29, 212–218. [Google Scholar] [CrossRef]
- Grubb, T.L.; Hogman, M.; Edner, A.; Frendin, J.H.; Heinonen, E.; Malavasi, L.M.; Frostell, C.G.; Ryden, A.; Alving, K.; Nyman, G.C. Physiologic responses and plasma endothelin-1 concentrations associated with abrupt cessation of nitric oxide inhalation in isoflurane-anesthetized horses. Am. J. Vet. Res. 2008, 69, 423–430. [Google Scholar] [CrossRef]
- Nyman, G.; Grubb, T.L.; Heinonen, E.; Frendin, J.; Edner, A.; Malavasi, L.M.; Frostell, C.; Hogman, M. Pulsed delivery of inhaled nitric oxide counteracts hypoxaemia during 2.5 h of inhalation anaesthesia in dorsally recumbent horses. Vet. Anaesth. Analg. 2012, 39, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Cuvelliez, S.G.; Eicker, S.W.; McLauchlan, C.; Brunson, D.B. Cardiovascular and respiratory effects of inspired oxygen fraction in halothane-anesthetized horses. Am. J. Vet. Res. 1990, 51, 1226–1231. [Google Scholar] [PubMed]
- Hubbell, J.A.; Aarnes, T.K.; Bednarski, R.M.; Lerche, P.; Muir, W.W. Effect of 50% and maximal inspired oxygen concentrations on respiratory variables in isoflurane-anesthetized horses. BMC Vet. Res. 2011, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Crumley, M.N.; McMurphy, R.M.; Hodgson, D.S.; Kreider, S.E. Effects of inspired oxygen concentration on ventilation, ventilatory rhythm, and gas exchange in isoflurane-anesthetized horses. Am. J. Vet. Res. 2013, 74, 183–190. [Google Scholar] [CrossRef]
- Taylor, A.H.; Seymour, C.J. Effect of low inspired oxygen fraction on respiratory indices in mechanically ventilated horses anaesthetised with isoflurane and medetomidine constant rate infusion. Vet. J. 2016, 211, 70–74. [Google Scholar] [CrossRef]
- Buchwald, H. Three helpful techniques for facilitating abdominal procedures, in particular for surgery in the obese. Am. J. Surg. 1998, 175, 63–64. [Google Scholar] [CrossRef]
- Perilli, V.; Sollazzi, L.; Bozza, P.; Modesti, C.; Chierichini, A.; Tacchino, R.M.; Ranieri, R. The effects of the reverse trendelenburg position on respiratory mechanics and blood gases in morbidly obese patients during bariatric surgery. Anesth. Analg. 2000, 91, 1520–1525. [Google Scholar] [CrossRef]
- Perilli, V.; Sollazzi, L.; Modesti, C.; Annetta, M.G.; Sacco, T.; Bocci, M.G.; Tacchino, R.M.; Proietti, R. Comparison of positive end-expiratory pressure with reverse trendelenburg position in morbidly obese patients undergoing bariatric surgery: Effects on hemodynamics and pulmonary gas exchange. Obes. Surg. 2003, 13, 605–609. [Google Scholar] [CrossRef]
- Perilli, V.; Sollazzi, L.; Modesti, C.; Sacco, T.; Bocci, M.G.; Ciocchetti, P.P.; Tacchino, R.M.; Proietti, R. Determinants of improvement in oxygenation consequent to reverse trendelenburg position in anesthetized morbidly obese patients. Obes. Surg. 2004, 14, 866–867. [Google Scholar] [CrossRef]
- Araujo, M.A.; Deschk, M.; Wagatsuma, J.T.; Floriano, B.P.; Siqueira, C.E.; Oliva, V.N.; Santos, P.S. Cardiopulmonary effects of reverse trendelenburg position at 5 degrees and 10 degrees in sevoflurane-anesthetized steers. Vet. Anaesth. Analg. 2017, 44, 854–864. [Google Scholar] [CrossRef]
- Binetti, A.; Mosing, M.; Sacks, M.; Duchateau, L.; Gasthuys, F.; Schauvliege, S. Impact of trendelenburg (head down) and reverse trendelenburg (head up) position on respiratory and cardiovascular function in anaesthetized horses. Vet. Anaesth. Analg. 2018, 45, 760–771. [Google Scholar] [CrossRef]
- Guedes, A.; Knych, H.; Tucker, L.; Almeida, D.C.; Baldo, C.F.; Wendt-Hornickle, E.; Allweiler, S. Pharmacokinetics and clinical effects of xylazine and dexmedetomidine in horses recovering from isoflurane anesthesia. J. Vet. Pharmacol. Ther. 2020, 43, 369–376. [Google Scholar] [CrossRef]
- Steffey, E.P.; Howland, D., Jr.; Giri, S.; Eger, E.I., 2nd. Enflurane, halothane, and isoflurane potency in horses. Am. J. Vet. Res. 1977, 38, 1037–1039. [Google Scholar]
- Enghoff, H. Volumen inefficax. Upsala. Lakaref. Forh. 1938, 44, 191–218. [Google Scholar]
- Araos, J.D.; Larenza, M.P.; Boston, R.C.; De Monte, V.; De Marzo, C.; Grasso, S.; Haskins, S.C.; Crovace, A.; Staffieri, F. Use of the oxygen content-based index, fshunt, as an indicator of pulmonary venous admixture at various inspired oxygen fractions in anesthetized sheep. Am. J. Vet. Res. 2012, 73, 2013–2020. [Google Scholar] [CrossRef]
- Menzies, M.P.; Ringer, S.K.; Conrot, A.; Theurillat, R.; Kluge, K.; Kutter, A.P.; Jackson, M.; Thormann, W.; Bettschart-Wolfensberger, R. Cardiopulmonary effects and anaesthesia recovery quality in horses anaesthetized with isoflurane and low-dose s-ketamine or medetomidine infusions. Vet. Anaesth. Analg. 2016, 43, 623–634. [Google Scholar] [CrossRef]
- Harrison, R.A.; Davison, R.; Shapiro, B.A.; Meyers, S.N. Reassessment of the assumed a-v oxygen content difference in the shunt calculation. Anesth. Analg. 1975, 54, 198–202. [Google Scholar] [CrossRef]
- Whitehair, K.J.; Steffey, E.P.; Woliner, M.J.; Willits, N.H. Effects of inhalation anesthetic agents on response of horses to three hours of hypoxemia. Am. J. Vet. Res. 1996, 57, 351–360. [Google Scholar]
- Clerbaux, T.; Gustin, P.; Detry, B.; Cao, M.L.; Frans, A. Comparative study of the oxyhaemoglobin dissociation curve of four mammals: Man, dog, horse and cattle. Comp. Biochem. Physiol. Comp. Physiol. 1993, 106, 687–694. [Google Scholar] [CrossRef]
- Heneghan, C.P.; Bergman, N.A.; Jones, J.G. Changes in lung volume and (PAO2-PaO2) during anaesthesia. Br. J. Anaesth. 1984, 56, 437–445. [Google Scholar] [CrossRef]
- Hubbell, J.A.E.; Muir, W.W. Anesthetic protocols and techniques for specific procedures. In Equine Anesthesia, 2nd ed.; Muir, W.W., Hubbell, J.A.E., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2009; Chapter 24; pp. 430–438. [Google Scholar]
- Lee, Y.H.; Clarke, K.W.; Alibhai, H.I.; Song, D. Effects of dopamine, dobutamine, dopexamine, phenylephrine, and saline solution on intramuscular blood flow and other cardiopulmonary variables in halothane-anesthetized ponies. Am. J. Vet. Res. 1998, 59, 1463–1472. [Google Scholar] [PubMed]
- Raisis, A.L. Skeletal muscle blood flow in anaesthetized horses. Part II: Effects of anaesthetics and vasoactive agents. Vet. Anaesth. Analg. 2005, 32, 331–337. [Google Scholar] [CrossRef]
- Naimark, A.; Wasserman, K. The effect of posture on pulmonary capillary blood flow in man. J. Clin. Investig. 1962, 41, 949–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sjostrand, T. Volume and distribution of blood and their significance in regulating the circulation. Physiol. Rev. 1953, 33, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, A.F.; Allaert, S.; Pletinckx, P.; Maes, J.W.; Heerman, J.; Vos, J.J.; Struys, M.M.R.F.; Scheeren, T.W.L. Phenylephrine increases cardiac output by raising cardiac preload in patients with anesthesia induced hypotension. J. Clin. Monit. Comput. 2018, 32, 969–976. [Google Scholar] [CrossRef]
- Duke, T.; Filzek, U.; Read, M.R.; Read, E.K.; Ferguson, J.G. Clinical observations surrounding an increased incidence of postanesthetic myopathy in halothane-anesthetized horses. Vet. Anaesth. Analg. 2006, 33, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Raisis, A.L.; Young, L.E.; Taylor, P.M.; Walsh, K.P.; Lekeux, P. Doppler ultrasonography and single-fiber laser doppler flowmetry for measurement of hind limb blood flow in anesthetized horses. Am. J. Vet. Res. 2000, 61, 286–290. [Google Scholar] [CrossRef]
- Dancker, C.; Hopster, K.; Rohn, K.; Kastner, S.B. Effects of dobutamine, dopamine, phenylephrine and noradrenaline on systemic haemodynamics and intestinal perfusion in isoflurane anaesthetised horses. Equine Vet. J. 2018, 50, 104–110. [Google Scholar] [CrossRef]
- Visser, L.C.; Scansen, B.A.; Bonagura, J.D. ECG of the month. J. Am. Vet. Med. Assoc. 2014, 245, 52–54. [Google Scholar] [CrossRef]
- Bélanger, C.; Visser, L.C.; Stern, J.A. ECG of the month. J. Am. Vet. Med. Assoc. 2016, 249, 1138–1140. [Google Scholar] [CrossRef]
- Schamroth, L.; Dubb, A. Escape-capture bigeminy. Mechanisms in S-A block, A-V block, and reversed reciprocal rhythm. Br. Heart J. 1965, 27, 667–669. [Google Scholar] [CrossRef]
- Hopster, K.; Wittenberg-Voges, L.; Geburek, F.; Hopster-Iversen, C.; Kastner, S.B.R. Effects of controlled hypoxemia or hypovolemia on global and intestinal oxygenation and perfusion in isoflurane anesthetized horses receiving an alpha-2-agonist infusion. BMC Vet. Res. 2017, 13, 361. [Google Scholar] [CrossRef]
- Hopster, K.; Muller, C.; Hopster-Iversen, C.; Stahl, J.; Rohn, K.; Kastner, S. Effects of dexmedetomidine and xylazine on cardiovascular function during total intravenous anaesthesia with midazolam and ketamine and recovery quality and duration in horses. Vet. Anaesth. Analg. 2014, 41, 25–35. [Google Scholar] [CrossRef]
- Chiang, S.T. A nomogram for venous shunt (Qs-Qt) calculation. Thorax 1968, 23, 563–565. [Google Scholar] [CrossRef]
- Briganti, A.; Portela, D.A.; Grasso, S.; Sgorbini, M.; Tayari, H.; Bassini, J.R.; Vitale, V.; Romano, M.S.; Crovace, A.; Breghi, G.; et al. Accuracy of different oxygenation indices in estimating intrapulmonary shunting at increasing infusion rates of dobutamine in horses under general anaesthesia. Vet. J. 2015, 204, 351–356. [Google Scholar] [CrossRef]
- Peiro, J.R.; Borges, A.S.; Goncalves, R.C.; Mendes, L.C. Evaluation of a portable clinical analyzer for the determination of blood gas partial pressures, electrolyte concentrations, and hematocrit in venous blood samples collected from cattle, horses, and sheep. Am. J. Vet. Res. 2010, 71, 515–521. [Google Scholar] [CrossRef]
| Variable | Horse | |||||
|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5* | #6 | |
| Breed | QH | Arabian | Mixed | QH | Mixed | QH |
| Sex | Gelding | Gelding | Gelding | Gelding | Gelding | Female |
| Age (years) | 9 | 10 | 5 | 6 | 13 | 8 |
| Body weight (kg) | 536 | 397 | 417 | 467 | 484 | 471 |
| Body shape | RB | RB | RB | RB | FB | RB |
| Thoracic depth (cm) | 75 | 74 | 67 | 70 | 72 | 72 |
| Thoracic width (cm) | 34 | 27 | 30 | 31 | 34 | 36 |
| Thoracic length (cm) | 99 | 93 | 91 | 95 | 141 | 93 |
| Thoracic circumference (cm) | 192 | 172 | 171 | 181 | 184 | 188 |
| Variable | Position | Minutes of Anesthesia | Overall Mean ± SD (95% CI) | |||
|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | |||
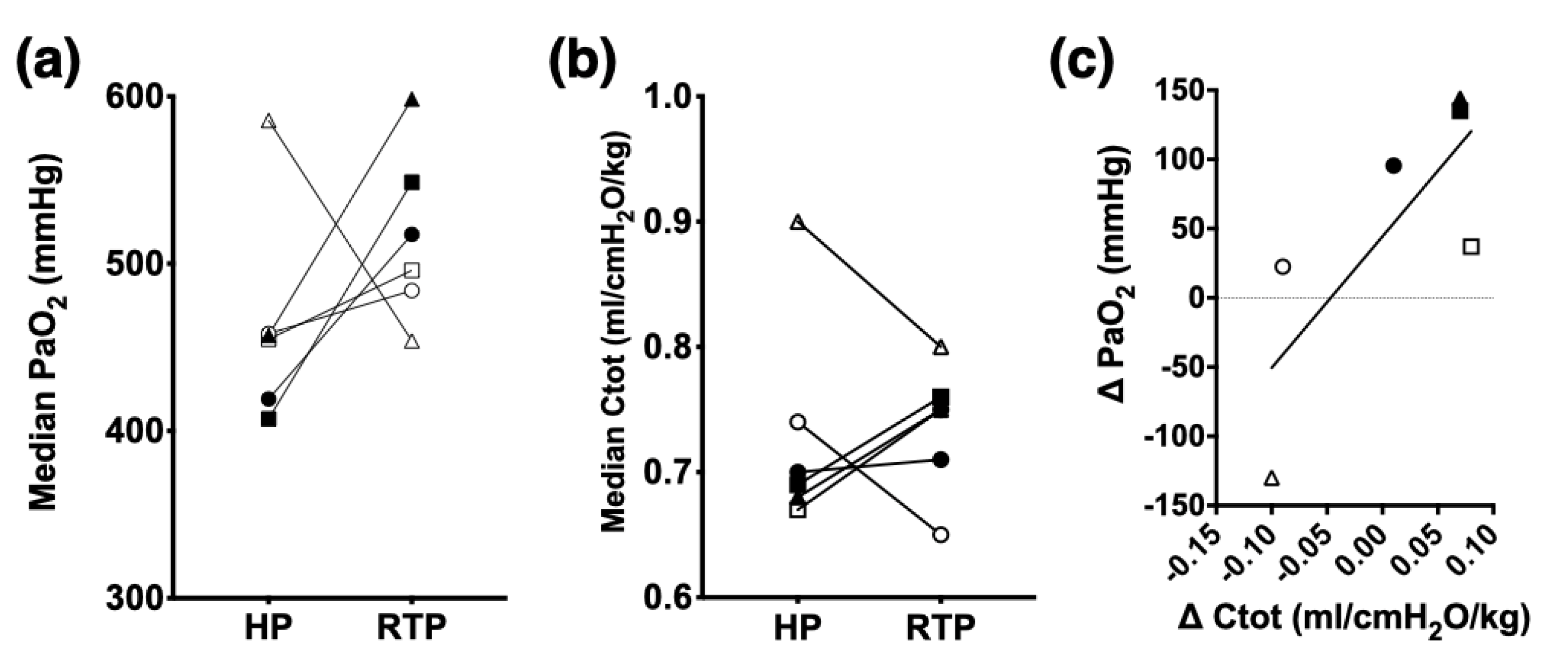
| PaO2 (mmHg) | HP | 415 ± 21 | 449 ± 32 | 413 ± 27 | 420 ± 33 | 424 ± 17 (398, 451) |
| RTP | 484 ± 53 | 486 ± 79 | 497 ± 60 * | 502 ± 52 * | 504 ± 14 (482, 525) | |
| P–F ratio (mmHg) | HP | 432 ± 25 | 466 ± 35 | 427 ± 27 | 433 ± 34 | 440 ± 18 (411, 468) |
| RTP | 510 ± 56 * | 505 ± 81 | 513 ± 62 * | 518 ± 53 * | 512 ± 5 (503, 520) | |
| F–shunt (%) | HP | 15 ± 1 | 13 ± 2 | 15 ± 1 | 15 ± 2 | 15 ± 1 (13, 17) |
| RTP | 10 ± 3 * | 10 ± 6 | 10 ± 4 * | 9 ± 3 * | 10 ± 0.3 (9, 10) | |
| PaCO2 (mmHg) | HP | 48 ± 7 | 53 ± 4 | 52 ± 6 | 51 ± 10 | 52 ± 2 (49, 55) |
| RTP | 49 ± 4 | 54 ± 7 | 54 ± 5 | 57 ± 6 | 53 ± 2 (49, 57) | |
| PETCO2 (mmHg) | HP | 35 ± 2 | 38 ± 2 | 36 ± 4 | 35 ± 6 | 36 ± 1 (34, 38) |
| RTP | 38 ± 3 | 39 ± 4 | 38 ± 3 | 37 ± 3 | 38 ± 1 (36, 39) | |
| Vd/Vt (%) | HP | 26 ± 8 | 29 ± 2 | 31 ± 3 | 32 ± 6 | 29 ± 3 (25, 33) |
| RTP | 24 ± 2 a | 28 ± 3 ab | 30 ± 4 b | 35 ± 4 b | 29 ± 5 (22, 36) | |
| Variable | Position | Horse | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | |||
| RT (°C) | HP | 36.0 (35–36.6) | 36.4 (36.1–37.3) | 36.5 (36.3–36.6) | 36.3 (36.0–36.8) | 36.3 (35.3–37.2) | 37.8 (36.6–37.8) | 36.4 (35.6–37.1) |
| RTP | 37.2 (36.2–37.2) | 36.3 (36.2–37.4) | 37.2 (36.2–37.4) | 36.4 (36.3–36.9) | 35.0 (34.2–36.6) | 36.7 (36.7–37.1) | 36.4 (36.0–36.9) | |
| Dobutamine (μg/kg/min) | HP | 1.4 | 2.1 | 1.5 | 1.7 | 0.5 | 1.2 | 1.5 |
| RTP | 2.1 | 4.8 | 1.5 | 1.1 | 3 | 1.8 | 1.8 | |
| Phenylephrine (μg/kg) | HP | 5.6 | 0 | 6.2 | 0.9 | 0 | 0 | 0.9 |
| RTP | 42.9 | 8.1 | 2.4 | 28.9 | 12.4 | 39.9 | 29 | |
| Body position during first anesthesia | - | HP | RTP | RTP | HP | RTP | HP | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucker, L.; Almeida, D.; Wendt-Hornickle, E.; Baldo, C.F.; Allweiler, S.; Guedes, A.G.P. Effect of 15° Reverse Trendelenburg Position on Arterial Oxygen Tension during Isoflurane Anesthesia in Horses. Animals 2022, 12, 353. https://doi.org/10.3390/ani12030353
Tucker L, Almeida D, Wendt-Hornickle E, Baldo CF, Allweiler S, Guedes AGP. Effect of 15° Reverse Trendelenburg Position on Arterial Oxygen Tension during Isoflurane Anesthesia in Horses. Animals. 2022; 12(3):353. https://doi.org/10.3390/ani12030353
Chicago/Turabian StyleTucker, Laura, Daniel Almeida, Erin Wendt-Hornickle, Caroline F. Baldo, Sandra Allweiler, and Alonso G. P. Guedes. 2022. "Effect of 15° Reverse Trendelenburg Position on Arterial Oxygen Tension during Isoflurane Anesthesia in Horses" Animals 12, no. 3: 353. https://doi.org/10.3390/ani12030353
APA StyleTucker, L., Almeida, D., Wendt-Hornickle, E., Baldo, C. F., Allweiler, S., & Guedes, A. G. P. (2022). Effect of 15° Reverse Trendelenburg Position on Arterial Oxygen Tension during Isoflurane Anesthesia in Horses. Animals, 12(3), 353. https://doi.org/10.3390/ani12030353







