Response of Long-Tailed Duck (Clangula hyemalis) to the Change in the Main Prey Availability in Its Baltic Wintering Ground
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
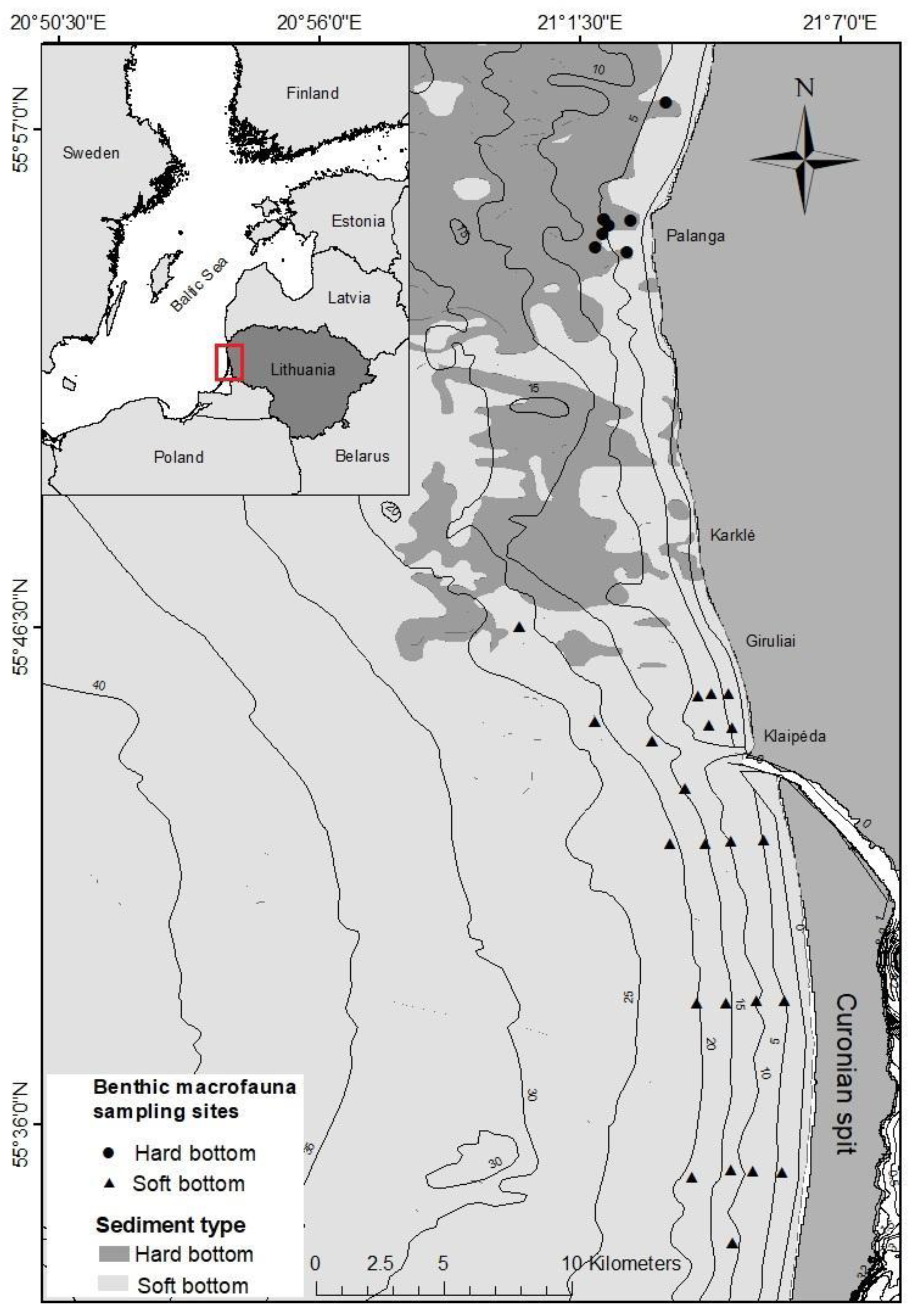
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Seabed Macrofauna Community
3.2. Stomach Data
3.2.1. Feeding of Juveniles
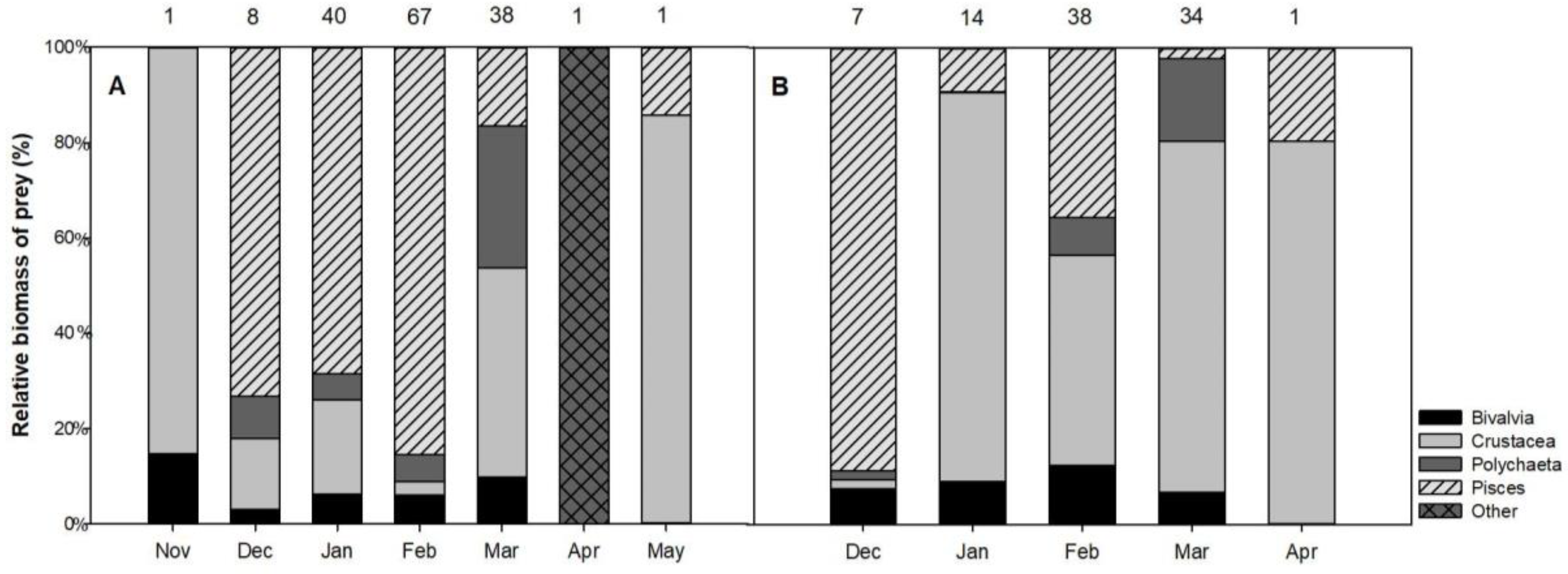
3.2.2. Monthly Variation in the Diet
3.3. Body Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellebaum, J.; Larsson, K.; Kube, J. Sea Ducks in the Baltic Sea. 2012. Available online: www.hgo.se/seaducks (accessed on 6 April 2021).
- Skov, H.; Heinänen, S.; Žydelis, R.; Bellebaum, J.; Bzoma, S.; Dagys, M.; Durinck, J.; Garthe, S.; Grishanov, G.; Hario, M.; et al. Waterbird populations and pressures in the Baltic Sea. TemaNord 2011, 550, 201. [Google Scholar]
- Hearn, R.; Harrison, A.; Cranswick, P. International Single Species Action Plan for the Conservation of the Long-tailed Duck Clangula hyemalis. In Agreement on the Conservation of African-Eurasian Migratory Waterbirds (AEWA); AEWA Technical Series No. 57; Wildfowl & Wetlands Trust: Slimbridge, UK, 2015. [Google Scholar]
- Žydelis, R. Habitat Selection of Waterbirds Wintering in Lithuanian Coastal Zone of the Baltic Sea. Ph.D. Thesis, Vilnius University, Vilnius, Lithuania, 2002. [Google Scholar]
- Bellebaum, J.; Kube, J.; Schulz, A.; Skov, H.; Wendeln, H. Decline of Long-tailed Duck Clangula hyemalis numbers in the Pomeranian Bay revealed by two different survey methods. Ornis Fenn. 2014, 91, 129–137. [Google Scholar]
- Karwinkel, T.; Pollet, I.L.; Vardeh, S.; Kruckenberg, H.; Glazov, P.; Loshchagina, J.; Kondratyev, A.; Merkel, B.; Bellebaum, J.; Quillfeldt, P. Year-round spatiotemporal distribution pattern of a threatened sea duck species breeding on Kolguev Island, south-eastern Barents Sea. BMC Ecol. BioMed Central 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, A.; Lindberg, V.; Larsson, K. Chemosphere Hydroxylated and methoxylated polybrominated diphenyl ethers in long-tailed ducks (Clangula hyemalis) and their main food, Baltic blue mussels (Mytilus trossulus × Mytilus edulis). Chemosphere 2016, 144, 1475–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long-Tailed Duck (Clangula hyemalis)—BirdLife Species Factsheet. Available online: http://datazone.birdlife.org/species/factsheet/long-tailed-duck-clangula-hyemalis/text (accessed on 26 November 2021).
- Larsson, K.; Tydén, L. Effects of oil spills on wintering Long-tailed Ducks Clangula hyemalis at Hoburgs bank in central Baltic Sea between 1996/97 and 2003/04. Ornis Svec. 2005, 15, 161–171. [Google Scholar]
- Nilsson, L. Habitat Selection, Food Choice, and Feeding Habits of Diving Ducks in Coastal Waters of South Sweden during the Non-Breeding Season. Ornis Scand. 1972, 3, 55–78. [Google Scholar] [CrossRef]
- Schorger, A.W. The deep diving of the loon and old-squaw and its mechanism. Wilson Bulletin 1947, 59, 151–159. [Google Scholar]
- Perry, M.C.; Osenton, P.C.; White, T.P. Atypical Feeding Behavior of Long-tailed Ducks in the Wake of a Commercial Fishing Boat while Clamming. Northeast. Nat. 2017, 24, 19–25. [Google Scholar] [CrossRef]
- Schummer, M.L.; PetrieS, A.; Bailey, R.C. Dietary Overlap of Sympatric Diving Ducks During Winter on Northeastern Lake Ontario. Auk 2008, 125, 425–433. [Google Scholar] [CrossRef]
- Stempniewicz, L. Feeding ecology of the long-tailed duck Clangula hyemalis wintering in the Gulf of Gdansk (southern Baltic Sea). Ornis Svec. 1995, 5, 133–142. [Google Scholar]
- Žydelis, R.; Ruškyte, D. Winter foraging of long-tailed ducks (Clangula hyemalis) exploiting different benthic communities in the Baltic Sea. Wilson Bulletin 2005, 117, 133–141. [Google Scholar] [CrossRef]
- White, T.P.; Veit, R.R. Spatial ecology of long-tailed ducks and white-winged scoters wintering on Nantucket Shoals. Ecosphere 2020, 11, e03002. [Google Scholar] [CrossRef] [Green Version]
- Backwell, P.R.Y.; O’Hara, P.D.; Christy, J.H. Prey availability and selective foraging in shorebirds. Anim. Behav. 1998, 55, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.P.; Quintana, F.; Hobson, V.J. Construction of energy landscapes can clarify the movement and distribution of foraging animals. Proc. Royal Soc. 2012, 279, 975–980. [Google Scholar] [CrossRef]
- Masello, J.F.; Kato, A.; Sommerfeld, J.; Mattern, T.; Quillfeldt, P. How animals distribute themselves in space: Variable energy landscapes. Front. Zool. 2017, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Lovvorn, J.R.; Richman, S.E.; Grebmeier, J.M.; Cooper, L.W. Diet and body condition of spectacled eiders wintering in pack ice of the Bering Sea. Polar Biol. 2003, 26, 259–267. [Google Scholar] [CrossRef]
- Drewitt, A.; Langston, R.H.W. Assessing the impacts of wind farms on birds. Ibis 2006, 148, 29–42. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Galanidi, M.; Showler, D.A.; Elliott, A.J.; Caldow, R.W.G.; Rees, E.I.S.; Stillman, R.A.; Sutherland, W.J. Distribution and behaviour of Common Scoter Melanitta nigra relative to prey resources and environmental parameters. Ibis 2006, 148, 110–128. [Google Scholar] [CrossRef]
- Perry, M.C. Foraging Behavior of Long-tailed Ducks in a Ferry Wake. Northeast Nat. 2012, 19, 135–139. [Google Scholar] [CrossRef]
- Furness, R.W.; Wade, H.M.; Masden, E.A. Assessing vulnerability of marine bird populations to offshore wind farms. J. Environ. Manag. 2013, 119, 56–66. [Google Scholar] [CrossRef]
- Kottsieper, J.; Schückel, U.; Schwemmer, P.; Fox, A.D.; Garthe, S. Comparison of bivalve communities between moulting and wintering areas used by Common Scoter Melanitta nigra in the German North Sea. Estuar. Coast. Shelf Sci. 2019, 229, 106398. [Google Scholar] [CrossRef]
- Kottsieper, J.; Schwemmer, P.; Markones, N.; Fox, A.D.; Garthe, S. An invasive alien bivalve apparently provides a novel food source for moulting and wintering benthic feeding sea ducks. Helgol. Mar. Res. 2019, 73, 11. [Google Scholar] [CrossRef]
- Skabeikis, A.; Morkūnė, R.; Bacevičius, E.; Lesutienė, J.; Morkūnas, J.; Poškienė, A.; Šiaulys, A. Effect of round goby (Neogobius melanostomus) invasion on blue mussel (Mytilus edulis trossulus) population and winter diet of the long-tailed duck (Clangula hyemalis). Biol. Invasions 2019, 21, 911–923. [Google Scholar] [CrossRef]
- Olenin, S.; Daunys, D. Coastal typology based on benthic biotope and community data: The Lithuanian case study. Baltic Sea Typology 2004, 4, 65–83. [Google Scholar]
- Van Franeker, J.A. Save the North. In Sea Fulmar-Litter-EcoQO Manual Part. 1: Collection and Dissection Procedures; Alterra-rapport 672; Wageningen University & Research (Alterra): Wageningen, The Netherlands, 2004; p. 40. [Google Scholar]
- Camphuysen, C.J.; Leopold, M.F. Diet study manual for stranded seabirds. In Technical Documents 4.1, Handbook on Oil Impact Assessment, version 1.0; EU: Maastricht, The Netherlands, 2007; Available online: www.oiledwildlife.eu (accessed on 26 November 2021).
- Mair, P.; Wilcox, R.R. Robust Statistical Methods in R Using the WRS2 Package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- Manly, B.; McDonald, L.; Thomas, D. Resource Selection by Animals, Statistical Design and Analysis for Field Studies; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Brey, T.; Rumohr, H.; Ankar, S. Energy Content of Macrobenthic Invertebrates: General Conversion Factors from Weight to Energy. J. Exp. Mar. Biol. Ecol. 1998, 117, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A.; Bourget, E. Weight-to-weight conversion factors for marine benthic macroinvertebrates. Mar. Ecol Prog Ser. 1998, 163, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Galasso, E.L.; Richard, M.; Lefebvre, S.; Aliaume, C.; Callier, M.D. Body size and temperature effects on standard metabolic rate for determining metabolic scope for activity of the polychaete Hediste (Nereis) diversicolor. PeerJ 2018, 6, e5675. [Google Scholar] [CrossRef] [Green Version]
- Stott, R.S.; Olson, D.P. Food-Habitat Relationship of Sea Ducks on the New Hampshire Coastline. Ecology 1973, 54, 996–1007. [Google Scholar] [CrossRef]
- Šaškov, A.; Šiaulys, A.; Bučas, M.; Daunys, D. Baltic herring (Clupea harengus membras) spawning grounds on the Lithuanian coast: Current status and shaping factors. Oceanologia 2014, 56, 789–804. [Google Scholar] [CrossRef] [Green Version]
- Wanless, S.; Harris, M.P.; Redman, P.; Speakman, J.R. Low energy values of fish as a probable cause of a major seabird breeding. Mar. Ecol. Prog. Ser. 2005, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Meissner, W.; Fischer, I.; Bzoma, S. Changes in the body composition of Velvet Scoters (Melanitta fusca) wintering in the Gulf of Gdańsk. Oceanol. Hydrobiol. Stud. 2012, 41, 11–16. [Google Scholar] [CrossRef]


| Soft-Bottom | Hard-Bottom | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Juvenile | Adult | Total | Sex | Juvenile | Adult | Total |
| Female | 4 | 27 | 31 | Female | 16 | 42 | 58 |
| Male | 2 | 62 | 64 | Male | 3 | 95 | 98 |
| Total | 6 | 89 | 95 | Total | 19 | 137 | 156 |
| Species/Taxa | Hard-Bottom | Soft-Bottom | ||||
|---|---|---|---|---|---|---|
| F (%) | Abundance (SE) | Biomass (SE) | F (%) | Abundance (SE) | Biomass (SE) | |
| M. edulis trossulus | 51% | 280.5 (64.8) | 3.0 (2.0) | 3% | 0.3 (0.3) | <0.1 (<0.1) |
| C. glaucum | 19% | 2.9 (1.3) | 0.2 (0.1) | 76% | 240.6 (83.3) | 67.8 (21.5) |
| L. balthica | 49% | 20.5 (14.3) | 5.5 (3.8) | 82% | 2080.3 (477.0) | 123.9 (25.5) |
| M. arenaria | 45% | 8.4 (2.4) | 0.2 (0.1) | 76% | 1274.2 (512.1) | 32.9 (9.3) |
| R. cuneata | 6% | 1.2 (0.9) | <0.1 (<0.1) | |||
| A. improvisus | 57% | 2584.9 (515.9) | 65.1 (22.9) | |||
| B.pilosa | 9% | 0.1 (<0.1) | <0.1 (<0.1) | 58% | 352.1 (156.3) | 0.2 (0.1) |
| Corophium sp. | 57% | 1056.0 (254.9) | 0.4 (0.2) | 79% | 174.2 (54.6) | 0.4 (0.1) |
| C. crangon | 3% | 0.3 (0.3) | 0.4 (0.4) | |||
| D. rathkei | 9% | 1.2 (0.7) | <0.1 (<0.1) | |||
| Gammaridea undet. | 55% | 2006.5 (612.7) | 0.5 (0.1) | 9% | 2.4 (1.8) | <0.1 (<0.1) |
| Mysidea undet. | 6% | 0.6 | <0.1 (<0.1) | |||
| I. balthica | 49% | 506.2 (181.3) | 0.1 (<0.1) | |||
| J. albifrons | 36% | 692.7 (331.1) | <0.1 (<0.1) | |||
| Ostracoda | 15% | 15.5 (8.8) | <0.1 (<0.1) | |||
| Palaemon elegans | 4% | 0.4 (0.2) | <0.1 (<0.1) | |||
| P. inermis | 6% | 0.7 (0.4) | <0.1 (<0.1) | |||
| Hydrobia sp. | 19% | 6.8 (4.3) | 0.1 (0.1) | 64% | 460.9 (152.1) | 2.8 (1.4) |
| Chironomidae | 9% | 0.9 (0.5) | <0.1 (<0.1) | |||
| Nematoda | 23% | 11.8 (4.2) | <0.1 (<0.1) | 48% | 70.0 (31.1) | <0.1 (<0.1) |
| Nemertea | 24% | 32.4 (12.9) | <0.1 (<0.1) | |||
| Oligochaeta | 68% | 268.4 (144.0) | 0.1 (0.1) | 88% | 1711.5 (394.5) | 1.0 (0.2) |
| F. sabella | 53% | 6261.3 (2748.7) | 0.2 (0.1) | |||
| H. diversicolor | 47% | 13.4 (3.7) | 2.9 (2.1) | 100% | 886.1 (170.9) | 26.1 (8.3) |
| Marenzellaria sp. | 85% | 121.7 (27.1) | 0.3 (0.2) | 100% | 2688.5 (429.2) | 22.2 (5.9) |
| Pygospio elegans | 68% | 34.8 (7.0) | <0.1 (<0.1) | 97% | 4639.4 (1524.2) | 1.7 (0.5) |
| S. shrubsolii | 73% | 428.2 (89.5) | 0.2 (0.1) | |||
| Factors/Parameters | Prey Taxonomic Diversity | Total Biomass | Macrofauna Biomass | Fish Biomass | Total Abundance |
|---|---|---|---|---|---|
| Sex | 0.0786 (0.780) | 3.6285 (0.059) | 1.7691 (0.188) | 1.5477 (0.219) | 1.1519 (0.289) |
| Substrate | 6.1957 (0.015) | 0.1046 (0.747) | 3.6010 (0.062) | 1.7287 (0.194) | 0.9362 (0.339) |
| Sex:Substrate | 3.8687 (0.052) | 0.1103 (0.741) | 1.9272 (0.169) | 1.5770 (0.215) | 0.0589 (0.810) |
| Species/Taxa | Hard-Bottom | Soft-Bottom | ||
|---|---|---|---|---|
| Average Biomass (g) | F (%) | Average Biomass (g) | F (%) | |
| Bivalves | ||||
| Bivalvia undet. | 0.4 (0.1) | 15% | 0.5 (0.1) | 21% |
| M. edulis trossulus | 0.7 (0.1) | 20% | 0.9 (0.1) | 6% |
| L. balthica | 0.4 (<0.1) | 8% | 0.3 (<0.1) | 14% |
| C. glaucum | 0.5 (0.1) | 3% | 0.2 (0.1) | 13% |
| M. arenaria | 0.9 (0.1) | 19% | 0.3 (0.1) | 15% |
| Crustacea | ||||
| G. zaddachi | 0.5 (0.1) | 1% | ||
| Gammaridae undet. | 1.3 (0.2) | 6% | <0.1 | 1% |
| D. villosus | 0.3 (<0.1) | 2% | ||
| S. entomon | 1.3 (0.2) | 22% | 4.5 (0.4) | 47% |
| N. integer | 1.2 (0.2) | 9% | ||
| Idothea sp. | 0.1 (<0.1) | 1% | ||
| A. improvisus | 0.4 (0.1) | 35% | 0.4 (0.1) | 11% |
| P. elegans | <0.1 (<0.1) | 1% | ||
| C. crangon | 1.6 (0.1) | 8% | 1.8 (0.3) | 4% |
| Crustacea undet. | 0.3 (<0.1) | 4% | ||
| P. lacustris | <0.1 | 1% | ||
| Mysidae undet. | <0.1 | 1% | <0.1 (<0.1) | 1% |
| Corophium undet. | <0.1 | 1% | <0.1 | 1% |
| Polychaeta | ||||
| Marenzelleria sp. | 1.2 (0.1) | 1% | ||
| H. diversicolor | 1.3 (0.4) | 45% | 0.6 (0.1) | 61% |
| Fishes | ||||
| O. eperlanus | 23.3 (1.6) | 18% | 6.1 (0.5) | 7% |
| A. tobianus | 0.2 (<0.1) | 1% | 2.1 (<0.1) | 2% |
| P. minutus | 0.7 | 1% | ||
| P. flesus | 11 (0.7) | 3% | 2.1 | 1% |
| G. aculeatus | 0.2 (<0.1) | 2% | <0.1 | 1% |
| N. melanostomus | <0.1 | 0% | <0.1 | 1% |
| M. scorpius | 1.0 | 1% | ||
| G. cernuus | 3.2 | 1% | ||
| C. harengus | <0.1 | 1% | ||
| Pisces (undetermined) | 2 (0.3) | 20% | 0.7(0.1) | 18% |
| Others | ||||
| Gastropoda undet. | <0.1 (<0.1) | 1% | <0.1 (<0.1) | 4% |
| Bottom macrophytes | <0.1 (<0.1) | 13% | <0.1 (<0.1) | 6% |
| G. aquaticus | <0.1 | 1% | ||
| Characteristics | Bottom Type | Žydelis and Ruškyte, 2005 | This Study, 2022 |
|---|---|---|---|
| Number of stomach samples per bottom type (n) | H | 119 | 156 |
| S | 89 | 95 | |
| Abundance of prey items in stomachs | H | 2.2 ± 1.1 | 5.8 ± 1.1 |
| S | 1.9 ± 1.2 | 4.7 ± 0.7 | |
| Number of prey items found in stomachs | H | 17 | 31 |
| S | 18 | 21 | |
| Frequency (%) of main prey in stomachs: | |||
| Mytilus edulis trossulus | H | 92.4% | 20% |
| Limecola balthica | S | 16.1% | 14% |
| Mya arenaria | S | 17.2% | 15% |
| Saduria entomon | H | 1.7% | 22% |
| S | 71.3% | 47% | |
| Osmerus eperlanus | H | 0% | 18% |
| S | 1.2% | 7% | |
| Hediste diversicolor | H | 0% | 45% |
| S | 14.9% | 61% | |
| Ivlev’s selectivity index: | |||
| Mytilus edulis trossulus | H | E = −0.05 | E = −0.007 |
| Saduria entomon | S | E = 0.73 | E = 0.99 |
| Occurrence of macrofauna in stomachs | 62% | 63.8% | |
| Body index | H | 6.9 ± 1.9 | 7.2 ± 1.9 |
| S | 7.4 ± 1.4 | 6.9 ± 1.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forni, P.; Morkūnas, J.; Daunys, D. Response of Long-Tailed Duck (Clangula hyemalis) to the Change in the Main Prey Availability in Its Baltic Wintering Ground. Animals 2022, 12, 355. https://doi.org/10.3390/ani12030355
Forni P, Morkūnas J, Daunys D. Response of Long-Tailed Duck (Clangula hyemalis) to the Change in the Main Prey Availability in Its Baltic Wintering Ground. Animals. 2022; 12(3):355. https://doi.org/10.3390/ani12030355
Chicago/Turabian StyleForni, Paola, Julius Morkūnas, and Darius Daunys. 2022. "Response of Long-Tailed Duck (Clangula hyemalis) to the Change in the Main Prey Availability in Its Baltic Wintering Ground" Animals 12, no. 3: 355. https://doi.org/10.3390/ani12030355
APA StyleForni, P., Morkūnas, J., & Daunys, D. (2022). Response of Long-Tailed Duck (Clangula hyemalis) to the Change in the Main Prey Availability in Its Baltic Wintering Ground. Animals, 12(3), 355. https://doi.org/10.3390/ani12030355







