Assessing Coagulation Parameters in Healthy Asian Elephants (Elephas maximus) from European and Thai Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Coagulation Time and Fibrinogen Measurements—Clinical Haemostasis Evaluations
2.2. Platelet Counts
2.3. Sample Collection for the Analysis of the Coagulation F7 Gene
2.4. DNA Extraction
2.5. Amplification and Sequencing of DNA
2.6. Data Selection and Analysis
3. Results
3.1. Overview of the Study Population
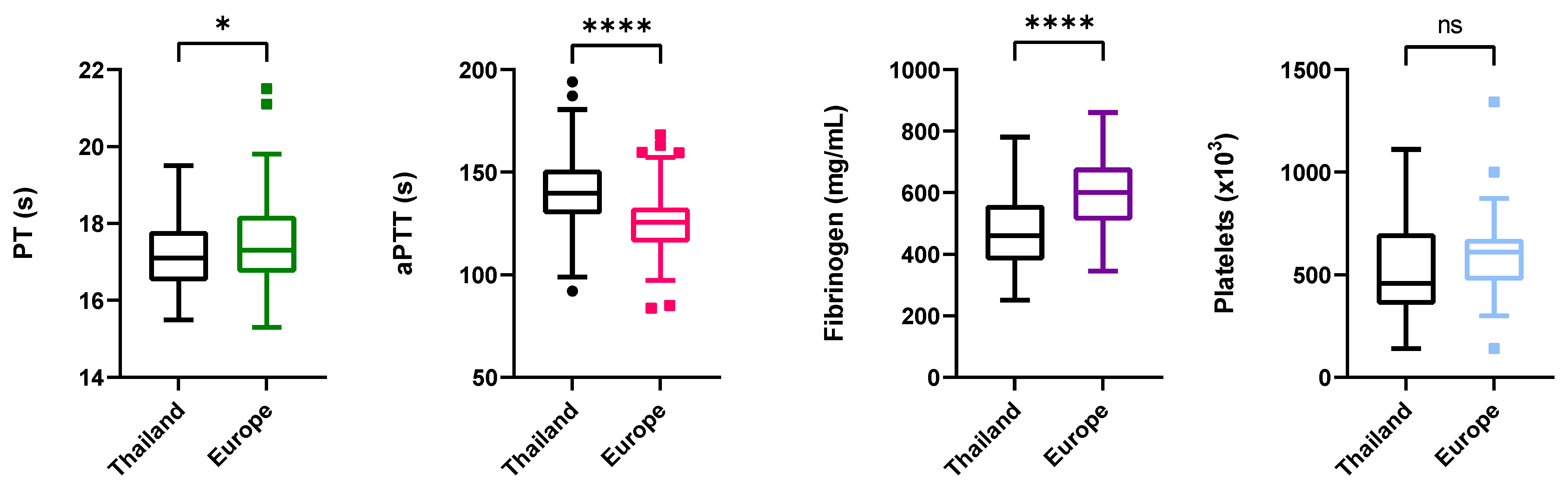
3.2. Influence of Location and EEHV-HD Status on Coagulation Time, Fibrinogen Concentration, and Platelet Counts
3.2.1. Coagulation Times
3.2.2. Fibrinogen
3.2.3. Platelet Counts
3.3. Influence of ender and ge lass on Coagulation Time, Fibrinogen Concentration, and Platelet Counts
3.3.1. Coagulation Times
3.3.2. Fibrinogen
3.3.3. Platelets
3.4. Coagulation Factor VII Gene (F7)
3.4.1. Analysis of F7 Gene Sequences
3.4.2. Distribution of Missense SNPs in the European and Thai Populations
3.4.3. Distribution of Missense SNPs between non-EEHV and EEHV Symptomatic Cases
4. Discussion
4.1. Fast Diagnostic Analyzer (VSPro)
4.2. Coagulation Times
4.3. Fibrinogen
4.4. Platelets
4.5. Genetic Analysis of F7 Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, C.; Tiwari, S.K.; Goswami, V.R.; De Silva, S.; Kumar, A.; Baskaran, N.; Yoganand, K.; Menon, V. Elephas maximus. The IUCN Red List of Threatened Species. 2020. Available online: https://www.asesg.org/PDFfiles/Asian%20Elephant%20Red%20List%20Assessment%202020.pdf (accessed on 12 August 2021).
- Zoetis VetScan Pro-utilization Guide. Available online: https://www.zoetisus.com/products/diagnostics/vetscan/pdf/vetscan-vspro-utilization-guide.pdf (accessed on 11 October 2021).
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, J.L.; Leung, L.L.; Landaw, S.A. Clinical Use of Coagulation Tests. Available online: https://somepomed.org/articulos/contents/mobipreview.htm?14/27/14769 (accessed on 12 August 2021).
- Fasano, A.; Sequeira, A. Blood Coagulation. In Modeling, Simulation and Applications; Hou, T., Le Bris, C., Patera, A.T., Zuazua, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 18. [Google Scholar] [CrossRef]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Norris, L.A. Blood coagulation. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 369–383. [Google Scholar] [CrossRef]
- Thornton, P.; Douglas, J. Coagulation in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 339–352. [Google Scholar] [CrossRef]
- Adams, R.L.C.; Bird, R.J. Review article: Coagulation cascade and therapeutics update: Relevance to nephrology. Part 1: Overview of coagulation, thrombophilias and history of anticoagulants. Nephrology 2009, 14, 462–470. [Google Scholar] [CrossRef]
- Mariani, G.; Bernardi, F. Factor VII deficiency. Semin. Thromb. Hemost. 2009, 35, 400–406. [Google Scholar] [CrossRef]
- Giansily-Blaizot, M.; Rallapalli, P.M.; Perkins, S.J.; Kemball-Cook, G.; Hampshire, D.J.; Gomez, K.; Ludlam, C.A.; McVey, J.H. The EAHAD blood coagulation factor VII variant database. Hum. Mutat. 2020, 41, 1209–1219. [Google Scholar] [CrossRef]
- McVey, J.H.; Rallapalli, P.M.; Kemball-Cook, G.; Hampshire, D.J.; Giansily-Blaizot, M.; Gomez, K.; Perkins, S.J.; Ludlam, C.A. The European Association for Haemophilia and Allied Disorders (EAHAD) Coagulation Factor Variant Databases: Important resources for haemostasis clinicians and researchers. Haemophilia 2020, 26, 306–313. [Google Scholar] [CrossRef]
- Susan, M. Cotter Coagulation Protein Disorders in Animals. Available online: https://www.msdvetmanual.com/circulatory-system/hemostatic-disorders/coagulation-protein-disorders-in-animals# (accessed on 1 October 2021).
- Lynch, M.; McGrath, K.; Raj, K.; McLaren, P.; Payne, K.; McCoy, R.; Giger, U. Hereditary factor VII deficiency in the Asian elephant (Elephas maximus) caused by a F7 missense mutation. J. Wildl. Dis. 2017, 53, 248–257. [Google Scholar] [CrossRef]
- Ossent, P.; Guscetti, F.; Metzler, A.E.; Lang, E.M.; Rübel, A.; Hauser, B. Acute and Fatal Herpesvirus Infection in a Young Asian Elephant ( Elephas maximus). Vet. Pathol. 1990, 27, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, W.; Reid, C.; Martina, B.; Fickel, J.; Osterhaus, A.D.M.E. Nonfatal clinical presentation of elephant endotheliotropic herpes virus discovered in a group of captive Asian elephants (Elephas maximus). J. Zoo Wildl. Med. 2010, 41, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Richman, L.K.; Montali, R.J.; Garber, R.L.; Kennedy, M.A.; Lehnhardt, J.; Hildebrandt, T.; Schmitt, D.; Hardy, D.; Alcendor, D.J.; Hayward, G.S. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science 1999, 283, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; Hildebrandt, T.B.; Marx, N.; Hunt, M.; Thy, N.; Reynes, J.M.; Schaftenaar, W.; Fickel, J. Endotheliotropic Elephant Herpes Virus (EEHV) infection. The first PCR-confirmed fatal case in Asia. Vet. Q. 2006, 28, 61–64. [Google Scholar] [CrossRef]
- Fickel, J.; Lieckfeldt, D.; Richman, L.K.; Streich, W.J.; Hildebrandt, T.B.; Pitra, C. Comparison of glycoprotein B (gB) variants of the elephant endotheliotropic herpesvirus (EEHV) isolated from Asian elephants (Elephas maximus). Vet. Microbiol. 2003, 91, 11–21. [Google Scholar] [CrossRef]
- Fickel, J.; Richman, L.K.; Montali, R.; Schaftenaar, W.; Göritz, F.; Hildebrandt, T.B.; Pitra, C. A variant of the endotheliotropic herpesvirus in Asian elephants (Elephas maximus) in European zoos. Vet. Microbiol. 2001, 82, 103–109. [Google Scholar] [CrossRef]
- Long, S.Y.; Latimer, E.M.; Hayward, G.S. Review of elephant endotheliotropic herpesviruses and acute hemorrhagic disease. ILAR J. 2016, 56, 283–296. [Google Scholar] [CrossRef]
- Howard, L.; Schaftenaar, W. Elephant endotheliotropic herpesvirus. In Fowler’s Zoo and Wild Animal Medicine, Current Therapy; Miller, R.E., Lamberski, N., Calle, P.P., Eds.; W.B. Saunders: Rhodes, NSW, Australia, 2019. [Google Scholar]
- Perrin, K.L.; Nielsen, S.S.; Martinussen, T.; Bertelsen, M.F. Quantification and risk factor analysis of elephant endotheliotropic herpesvirus-haemorrhagic disease fatalities in Asian elephants Elephas maximus in Europe (1985–2017). Res. Artic. J. Zoo Aquarium Res. 2021, 9, 8–13. [Google Scholar] [CrossRef]
- Jesus, S.A.; Doherr, M.G.; Hildebrandt, T.B. Elephant endotheliotropic herpesvirus impact in the european asian elephant (Elephas maximus) population: Are hereditability and zoo-associated factors linked with mortality? Animals 2021, 11, 2816. [Google Scholar] [CrossRef]
- Gentry, P.A.; Ross, M.L.; Yamada, M. Blood coagulation profile of the Asian elephant (Elephas maximus). Zoo Biol. 1996, 15, 413–423. [Google Scholar] [CrossRef]
- Kaye, S.; Abou-Madi, N.; Fletcher, D.J. EFFECT of ϵ-AMINOCAPROIC ACID on FIBRINOLYSIS in PLASMA of ASIAN ELEPHANTS (ELEPHAS MAXIMUS). J. Zoo Wildl. Med. 2016, 47, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Flanders, J.A.; Wendy, K.; Isaza, R.; Schmitt, D. Use of Thromboelastography in the Clinical Management of Eehv1 Infection in a Young Female Asian Elephant (Elephas maximus). In Proceedings of the Joint EAZWV/AAZV/Leibniz-IZW Conference Proceedings, Prague, Czech Republic, 10 October 2018; p. 112. [Google Scholar]
- McCann, R.; Hanzlicek, A.; Wallis, M.; di Girolamo, N.; Cole, G.A.; D’Agostino, J.; Backues, K.; Brandão, J. Blood coagulation assessment of captive Asian elephants (Elephas maximus) using viscoelastic point-of-care units. In Proceedings of the zoo and wildlife health conference, Kolmården, Sweden, 12–15 June 2019; p. 15. [Google Scholar]
- Perrin, K.L.; Krogh, A.K.; Kjelgaard-Hansen, M.; Howard, L.; Bochsen, L.; Kiso, W.K.; Schmitt, D.; Kristensen, A.T.; Bertelsen, M.F. Thromboelastography in the healthy Asian elephant (Elephas maximus): Reference intervals and effects of storage. J. Zoo Wildl. Med. 2018, 49, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Condrey, J.A.; Flietstra, T.; Nestor, K.M.; Schlosser, E.L.; Coleman-Mccray, J.D.; Genzer, S.C.; Welch, S.R.; Spengler, J.R. Prothrombin time, activated partial thromboplastin time, and fibrinogen reference intervals for inbred strain 13/n guinea pigs (Cavia porcellus) and validation of low volume sample analysis. Microorganisms 2020, 8, 1127. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, C.E.; Brainard, B.M. Point of Care Assessment of Coagulation. Top. Companion Anim. Med. 2016, 31, 11–17. [Google Scholar] [CrossRef]
- Schmidt, H.; Kappelhof, J. Review of the management of the Asian elephant Elephas maximus EEP: Current challenges and future solutions. Int. Zoo Yearb. 2019, 53, 31–44. [Google Scholar] [CrossRef]
- Vaser, R.; Adusumalli, S.; Leng, S.N.; Sikic, M.; Ng, P.C. SIFT missense predictions for genomes. Nat. Protoc. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Dixon-Jimenez, A.C.; Brainard, B.M.; Cathcart, C.J.; Koenig, A. Evaluation of a point-of-care coagulation analyzer (Abaxis VSPro) for identification of coagulopathies in dogs. J. Vet. Emerg. Crit. Care (San Antonio) 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Nevitt, B.N.; Chinnadurai, S.K.; Watson, M.K.; Langan, J.N.; Adkesson, M.J. Prothrombin time and activated partial thromboplastin time using a point-of-care analyser (Abaxis VSpro®) in Bennett’s wallabies (Macropus rufogriseus). Aust. Vet. J. 2016, 94, 384–386. [Google Scholar] [CrossRef]
- Levy, J.H.; Szlam, F.; Wolberg, A.S.; Winkler, A. Clinical use of the activated partial thromboplastin time and prothrombin time for screening: A review of the literature and current guidelines for testing. Clin. Lab. Med. 2014, 34, 453–477. [Google Scholar] [CrossRef]
- Salakij, J.; Salakij, C. Hematology, cytochemistry and ultrastructure of blood cells from Asian elephant (Elephas maximus). Kasetsart J. Nat. Sci. 2005, 39, 482–493. [Google Scholar]
- Silva, I.D.; Kuruwita, V.Y. Hematology, Plasma, and Serum Biochemistry Values in Free-Ranging Elephants (Elephas maximus ceylonicus) in Sri Lanka. J. Zoo Wildl. Med. 1993, 24, 434–439. [Google Scholar]
- Thitaram, C.; Pongsopawijit, P.; Thongtip, N.; Angkavanich, T.; Chansittivej, S.; Wongkalasin, W.; Somgird, C.; Suwankong, N.; Prachsilpchai, W.; Suchit, K.; et al. Dystocia following prolonged retention of a dead fetus in an Asian elephant (Elephas maximus). Theriogenology 2006, 66, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Hermes, R.; Saragusty, J.; Schaftenaar, W.; Göritz, F.; Schmitt, D.L.; Hildebrandt, T.B. Obstetrics in elephants. Theriogenology 2008, 70, 131–144. [Google Scholar] [CrossRef]
- Schaftenaar, W. Delayed postpartum fetotomy in an Asian elephant (elephas maximus). J. Zoo Wildl. Med. 2013, 44, 130–135. [Google Scholar] [CrossRef]
- Niemuller, C.; Gentry, P.A.; Liptrap, R.M. Longitudinal study of haematological and biochemical constituents in blood of the Asian elephant (Elephas maximus). Comp. Biochem. Physiol. Part A Physiol. 1990, 96, 131–134. [Google Scholar] [CrossRef]
- Pich, A.A.; Nevitt, D.; Sanchez, C.R. Knowing your platelets: The role of platelets in elephant endotheliotropic herpesvirus and routine monitoring method to aid in early detection in Asian elephants (Elephas maximus). In Proceedings of the 36th Annual Association of Zoo Veterinary Technicians Conference, Tulsa, OK, USA, 14–17 September 2016. [Google Scholar]
- Lewis, J.H. Comparative hematology: Studies on elephants, Elephas maximus. Comp. Biochem. Physiol. Part A Physiol. 1974, 49, 175–181. [Google Scholar] [CrossRef]
| F7e1_F | GAGCAGCTGAGGAACTTAGC | F7e1_R | CCCACTTTCCAGATTTGAGG |
| F7e2_F | TACAAGCCAGGAGAAGGAGC | F7e2_R | ATGGACTCCAGGAGACATGG |
| F7e3_F | TCTGTGGCTGACTTGTTTGC | F7e3_R | AGAAGGGGGTGAGGTAGGG |
| F7e4_F | AACTCACCGCCATCTCTCC | F7e4_R1 | TCAACACTCTCAGATTGGAAGG |
| F7e5_F | CTGTACCAGCTGCTTTTCCC | F7e5_R1 | TCAGTAAAGGTTATGCCCGC |
| F7e6_F | AGCTCAGGCAGATGTAACCC | F7e6_R1 | GCTGACCTGCCATTTTTCTC |
| F7e7_F | GCCAGATAAGAGGGCAGTTG | F7e7_R1 | CGATAGCAGAGAGGTTTGCC |
| F7e8_F1 | TGACAGGCCAAAGACACAAC | F7e8_R1 | GTCCCATCCAGGTAGCCAG |
| F7e8_F2 | ACGTAGTGCCCCTCTGTTTG | F7e8_R2 | GCAGCAGCAGCTTTATTTCC |
| F7e8_F3 | TCTCCCGGTACATTGAGTGG | F7e8_R3 | GACGTCCATCTCTCTCAGCC |
| PT (s) | aPTT (s) | Fibrinogen (mg/dL) | Platelet Count (×103/μL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EEHV | REGION | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N |
| No EEHV-HD | Thailand | 17.13 | 0.86 | 57 | 143.80 | 18.68 | 57 | 467 | 112 | 57 | 540 | 274 | 49 |
| Europe | 17.51 | 1.23 | 62 | 126.10 | 17.31 | 62 | 601 | 179 | 54 | 604 | 173 | 58 | |
| Total | 17.33 | 1.08 | 119 | 134.58 | 19.98 | 119 | 530 | 167 | 111 | 575 | 226 | 107 | |
| EEHV-HD survivors | Thailand | 17.45 | 0.54 | 6 | 123.40 | 22.14 | 6 | 481 | 162 | 6 | 701 | 218 | 6 |
| Europe | 18.70 | 0.71 | 2 | 109.15 | 2.90 | 2 | 560 | . | 1 | 281 | 198 | 2 | |
| Total | 17.76 | 0.79 | 8 | 119.84 | 19.87 | 8 | 492 | 151 | 7 | 596 | 278 | 8 | |
| Total between Groups | Thailand | 17.16 | 0.84 | 63 | 141.86 | 19.77 | 63 | 468 | 116 | 63 | 558 | 271 | 55 |
| Europe | 17.55 | 1.23 | 64 | 125.57 | 17.30 | 64 | 601 | 111 | 54 * | 594 | 182 | 60 | |
| Total | 17.36 | 1.07 | 127 | 133.65 | 20.22 | 127 | 530 | 132 | 117 | 576 | 229 | 115 | |
| Gender | AGE class | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N |
| F | 1 | 16.88 | 0.74 | 4 | 128.43 | 2.08 | 4 | 526 | 153 | 4 | 433 | 215 | 3 |
| 2 | 17.12 | 1.40 | 9 | 125.61 | 21.56 | 9 | 423 | 103 | 9 | 693 | 408 | 6 | |
| 3 | 17.46 | 0.91 | 11 | 133.43 | 16.45 | 11 | 555 | 135 | 11 | 500 | 167 | 10 | |
| 4 | 17.46 | 1.13 | 37 | 132.18 | 18.67 | 37 | 560 | 116 | 35 | 627 | 216 | 36 | |
| 5 | 17.26 | 1.14 | 24 | 130.42 | 17.20 | 24 | 661 | 247 | 22 | 522 | 181 | 24 | |
| Total | 17.34 | 1.11 | 85 | 130.97 | 17.66 | 85 | 570 | 176 | 81 | 577 | 225 | 79 | |
| M | 1 | 18.00 | 1.03 | 4 | 118.25 | 10.66 | 4 | 597 | 35 | 3 | 555 | 336 | 4 |
| 2 | 16.98 | 0.43 | 4 | 133.05 | 17.02 | 4 | 578 | 119 | 3 | 615 | 58 | 4 | |
| 3 | 17.38 | 0.68 | 9 | 141.07 | 15.36 | 9 | 503 | 140 | 8 | 646 | 249 | 7 | |
| 4 | 17.53 | 1.99 | 6 | 130.13 | 35.49 | 6 | 533 | 57 | 4 | 448 | 116 | 4 | |
| 5 | 17.28 | 0.51 | 4 | 137.90 | 12.53 | 4 | 470 | 162 | 4 | 394 | 210 | 3 | |
| Total | 17.43 | 1.08 | 27 | 133.60 | 20.99 | 27 | 525 | 119 | 22 | 553 | 223 | 22 | |
| Total between “age classes” | 1 | 17.44 | 1.03 | 8 | 123.34 | 8.95 | 8 | 556 | 116 | 7 | 503 | 276 | 7 |
| 2 | 17.08 | 1.17 | 13 | 127.90 | 19.88 | 13 | 462 | 123 | 12 | 662 | 308 | 10 | |
| 3 | 17.43 | 0.80 | 20 | 136.87 | 16.03 | 20 | 533 | 136 | 19 | 560 | 211 | 17 | |
| 4 | 17.47 | 1.25 | 43 | 131.89 | 21.19 | 43 | 558 | 111 | 39 | 609 | 215 | 40 | |
| 5 | 17.27 | 1.07 | 28 | 131.49 | 16.63 | 28 | 632 | 244 | 26 | 508 | 185 | 27 | |
| Total | 17.36 | 1.10 | 112 | 131.60 | 18.45 | 112 | 561 | 166 | 103 | 572 | 223 | 101 | |
| Exon | SNP Position | Codon Change | Type of Mutation |
|---|---|---|---|
| 2 | C142G | CTG > GTG | missense |
| 4 | C281T | CCG > CTG | missense |
| G294C | GGG >GGC | silent | |
| G300C | CTG > CTC | silent | |
| 5 | T386A | CTG > CAG | missense |
| G458A | CGA > CAA | missense | |
| T489C | GAT > GAC | silent | |
| 8 | C870T | CGC > CGT | silent |
| C975T | AGC > AGT | silent | |
| T1161C | AGT > AGC | silent |
| Missense SNP | Amino Acid | SIFT | PROVEAN | State | Thailand | Europe | Total | No EEHV-HD | EEHV-HD | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| exon2, C142G | ||||||||||
| C | wild-type * | 50 | 17 | 67 | 62 | 5 | 67 | |||
| C/G | Leu48Val | Tolerated | Neutral | Heterozygous | 22 | 34 | 56 | 50 | 6 | 56 |
| G | Leu48Val | Homozygous different | 4 | 9 | 13 | 11 | 2 | 13 | ||
| Total | 76 | 60 | 136 | 123 | 13 | 136 | ||||
| exon4, C281T | ||||||||||
| C | wild-type | 70 | 49 | 119 | 108 | 11 | 119 | |||
| C/T | Pro94Leu | Not Tolerated | Deleterious | Heterozygous | 6 | 2 | 8 | 7 | 1 | 8 |
| Total | 76 | 51 | 127 | 115 | 12 | 127 | ||||
| exon5, T386A | ||||||||||
| T | wild-type | 0 | 0 | 0 | 0 | 0 | 0 | |||
| A | Leu129Gln | Homozygous | 75 | 63 | 138 | 126 | 12 | 138 | ||
| A/T | Leu129Gln | Not Tolerated | Deleterious | Heterozygous | 1 | 2 | 3 | 3 | 0 | 3 |
| Total | 76 | 65 | 141 | 129 | 12 | 117 | ||||
| exon5, G458A | ||||||||||
| G | wild-type | 75 | 63 | 138 | 126 | 12 | 138 | |||
| G/A | Arg153Gln | Tolerated | Neutral | Heterozygous | 0 | 2 | 2 | 2 | 0 | 2 |
| A | Arg153Gln | Tolerated | Neutral | Homozygous | 1 | 0 | 1 | 1 | 0 | 1 |
| Total | 76 | 65 | 141 | 129 | 12 | 141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, S.A.; Schmidt, A.; Fickel, J.; Doherr, M.G.; Boonprasert, K.; Thitaram, C.; Sariya, L.; Ratanakron, P.; Hildebrandt, T.B. Assessing Coagulation Parameters in Healthy Asian Elephants (Elephas maximus) from European and Thai Populations. Animals 2022, 12, 361. https://doi.org/10.3390/ani12030361
Jesus SA, Schmidt A, Fickel J, Doherr MG, Boonprasert K, Thitaram C, Sariya L, Ratanakron P, Hildebrandt TB. Assessing Coagulation Parameters in Healthy Asian Elephants (Elephas maximus) from European and Thai Populations. Animals. 2022; 12(3):361. https://doi.org/10.3390/ani12030361
Chicago/Turabian StyleJesus, Sónia A., Anke Schmidt, Jörns Fickel, Marcus G. Doherr, Khajohnpat Boonprasert, Chatchote Thitaram, Ladawan Sariya, Parntep Ratanakron, and Thomas B. Hildebrandt. 2022. "Assessing Coagulation Parameters in Healthy Asian Elephants (Elephas maximus) from European and Thai Populations" Animals 12, no. 3: 361. https://doi.org/10.3390/ani12030361
APA StyleJesus, S. A., Schmidt, A., Fickel, J., Doherr, M. G., Boonprasert, K., Thitaram, C., Sariya, L., Ratanakron, P., & Hildebrandt, T. B. (2022). Assessing Coagulation Parameters in Healthy Asian Elephants (Elephas maximus) from European and Thai Populations. Animals, 12(3), 361. https://doi.org/10.3390/ani12030361







