Simple Summary
Several studies point out that the use of local forage legumes, such as sainfoin, can be appropriate for feeding sheep autochthonous breeds, with additional benefits also for the soil. Besides, sainfoin has a medium content of proanthocyanidins (PAC), also known as condensed tannins, the effects of which have been studied in fattening lambs but seldom on suckling lambs. The aim of the study was to evaluate the effect of PAC of sainfoin fed to dams on the productive traits, weight of the digestive organs, and on carcass and meat quality of their suckling lambs. The inclusion of PAC from sainfoin in the dam diet did not produce detrimental changes on the growth and carcass and meat characteristics of their suckling lambs. Therefore, sainfoin can be fed to ewes during lactation to produce suckling lambs, achieving good performances and meat quality.
Abstract
Sainfoin (Onobrychis viciifolia) is a forage legume with a medium content of proanthocyanidins (PAC), which may affect animal performance and product quality. The objective of the present study was to assess the effect of PAC from sainfoin fed to dams, using polyethylene glycol (PEG) as a blocking agent, on the performance and carcass and meat quality of their suckling male lambs. After lambing, twenty lactating dams were fed fresh sainfoin ad libitum plus 200 g per day of barley; ten were orally dosed with water (Sainfoin), and ten were dosed orally with a water dilution of 100 g PEG (Sainfoin + PEG). Their lambs (4.1 ± 0.64 kg at birth) suckled ad libitum until they reached the target slaughter weight of 10–12 kg. The presence of PAC in the dams’ diet did not affect the growth, blood metabolites and carcass weight and fatness of the suckling lambs but decreased the lightness of caudal fat (p < 0.05) and increased the weight of the digestive compartments (p < 0.05). Regarding the meat characteristics, PAC only decreased polyphenols content (p < 0.05). In conclusion, the presence of PAC in the dams’ diet had not significant effects on the performance and product quality of their suckling lambs.
1. Introduction
Nowadays, there is increasing social pressure for livestock production systems to minimize their negative environmental impacts, and to reduce the inclusion of feed components that compete for land use with human food crops [1]. In the last decades, the European Union has encouraged the use of local legumes for animal feeding in order to reduce the dependency on soybean meal, and to benefit from their positive environmental effects [2,3]. Among legume forages, sainfoin (Onobrychis viciifolia) has proven to be an excellent forage to be fed during lactation in ewes [4]. Furthermore, consumers are increasingly aware of the importance of food quality on human health, which has increased the demand for products obtained from forage-fed animals as they are considered healthier than those obtained from concentrate-fed diets [4,5], as well as more respectful with animal welfare.
In the Mediterranean area, the traditional production of suckling lambs —slaughtered at 10–12 kg body weight (BW)—is based on a system in which dams are fed diets mainly composed by straw, cereals and byproducts, and lambs are fed exclusively on their dams’ milk. In this framework, the inclusion of high proportions of fresh forage in the diet of the ewes could be an interesting alternative. In fact, grazing sainfoin during lactation improved the meat quality of light lambs even after a finishing period on concentrates, when compared to lambs reared with dams grazing alfalfa [6]. This could be due to a possible synergy between dietary proanthocyanidins (PAC) present in sainfoin and other antioxidant components in the muscle [7] or the milk [8].
Previous research concerning the effect of PAC on lamb performance is not conclusive, as some studies reported that weight increased [9,10], decreased [5] or did not change [11]. Regarding meat quality, there is also no consensus about the relation between PAC in lamb diets and the color parameters and heme pigments contents [12,13]. On the other hand, improvements in the antioxidant capacity of tissues due to the action of PAC have been observed [14,15]. Therefore, this great variability of results may depend on molecular weight, structure and degree of polymerization of PAC, as well as on the type of diet and animal studied [16].
Most of the studies regarding the inclusion of PAC have been carried out in fattening lambs with limited research on suckling lambs, the diet of which is based almost exclusively in milk. Therefore, to study the effect of PAC on the meat of suckling lambs, the source of PAC has to be included in their dam’s diet, and results cannot be extrapolated from those obtained in fattening lambs. Hence, the aim was to evaluate the effect of PAC of fresh sainfoin fed to dams on productive parameters, weight of the digestive organs, and carcass and meat quality of suckling lambs.
2. Materials and Methods
2.1. Experimental Site
The experimental procedures (CEEA, 2017–07), which were in compliance with the guidelines of the Directive 2010/63/EU of the European Parliament and of the Council of 22 September on the protection of animals used for experimental purposes, were approved by The Animal Ethics Committee of the Centro de Investigación y Tecnología Agroalimentaria de Aragón (CITA).
2.2. Animal Management and Experimental Design
The experiment was conducted in the facilities of CITA in Zaragoza, Spain —41°3′ N, 0°47′ W and 216 m above sea level— in spring 2019, during 28 days. All the methodology carried out during the experiment has been explained in detail in a previous study [17]. Briefly, after lambing twenty multiparous Rasa Aragonesa ewes with their male lambs were assigned into two homogeneous groups according to ewe body weight (BW; 61 ± 6.2 kg), body condition score (BCS; 3.3 ± 0.57), lambing date (April 6 ± 0.1 d) and lamb body weight at birth (4.1 ± 0.64 kg). All dams were fed fresh sainfoin (Onobrychis viciifolia cv Reznos) —dry matter (DM): 213 g/kg; crude protein (CP): 116 g/kg DM; neutral detergent fiber (NDF): 369 g/kg DM; acid detergent fiber (ADF): 264 g/kg DM; total PAC: 38.8 g eq. PAC sainfoin/kg DM—, water and mineral blocks ad libitum and 200 g/head/day of barley —DM: 912 g/kg; NDF: 250 g/kg DM; ADF: 87 g/kg DM; CP: 95 g/kg DM— distributed in two meals. Before each meal, ten ewes were drenched with 100 mL of water (Sainfoin), whereas ten ewes were orally dosed with 100 mL of polyethylene glycol (PEG) solution (50 g of PEG 4000/100 mL; Sainfoin + PEG to inactivate the effects of PAC. Each pair of dam-lamb was placed in an individual pen (2.2 m2). Lambs exclusively suckled their dams ad libitum until they reached the target slaughter weight of 10–12 kg BW.
The detailed chemical composition of feedstuffs and milk has been reported in a previous study [17]. The dry matter intake (1879.5 ± 281.3 g DM/d) and the milk yield and chemical composition was similar between groups —milk yield: 1.25 L/d; crude fat: 6.54%, CP: 5.02%, lactose: 5.28%—, except for the polyphenols (42.3 vs. 51.8 µg eq. [gallic acid]/g fresh sample, for Sainfoin and Sainfoin + PEG, respectively) and urea (275 vs. 338 mg/L, for Sainfoin and Sainfoin + PEG, respectively).
2.3. Measurements and Sampling Procedures
Lambs were weighed weekly at 8:00 h with an electronic scale (0.1 kg precision) to calculate the average daily gain (ADG). Blood samples were obtained the day of slaughter, from the jugular vein into heparin tubes (Vaccuette, Madrid, Spain). Samples were immediately centrifuged—3000 × g for 15 min at 4 °C—and stored at −20 °C until the metabolites analyses were performed.
When lambs reached the target weight of 10–12 kg BW, they were stunned by a captive bolt pistol and exsanguinated in the experimental abattoir of the Research Centre, using standard commercial procedures and according to Council Regulation (EC) Nº 1099/2009. The contents of the digestive tract corresponding to the sections of reticulum-rumen, omasum-abomasum and duodenum-jejunum were extracted and weighed. Then, the empty digestive compartments were weighed. Hot carcass weight (HCW) was recorded without head and offal. After 24 h chilling at 4 °C in total darkness, the cold carcass weight (CCW) was obtained. The dressing percentage was calculated as:
and the carcass shrinkage was calculated as:
The fatness degree of the carcasses was determined following the Community Scale for Classification of Carcasses of Ovine Animals and of Light Lambs [18] and scored from 1 (1−, very low) to 4 (4+, very high) following the scale of 1 (low), 2 (slight), 3 (average), and 4 (high). Caudal subcutaneous fat color was measured on tail root using a Minolta CM-2006 d spectrophotometer (Konica Minolta Holdings, Inc., Osaka, Japan), registering lightness (L*), redness (a*), and yellowness (b*), which were used to calculate hue angle (hab), and chroma (C*ab). The absolute value of the summation of the translated spectrum (SUM) was calculated as:
where TRi was the reflectance value at i nm. An extensive explanation of the baselines of the method is exposed in Prache and Theriez [19].
After that, the carcass was carefully split longitudinally into the two half carcasses and the longissimus thoracis et lumborum (LTL) muscles of both sides were collected. Perirenal fat deposit was extracted and weighed.
2.4. Meat Quality
The LTL muscles from 4th–6th lumbar vertebrae of the left side were used to measure the pH with a pH-meter equipped with a Crison 507 penetrating electrode (Crison Instruments, S.A., Barcelona, Spain) and to estimate the chemical composition by NIRs (FoodScanTM2, Foss Analytics, Hilleroed, Denmark). From 6th to 13th thoracic vertebrae from both sides were sliced into 2.5 cm-hick samples, the left ones were assigned to days 0, 2 and 7 of display and the right ones for days of display 5 and 9. The slices were placed in trays, wrapped with oxygen permeable polyvinyl chloride film, and kept in darkness at 4 °C until being measured for color and heme pigment estimations. LTL color was measured as had been explained above, while heme pigments were measured as described in Lobón, Blanco, Sanz, Ripoll, Bertolín and Joy [4]. The 0-d samples were allowed to bloom also in darkness at 4 °C for 1 h before being measured. After that, meat was freeze-dried and vacuum-stored in total darkness at −80 °C until the analysis of polyphenols and a 2,2-azinobis-3-ethylbensothiazoline-6-sulfonic acid (ABTS) assay, which were determined according to Leal, et al. [20] and Vázquez, et al. [21], respectively.
2.5. Plasma Analysis
Plasma concentrations of creatinine and urea (kinetic methods) were analyzed with an automatic analyzer (GernonStar, RAL/TRANSASIA, Dabhel, India). The methodology to determine antioxidant activity of plasma regarding to polyphenols concentration, super-oxide dismutase (SOD) and ABTS and the method of the determination of lipid oxidation (measured as malondialdehyde; MDA) are described in Baila, et al. [17].
2.6. Statistical Analysis
Data were analysed with the SAS [22] using the lamb as the experimental unit. The productive traits—animal performances and plasmatic metabolites—, carcass characteristics and meat chemical composition of the suckling lambs were analyzed through an variance analysis with a general linear model, with the presence of PAC as the fixed effect. Color and heme pigments of LTL muscle were analysed with mixed models—MIXED procedure—with presence of PAC, time of display and their interactions as fixed effects and the lamb as the random effect. The degrees of freedom were adjusted with the Kenward-Rodger correction. Results were reported as least square means and their associated standard errors of the means, and the Tukey correction was applied for pair-wise comparisons. The effects were considered significant at p < 0.05.
3. Results
3.1. Lamb Performance and Plasma Metabolites
The productive traits of the suckling lambs are shown in Table 1. The presence of PAC in the dams’ diet did not affect any productive trait studied, such as average daily gain, age and weight at slaughter. Regarding the plasma metabolites at slaughter, the presence of PAC in the dams’ diet did not affect creatinine and urea plasmatic concentrations of the suckling lambs.

Table 1.
Effect of the presence of proanthocyanidins (PAC) in the dams’ diet 1 on the performance, plasma metabolites and antioxidant (AO) status of their suckling lambs.
In the same line, the plasma polyphenols concentration and the antioxidant activity measured as SOD, ABTS and MDA were not affected by the presence of PAC in the dams’ diet (p > 0.05).
3.2. Digestive Compartments and Carcass Traits
The presence of PAC in the dams’ diet significantly increased the weight of the content in the reticulum-rumen (p < 0.05) and, concomitantly, in the forestomach (p < 0.01) and increased the weight of the digestive compartments (p < 0.05), except for the omasum-abomasum (Table 2). Nevertheless, most of the carcass characteristics were not affected by the treatment (Table 2). The color of subcutaneous fat of the suckling lambs was similar between groups, except for lightness, which was decreased with the presence of PAC in the dams’ diet (p < 0.01).

Table 2.
Effect of the presence of proanthocyanidins (PAC) in the dams’ diet 1 on the weights of the digestive compartments, the carcass characteristics and the color of caudal fat deposits of their suckling lambs.
3.3. Meat Quality
There were no differences between groups in the pH values of LTL at 24 h post-mortem, DM, CP, intramuscular fat, total collagen and ash contents (Table 3). The polyphenol content of meat significantly decreased with the presence of PAC in the dams’ diet (p < 0.05) but did not affect the total antioxidant capacity estimated by ABTS content.

Table 3.
Effect of the presence of proanthocyanidins (PAC) in the dams’ diet 1 on the pH, chemical composition and total antioxidant (AO) capacity of the meat of their suckling lambs.
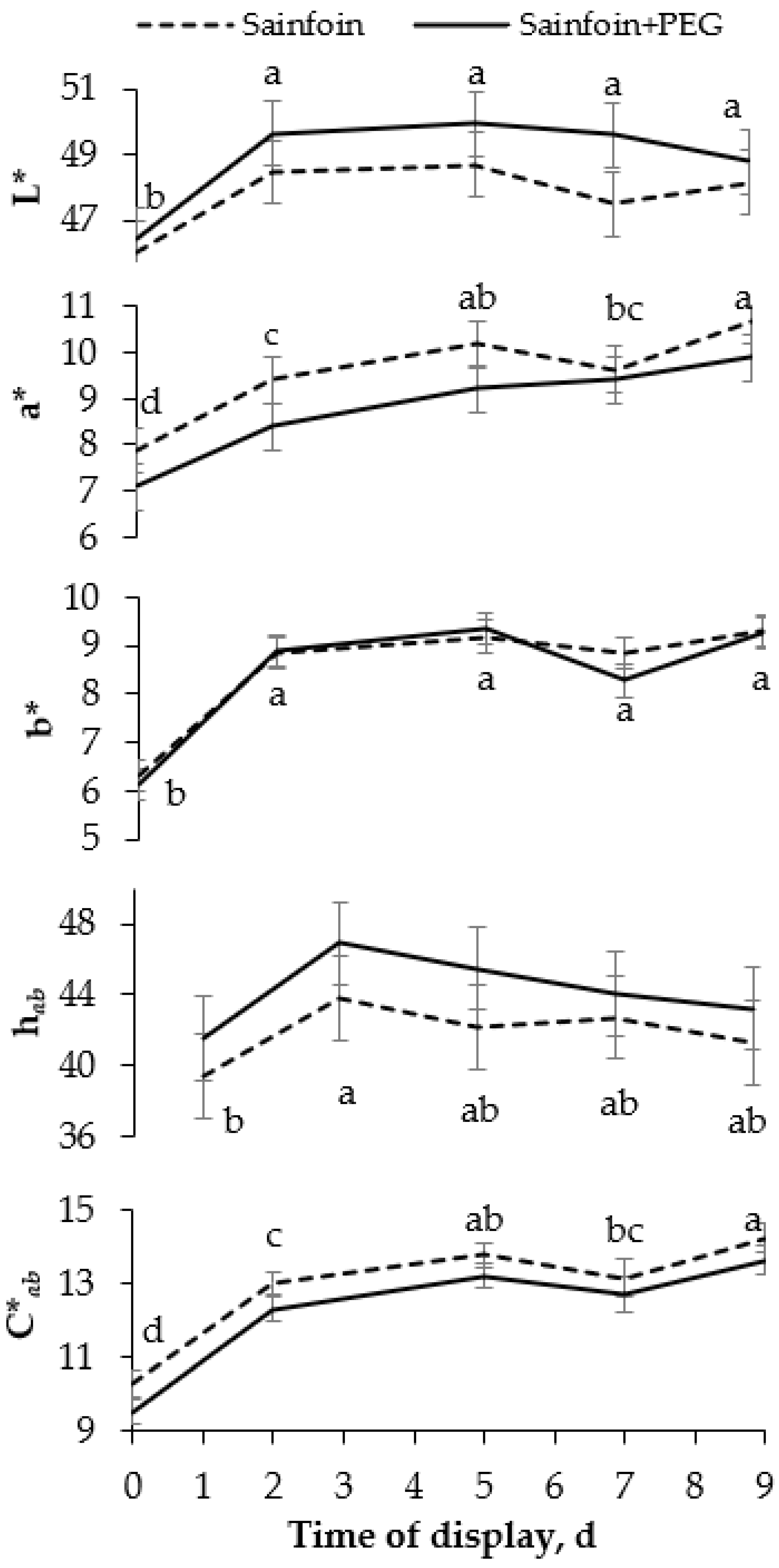
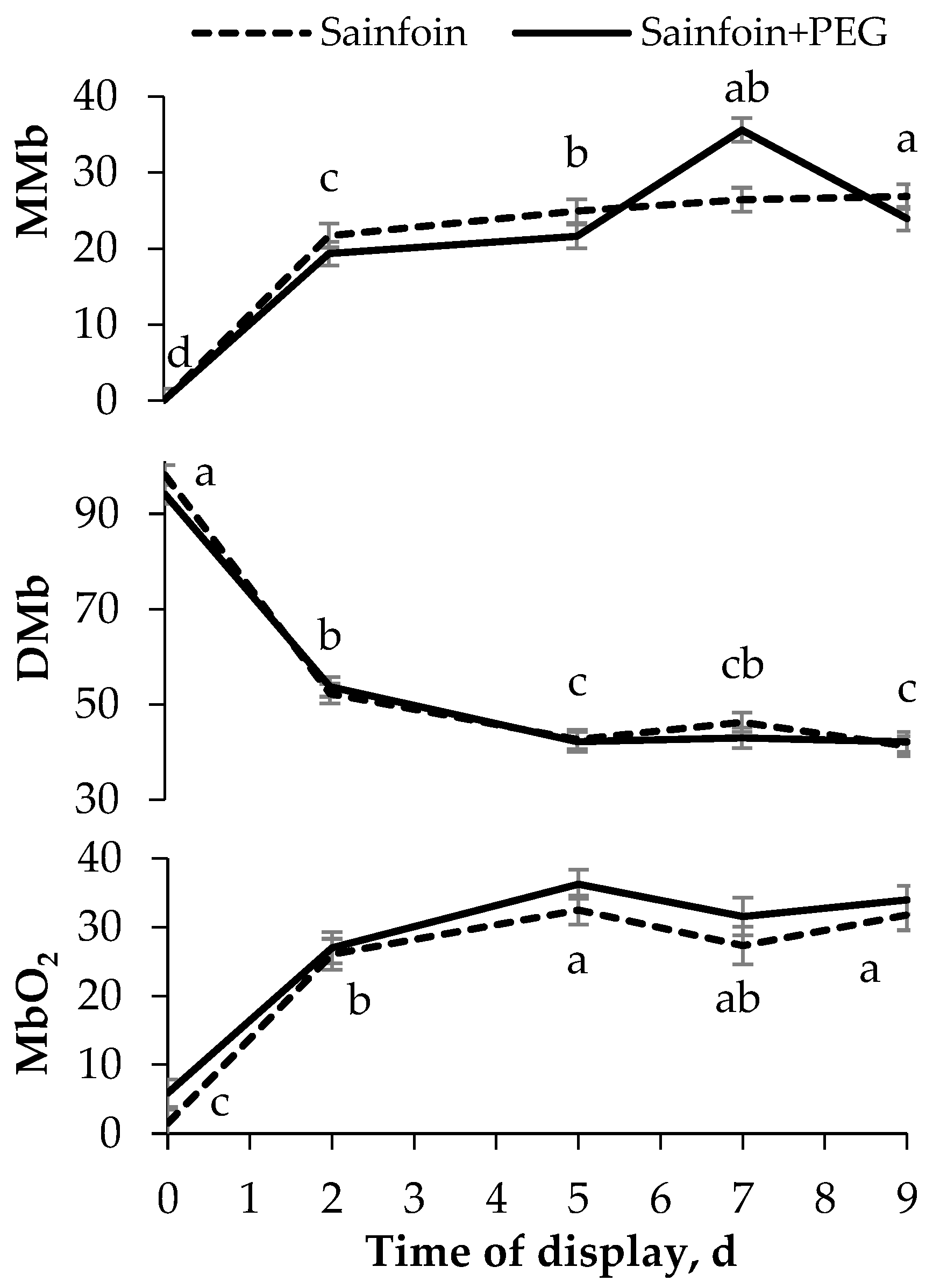
No significant interactions were observed between the presence of PAC in the dams’ diet and the time of display of meat. The LTL color (Figure 1) was not modified by the presence of PAC on the dams’ diet, but it was affected by the time of display (p < 0.05). All color variables increased from day 0 to day 2 (p < 0.05), and thereafter L* and b* remained steady, whereas C*ab and a* increased until day 5, remaining unchanged onwards (p < 0.001). Similarly to the color parameters, the presence of PAC in the dams’ diet had no significant effect on heme pigments (Figure 2), but the day of display had a significant effect (p < 0.05). Metmyoglobin and oxymyoglobin showed a similar evolution over time, increasing until day 5 (p < 0.001), whereas deoxymyoglobin followed the inverse pattern, decreasing until 5 day (p < 0.001) and remaining steady thereafter.
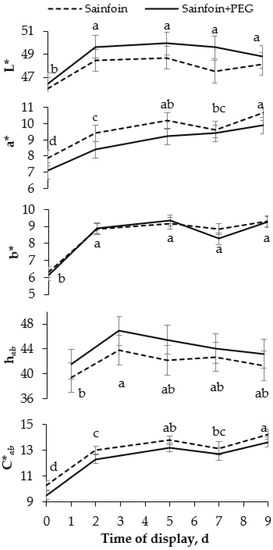
Figure 1.
Evolution of instrumental color [lightness (L*), redness (a*), yellowness (b*), hue angle (hab), and chroma (C*ab)] of meat of suckling lambs according to the presence of proanthocyanidins (PAC) in their dams’ diet. Within a parameter, different letters mean differences at p < 0.05 among days. Vertical bars indicate the standard error of the mean.
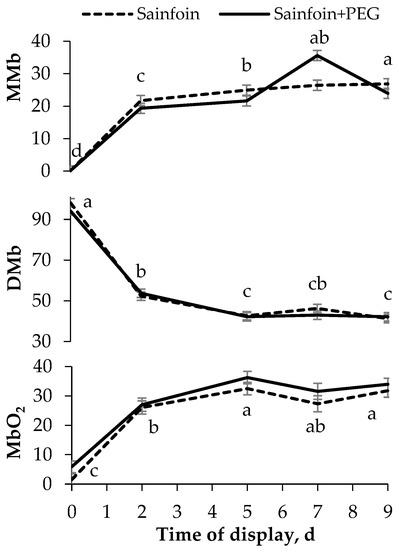
Figure 2.
Evolution of heme pigments [metmyoglobin (MMb), deoxymyoglobin (DMb), and oxymyoglobin (MbO2)] of meat of suckling lambs according to the presence of proanthocyanidins (PAC) in their dams’ diet. Within a parameter, different letters mean differences at p < 0.05 among days. Vertical bars indicate the standard error of the mean.
4. Discussion
The lack of effect of the presence of PAC from sainfoin in the diet of lactating ewes on the weight gains and carcass characteristics of their suckling lambs in the current study can be related to the similar milk production and quality of the dams, as previously shown. Milk yield and composition are the main factors responsible for suckling lamb growth [23], the protein intake being the most determinant [24]. A previous study [10] reported a higher ADG in suckling lambs whose dams received polyphenols of grape seed extract, but not when the supplementation was given directly to fattening lambs. Therefore, it is possible that polyphenols elicit a more pronounced effect during the suckling period compared to the post-weaning phase, as shown in lambs supplemented with grape pomace [25]. In a meta-analysis, it was reported that the performance of weaned lambs was not modified when the concentrations of PAC in their diet ranged between 16 and 25 g PAC/kg DM [26].
The growth of the suckling lambs of this study was comparable to that observed when dams were fed concentrates indoors, and higher than those obtained with dams fed on pasture during lactation [27]. Therefore, in this study, a diet based mainly on fresh sainfoin with only a 10% of supplementation was sufficient to achieve good performances in rearing a male lamb of this autochthonous breed. This alternative feeding management could be advisable to allow diversification of the production system and to increase system resilience in an unfavorable situation from a meteorological, social and economic point of view [28].
The similar plasma urea concentration at slaughter of lambs of both treatments was unexpected, because dams from the Sainfoin group had lower urea concentrations both in the plasma and the milk [17]. The Sainfoin + PEG suckling lambs ingested a greater quantity of urea from their dam’s milk, but it was not reflected in their plasma concentration, which suggests that the protein metabolism could be different between groups. The plasma creatinine concentration in suckling lambs was similar between treatments, indicating a similar metabolism of muscle mass [29], so that the PAC of dams’ diet had no effect on the use of amino acids of their lambs by reducing the ruminal degradation of dietary protein [16].
The antioxidant effect of PAC is well known [14,15], and some studies even report the transfer of dietary phenolic compounds from the milk to the meat of the suckling lamb, where they act as antioxidants [30]. However, in this study it was not observed in dams’ milk, which was reflected in the similar plasma antioxidant activity of lambs from both treatments. The metabolism of PAC along the digestive tract is complex [31], and the fact that the milk ingested by the lambs has already been processed in the mammary gland complicates the mechanism even further. In addition, Leparmarai, et al. [8] suggested that most of the phenolic compounds ingested by sheep were catabolized before reaching the milk or blood, so that the actual amount received by the lambs through the milk could be very low.
The carcass characteristics, dressing percentage and fat cover was similar in the suckling lambs of both experimental groups. Differences observed in carcass weight, fatness score and perirenal fat weight are usually related to the quality of feeding sources [32,33], dry matter intake [5,34,35], and/or age and weight at slaughter [33], all of which were similar in the present study. On average, the dressing percentage obtained in the suckling lambs was greater than the observed in fattened lambs, due to their lower development of the digestive tract of the former [5,33].
Unexpectedly, the reticulum-rumen and forestomach contents and the empty digestive compartments were heavier in lambs of the Sainfoin group. The higher weight of the digestive tract is usually associated with a worst dressing percentage [36]. In this case, the greater weight of the digestive organs of the Sainfoin group lambs produced a numerically lower dressing percentage, although the differences were not statistically significant. On the other hand, the digestive content is closely related with the intake. Since the milk intake of the lambs of both treatments was similar, the cause of this result remains unclear.
Yellowness and SUM (an estimator of carotenoids) in animal fat and meat are mainly influenced by the carotenoid pigments coming from feedstuffs [5,37]. The lack of differences observed in these parameters between treatments is ascribed to the similar sainfoin intake of their dams, and the consequent similar intake of secondary compounds. The lower L* value of caudal fat in Sainfoin lambs disagrees with the results observed by Rivaroli et al. [35], who concluded that the mechanisms of PAC modifying the L* of caudal fat deposits are unclear. Brainard [38] developed a method to estimate if instrumental color differences (expressed as ) are perceptible by human vision. In relation to this, Carrasco, et al. [39] reported that differences of color () of caudal fat lower than 5.2 between carcasses were imperceptible. In the present study, the difference between both treatments was 2.6; therefore, the practical implications were minimal. Fat L* values in suckling lambs of Churra Tensina, a similar local breed, ranged between 69 and 72 when their dams received a diet based on fresh forage or hay [40]. On the other hand, the color parameters were similar to those obtained by Lobón et al. [41] in fattened lambs previously raised by dams grazing sainfoin, despite their post-weaning finishing period and, consequently, heavier slaughter weight (23.4 kg BW).
Regarding the meat quality, the pH value of meat was within the normal range, close to 5.50 [27]. The presence of PAC in the dams’ diet did not have any effect on the chemical composition of the meat of their suckling lambs, as Gómez-Cortés et al. [42] reported when grape pomace was included as a source of PAC in the diet of lactating ewes. The content of fat and protein in the meat were similar to those obtained in suckling lambs of dams fed with pasture [27] or supplemented with polyphenols [10].
In this study, the content of polyphenol in the meat of suckling lambs reflects the concentration in the milk of their dams. Proanthocyanidins and polyphenols are characterized by providing a great antioxidant power [14] and studies show an improvement of antioxidant status which increases the shelf life of lamb meat [7,43]. However, this result was not reflected on the antioxidant capacity, probably because it was only measured on the first day post slaughter, when the oxidation process had hardly started.
In relation to heminic pigments in LTL, Vieira et al. [30] observed a reduction of MMb in the meat of suckling lambs whose dams were fed grape pomace (containing PAC). However, this reduction was observed from day 10 of meat storage, so the time of the present study could have been insufficient to show effects. Contrary to the present results of color, Vasta, et al. [44] in a review pointed out that the meat of lambs fed with PAC was lighter than that of their counterparts fed with PEG, concluding that the mechanisms of action of PAC on meat color are still unclear. Besides in the abovementioned review most of studies involved weaned lambs, whereas here we studied suckling lambs fed exclusively with maternal milk.
5. Conclusions
The inclusion of PAC from sainfoin in the dams’ diet had no significant effect on the ADG, plasmatic antioxidant activity, and carcass and meat quality of their suckling lambs. Therefore, fresh sainfoin can be fed to ewes during lactation to produce suckling lambs, achieving good performances and meat quality.
Author Contributions
Conceptualization, M.J., S.L., I.C. and M.B.; formal analysis, C.B. and S.L.; chemical analysis, C.B. and G.R.; investigation, M.J., S.L., I.C., M.B. and C.B., writing-original draft preparation, C.B., review and editing, M.J., M.B., I.C., G.R. and S.L.; project administration, M.J.; funding acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this project was provided by the Spanish Ministry of Economy and Competitiveness (RTA2017-00008-C02-01) and the Government of Aragón (Grant Research Group Funds, Group A17_20R). C. Baila had a predoctoral grant from AEI (PRE2018-086670).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Centro de Investigación y Tecnología Agroalimentaria de Aragón (CITA) (CEEA, 2017–07).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed in the present study are available from the corresponding author on reasonable request.
Acknowledgments
Special thanks are due to the CITA-Department of Animal, to the Servicio General de Apoyo a la Investigación-SAI, Universidad de Zaragoza and to J.R. Bertolín, M.A. Legua and A. Domínguez for helping with the laboratory analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Calabrò, S.; Tudisco, R.; Balestrieri, A.; Piccolo, G.; Infascelli, F.; Cutrignelli, M.I. Fermentation characteristics of different grain legumes cultivars with the in vitro gas production technique. Ital. J. Anim. Sci. 2009, 8, 280–282. [Google Scholar] [CrossRef][Green Version]
- Guyader, J.; Janzen, H.H.; Kroebel, R.; Beauchemin, K.A. Forage use to improve environmental sustainability of ruminant production. J. Anim. Sci. 2016, 94, 3147–3158. [Google Scholar] [CrossRef] [PubMed]
- Lobón, S.; Blanco, M.; Sanz, A.; Ripoll, G.; Bertolín, J.; Joy, M. Meat quality of light lambs is more affected by the dam’s feeding system during lactation than by the inclusion of quebracho in the fattening concentrate. J. Anim. Sci. 2017, 95, 4998–5011. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Dohme-Meier, F.; Silacci, P.; Ampuero Kragten, S.; Kreuzer, M.; Bee, G. Forage legumes rich in condensed tannins may increase n-3 fatty acid levels and sensory quality of lamb meat. J. Sci. Food Agric. 2015, 96, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Lobón, S.; Joy, M.; Sanz, A.; Álvarez-Rodríguez, J.; Blanco, M. The fatty acid composition of ewe milk or suckling lamb meat can be used to discriminate between ewes fed different diets. Anim. Prod. Sci. 2019, 59, 1108–1118. [Google Scholar] [CrossRef]
- Valenti, B.; Natalello, A.; Vasta, V.; Campidonico, L.; Roscini, V.; Mattioli, S.; Pauselli, M.; Priolo, A.; Lanza, M.; Luciano, G. Effect of different dietary tannin extracts on lamb growth performances and meat oxidative stability: Comparison between mimosa, chestnut and tara. Animal 2018, 13, 435–443. [Google Scholar] [CrossRef]
- Leparmarai, P.T.; Sinz, S.; Kunz, C.; Liesegang, A.; Ortmann, S.; Kreuzer, M.; Marquardt, S. Transfer of total phenols from a grapeseed-supplemented diet to dairy sheep and goat milk, and effects on performance and milk quality. J. Anim. Sci. 2019, 97, 1840–1851. [Google Scholar] [CrossRef]
- Valderrábano, J.; Calvete, C.; Uriarte, J. Effect of feeding bioactive forages on infection and subsequent development of Haemonchus contortus in lamb faeces. Vet. Parasitol. 2010, 172, 89–94. [Google Scholar] [CrossRef]
- Giller, K.; Sinz, S.; Messadene-Chelali, J.; Marquardt, S. Maternal and direct dietary polyphenol supplementation affect growth, carcass and meat quality of sheep and goats. Animal 2021, 15, 100333. [Google Scholar] [CrossRef]
- Peng, K.; Shirley, D.C.; Xu, Z.; Huang, Q.; McAllister, T.A.; Chaves, A.V.; Acharya, S.; Liu, C.; Wang, S.; Wang, Y. Effect of purple prairie clover (Dalea purpurea Vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim. Feed Sci. Technol. 2016, 222, 100–110. [Google Scholar] [CrossRef]
- Luciano, G.; Monahan, F.; Vasta, V.; Biondi, L.; Lanza, M.; Priolo, A. Dietary tannins improve lamb meat colour stability. Meat Sci. 2009, 81, 120–125. [Google Scholar] [CrossRef]
- Seoni, E.; Battacone, G.; Silacci, P.; Kragten, S.A.; Chelali, J.M.; Dohme-Meier, F.; Bee, G. Effect of condensed tannins from Birdsfoot trefoil and dietary protein level on growth performance, carcass composition and meat quality of ram lambs. Small Rumin. Res. 2018, 169, 118–126. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- López-Andrés, P.; Luciano, G.; Vasta, V.; Gibson, T.M.; Biondi, L.; Priolo, A.; Mueller-Harvey, I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 2013, 110, 632–639. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Baila, C.; Joy, M.; Blanco, M.; Casasús, I.; Bertolín, J.R.; Lobón, S. Effects of feeding sainfoin proanthocyanidins to lactating ewes on intake, milk production and plasma metabolites. Animal 2022, 16, 100438. [Google Scholar] [CrossRef]
- EEC. 461/93; Community Scale for the Classification of Carcasses of Light Lambs. Directorate-General for Agriculture and Rural Development (European Commission): Brussels, Belgium, 1993.
- Prache, S.; Theriez, M. Traceability of lamb production systems: Carotenoids in plasma and adipose tissue. Anim. Sci. 1999, 69, 29–36. [Google Scholar] [CrossRef]
- Leal, L.N.; Jordán, M.J.; Bello, J.M.; Otal, J.; den Hartog, L.A.; Hendriks, W.H.; Martín-Tereso, J. Dietary supplementation of 11 different plant extracts on the antioxidant capacity of blood and selected tissues in lightweight lambs. J. Sci. Food Agric. 2019, 99, 4296–4303. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Martínez, R.A.F.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin–Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef]
- SAS Statistical Software, v.9.3. EE.UU. SAS Institute Inc.: Cary, NC, USA, 2012.
- Gallardo, B.; Gómez-Cortés, P.; Mantecón, A.; Juárez, M.; Manso, T.; de la Fuente, M. Effects of olive and fish oil Ca soaps in ewe diets on milk fat and muscle and subcutaneous tissue fatty-acid profiles of suckling lambs. Animal 2014, 8, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, A.; Caja, G.; Casals, R.; Mezghani, I. Lactational evaluation of effects of calcium soap of fatty acids on dairy ewes. Small Rumin. Res. 2006, 66, 1–10. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Petrotos, K.; Goulas, P.; Kouretas, D. Effects of dietary grape pomace supplementation on performance, carcass traits and meat quality of lambs. In Vivo 2018, 32, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rodríguez, J.; Serrano-Pérez, B.; Villalba, D.; Joy, M.; Bertolín, J.R.; Molina, E. ¿Afectan los taninos condensados de la dieta a los resultados productivos, la composición de ácidos grasos y el color de la carne de cordero? Itea-Inf. Tec. Econ. Ag. 2020, 116, 116–130. [Google Scholar] [CrossRef]
- Fusaro, I.; Giammarco, M.; Chincarini, M.; Odintsov Vaintrub, M.; Palmonari, A.; Mammi, L.M.E.; Formigoni, A.; di Giuseppe, L.; Vignola, G. Effect of ewe diet on milk and muscle fatty acid composition of suckling lambs of the protected geographical origin abbacchio romano. Animals 2020, 10, 25. [Google Scholar] [CrossRef]
- Ripoll-Bosch, R. Diagnóstico de los Sistemas Ganaderos Ovinos en Áreas Desfavorecidas: Caracterización Productiva de la raza Ojinegra de Teruel, Análisis Integrado de Sostenibilidad y Evaluación de la Huella de Carbono; Universidad de Zaragoza: Zaragoza, Spain, 2013. [Google Scholar]
- Hegarty, R.; McFarlane, J.R.; Banks, R.; Harden, S. Association of plasma metabolites and hormones with the growth and composition of lambs as affected by nutrition and sire genetics. Aust. J. Agric. Res. 2006, 57, 683–690. [Google Scholar] [CrossRef]
- Vieira, C.; Guerra-Rivas, C.; Martínez, B.; Rubio, B.; Manso, T. Effects of grape pomace supplementation on the diet of lactating ewes as compared to vitamin E on the meat shelf life of suckling lambs. Meat Sci. 2022, 184, 108666. [Google Scholar] [CrossRef]
- Quijada, J.; Drake, C.; Gaudin, E.; El-Korso, R.; Hoste, H.; Mueller-Harvey, I. Condensed tannin changes along the digestive tract in lambs fed with sainfoin pellets or hazelnut skins. J. Agric. Food Chem. 2018, 66, 2136–2142. [Google Scholar] [CrossRef]
- Karnezos, T.; Matches, A. Fatty acid composition of dock-fat from lambs grazing alfalfa, sainfoin, wheatgrass. J. Food Qual. 1993, 16, 409–415. [Google Scholar] [CrossRef]
- Bonanno, A.; di Miceli, G.; di Grigoli, A.; Frenda, A.; Tornambè, G.; Giambalvo, D.; Amato, G. Effects of feeding green forage of sulla (Hedysarum coronarium L.) on lamb growth and carcass and meat quality. Animal 2011, 5, 148–154. [Google Scholar] [CrossRef]
- Copani, G.; Niderkorn, V.; Anglard, F.; Quereuil, A.; Ginane, C. Silages containing bioactive forage legumes: A promising protein-rich feed source for growing lambs. Grass Forage Sci. 2016, 71, 622–631. [Google Scholar] [CrossRef]
- Rivaroli, D.; Prunier, A.; Meteau, K.; do Prado, I.N.; Prache, S. Tannin-rich sainfoin pellet supplementation reduces fat volatile indoles content and delays digestive parasitism in lambs grazing alfalfa. Animal 2019, 13, 1883–1890. [Google Scholar] [CrossRef]
- Díaz, M.; Velasco, S.; Caneque, V.; Lauzurica, S.; de Huidobro, F.R.; Pérez, C.; González, J.; Manzanares, C. Use of concentrate or pasture for fattening lambs and its effect on carcass and meat quality. Small Rumin. Res. 2002, 43, 257–268. [Google Scholar] [CrossRef]
- Prache, S.; Aurousseau, B.; Theriez, M.; Renerre, M. Discoloration of sheep carcass subcutaneous fat. INRA Prod. Anim. 1990, 3, 275–285. [Google Scholar] [CrossRef]
- Brainard, D.H. Color appearance and color difference specification. In The Science of Color, 2nd ed.; Shevell, S.K., Ed.; Elsevier: Oxford, UK, 2003; p. 340. [Google Scholar]
- Carrasco, S.; Panea, B.; Ripoll, G.; Sanz, A.; Joy, M. Influence of feeding systems on cortisol levels, fat colour and instrumental meat quality in light lambs. Meat Sci. 2009, 83, 50–56. [Google Scholar] [CrossRef]
- Joy, M.; Sanz, A.; Ripoll, G.; Panea, B.; Ripoll-Bosch, R.; Blasco, I.; Alvarez-Rodriguez, J. Does forage type (grazing vs. hay) fed to ewes before and after lambing affect suckling lambs performance, meat quality and consumer purchase intention? Small Rumin. Res. 2012, 104, 1–9. [Google Scholar]
- Lobón, S.; Blanco, M.; Sanz, A.; Ripoll, G.; Joy, M. Effects of feeding strategies during lactation and the inclusion of quebracho in the fattening on performance and carcass traits in light lambs. J. Sci. Food Agric. 2019, 99, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Guerra-Rivas, C.; Gallardo, B.; Lavín, P.; Mantecón, A.; de la Fuente, M.; Manso, T. Grape pomace in ewes diet: Effects on meat quality and the fatty acid profile of their suckling lambs. Food Res. Int. 2018, 113, 36–42. [Google Scholar] [CrossRef]
- Vasta, V.; Luciano, G. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Rumin. Res. 2011, 101, 150–159. [Google Scholar] [CrossRef]
- Vasta, V.; Nudda, A.; Cannas, A.; Lanza, M.; Priolo, A. Alternative feed resources and their effects on the quality of meat and milk from small ruminants. Anim. Feed Sci. Technol. 2008, 147, 223–246. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).