Metabolic Profiling Reveals That the Olfactory Cues in the Duck Uropygial Gland Potentially Act as Sex Pheromones
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Sampling
2.2. Metabolite Extraction
2.3. Chromatographic Conditions
2.4. Mass Spectrometry Conditions
2.5. Qualitative and Quantitative Analysis of Metabolites
2.6. Differential Metabolite Screening and Functional Analysis
3. Results
3.1. The Chemical Components of the Uropygial Glands Can Help Distinguish the Ducks with Different Genders
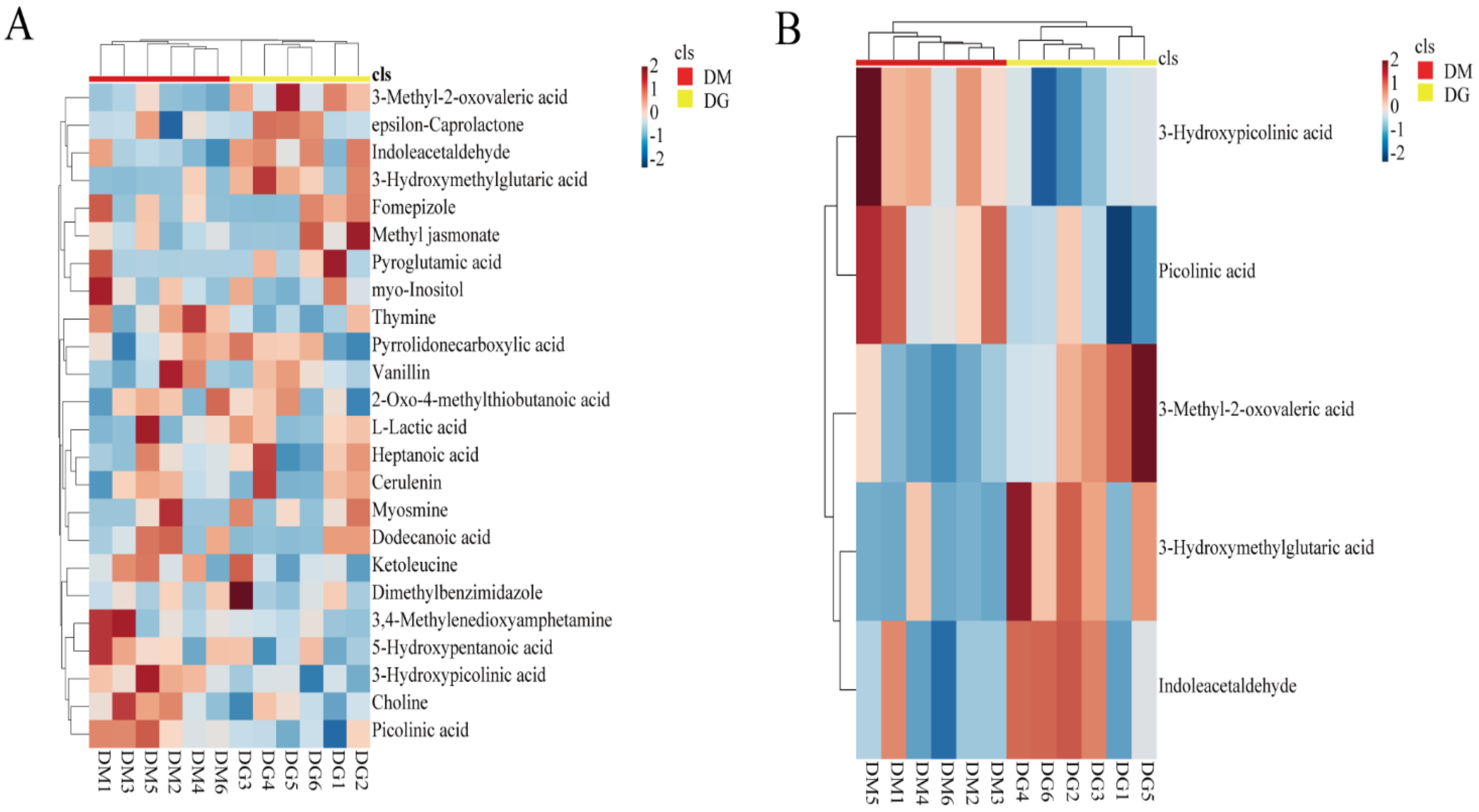
3.2. Screening Differential Metabolites in Duck Uropygial Gland between Two Sexes
3.3. KEGG Enrichment Based on the Differential Annotated Metabolites
3.4. The Enrichment of Volatile Substances Potentially as Olfactory Cues in Duck Uropygial Gland for Chemical Signals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, E.O. Chemical communication among workers of the fire ant Solenopsis saevissima (Fr. Smith) 1. The Organization of Mass-Foraging. Anim. Behav. 1962, 10, 134–147. [Google Scholar] [CrossRef]
- Takahashi, D.Y.; Narayanan, D.Z.; Ghazanfar, A.A. Coupled Oscillator Dynamics of Vocal Turn-Taking in Monkeys. Curr. Biol. 2013, 23, 2162–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strandburg-Peshkin, A.; Twomey, C.R.; Bode, N.W.; Kao, A.B.; Katz, Y.; Ioannou, C.C.; Rosenthal, S.B.; Torney, C.J.; Wu, H.S.; Levin, S.A.; et al. Visual sensory networks and effective information transfer in animal groups. Curr. Biol. 2013, 23, R709–R711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caro, S.P.; Balthazart, J. Pheromones in birds: Myth or reality? J. Comp. Physiol. A 2010, 196, 751–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, B.G.; Jones, T.M. The role of chemical communication in mate choice. Biol. Rev. 2007, 82, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Factory, L.H.C. Behavioral Responses of Losiderma Serricorne and Chemical Composition of Essential Oil Isolated from Nicotiana Tabacum Leaves; Yunnan Agricultural University: Kunming, China, 2017. [Google Scholar]
- Saavedra, I.; Amo, L. Insectivorous birds eavesdrop on the pheromones of their prey. PLoS ONE 2018, 13, e0190415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houck, L.D.; Watts, R.A.; Arnold, S.J.; Bowen, K.E.; Kiemnec, K.M.; Godwin, H.A.; Feldhoff, P.W.; Feldhoff, R.C. A recombinant courtship pheromone affects sexual receptivity in a plethodontid salamander. Chem. Senses 2008, 33, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Rennie, P.J.; Gower, D.; Holland, K.; Mallet, A.; Watkins, W. The skin microflora and the formation of human axillary odour. Int. J. Cosmet. Sci. 1990, 12, 197–207. [Google Scholar] [CrossRef]
- de Catanzaro, D. Sex steroids as pheromones in mammals: The exceptional role of estradiol. Horm. Behav. 2015, 68, 103–116. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.; Zhang, L.; Gaur, U.; Ma, T.; Jie, H.; Zhao, G.; Wu, N.; Xu, Z.; Xu, H. The musk chemical composition and microbiota of Chinese forest musk deer males. Sci. Rep. 2016, 6, 18975. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, M.; Sreedevi, M. Captive breeding of the Small Indian Civet Viverricula indica (É. Geoffroy Saint-Hilaire, 1803). Small Carniv. Conserv. 2007, 36, 5–8. [Google Scholar]
- Daniel, W.; Bekele, A.; Balakrishnan, M.; Belay, G. Collection of African Civet Civettictis civetta perineal gland secretion from naturally scent-marked sites. Small Carniv. Conserv. 2011, 44, 14–18. [Google Scholar]
- Burger, B.V.; Reiter, B.; Borzyk, O.; Plessis, M. Avian Exocrine Secretions. I. Chemical Characterization of the Volatile Fraction of the Uropygial Secretion of the Green Woodhoopoe, Phoeniculus purpureus. J. Chem. Ecol. 2004, 30, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Balthazart, J.; Schoffeniels, E. Sex differences in the chemical composition of uropygial gland waxes in domestic ducks. Biochem. Syst. Ecol. 1979, 7, 149–153. [Google Scholar] [CrossRef]
- Karlsson, A.-C.; Jensen, P.; Elgland, M.; Laur, K.; Fyrner, T.; Konradsson, P.; Laska, M. Red junglefowl have individual body odors. J. Exp. Biol. 2010, 213, 1619–1624. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, D.J.; Gerlach, N.M.; Soini, H.A.; Novotny, M.V.; Ketterson, E.D. Bird odour predicts reproductive success. Anim. Behav. 2013, 86, 697–703. [Google Scholar] [CrossRef]
- Pageat, P. Avian Appeasing Pheromones to Decrease Stress, Anxiety and Aggressiveness. U.S. Patent 7,723,388, 25 May 2010. [Google Scholar]
- Hirao, A.; Aoyama, M.; Sugita, S. The role of uropygial gland on sexual behavior in domestic chicken Gallus gallus domesticus. Behav. Processes 2009, 80, 115–120. [Google Scholar] [CrossRef]
- Jawad, H.S.; Idris, L.; Bakar, Z.; Kassim, A. Partial ablation of uropygial gland effect on carcass characteristics of Akar Putra chicken. Poult. Sci. 2016, 95, 1966–1971. [Google Scholar] [CrossRef]
- Giraudeau, M.; Czirják, G.Á.; Duval, C.; Bretagnolle, V.; Eraud, C.; McGraw, K.J.; Heeb, P. Effect of restricted preen-gland access on maternal self maintenance and reproductive investment in mallards. PLoS ONE 2010, 5, e13555. [Google Scholar] [CrossRef] [Green Version]
- Kolattukudy, P.; Bohnet, S.; Rogers, L. Diesters of 3-hydroxy fatty acids produced by the uropygial glands of female mallards uniquely during the mating season. J. Lipid Res. 1987, 28, 582–588. [Google Scholar] [CrossRef]
- Amo, L.; Avilés, J.M.; Parejo, D.; Peña, A.; Rodríguez, J.; Tomás, G. Sex recognition by odour and variation in the uropygial gland secretion in starlings. J. Anim. Ecol. 2012, 81, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Stallcup, R.; Evens, J. Field Guide to Birds of the Northern California Coast; University of California Press: Oakland, CA, USA, 2014. [Google Scholar]
- Balthazart, J.; Schoffeniels, E. Pheromones are involved in the control of sexual behaviour in birds. Naturwissenschaften 1979, 66, 55–56. [Google Scholar] [CrossRef]
- Reuhs, B.L. High-performance liquid chromatography. In Food Analysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 213–226. [Google Scholar]
- Sangster, T.; Major, H.; Plumb, R.; Wilson, A.J.; Wilson, I.D. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis. Analyst 2006, 131, 1075–1078. [Google Scholar] [CrossRef]
- Ng, J.S.Y.; Ryan, U.; Trengove, R.D.; Maker, G.L. Development of an untargeted metabolomics method for the analysis of human faecal samples using Cryptosporidium-infected samples. Mol. Biochem. Parasitol. 2012, 185, 145–150. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Rodríguez, M.; Valdivia, E.; Soler, J.J.; Martín-Vivaldi, M.; Martín-Platero, A.; Martínez-Bueno, M. Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. J. Exp. Biol. 2009, 212, 3621–3626. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.Y.; Hansen, I.A. Lipogenesis in chicken uropygial glands. Eur. J. Biochem. 1972, 31, 372–377. [Google Scholar] [CrossRef]
- Leclaire, S.; Merkling, T.; Raynaud, C.; Giacinti, G.; Bessière, J.-M.; Hatch, S.A.; Danchin, É. An individual and a sex odor signature in kittiwakes? Study of the semiochemical composition of preen secretion and preen down feathers. Naturwissenschaften 2011, 98, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Wei, W.; Zhang, J.-H.; Yang, W.-H. Uropygial gland-secreted alkanols contribute to olfactory sex signals in budgerigars. Chem. Senses 2010, 35, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Stralendorff, F.v. Partial chemical characterization of urinary signaling pheromone in tree shrews (Tupaia belangen). J. Chem. Ecol. 1987, 13, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Van Emden, H.; Hagen, K. Olfactory reactions of the green lacewing, Chrysopa carnea, to tryptophan and certain breakdown products. Environ. Entomol. 1976, 5, 469–473. [Google Scholar] [CrossRef]
- Poulin, R.X.; Lavoie, S.; Siegel, K.; Gaul, D.A.; Weissburg, M.J.; Kubanek, J. Chemical encoding of risk perception and predator detection among estuarine invertebrates. Proc. Natl. Acad. Sci. USA 2018, 115, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-X.; Sun, L.; Zuo, M.-X. Uropygial gland volatiles may code for olfactory information about sex, individual, and species in Bengalese finches Lonchura striata. Curr. Zool. 2009, 55, 357–365. [Google Scholar] [CrossRef]
- Asnani, M.; Ramachandran, A. Roles of adrenal and gonadal steroids and season in uropygial gland function in male pigeons, Columba livia. Gen. Comp. Endocrinol. 1993, 92, 213–224. [Google Scholar] [CrossRef]
- Whelan, R.J.; Levin, T.C.; Owen, J.C.; Garvin, M.C. Short-chain carboxylic acids from gray catbird (Dumetella carolinensis) uropygial secretions vary with testosterone levels and photoperiod. Comp. Biochem. Physiol. Part B 2010, 156, 183–188. [Google Scholar] [CrossRef]
- Takeuchi, S.; Teshigawara, K.; Takahashi, S. Molecular cloning and characterization of the chicken pro-opiomelanocortin (POMC) gene. Biochim. Biophys. Acta BBA Mol. Cell Res. 1999, 1450, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, S.; Takahashi, S. Melanocortin receptor genes in the chicken—Tissue distributions. Gen. Comp. Endocrinol. 1998, 112, 220–231. [Google Scholar] [CrossRef]

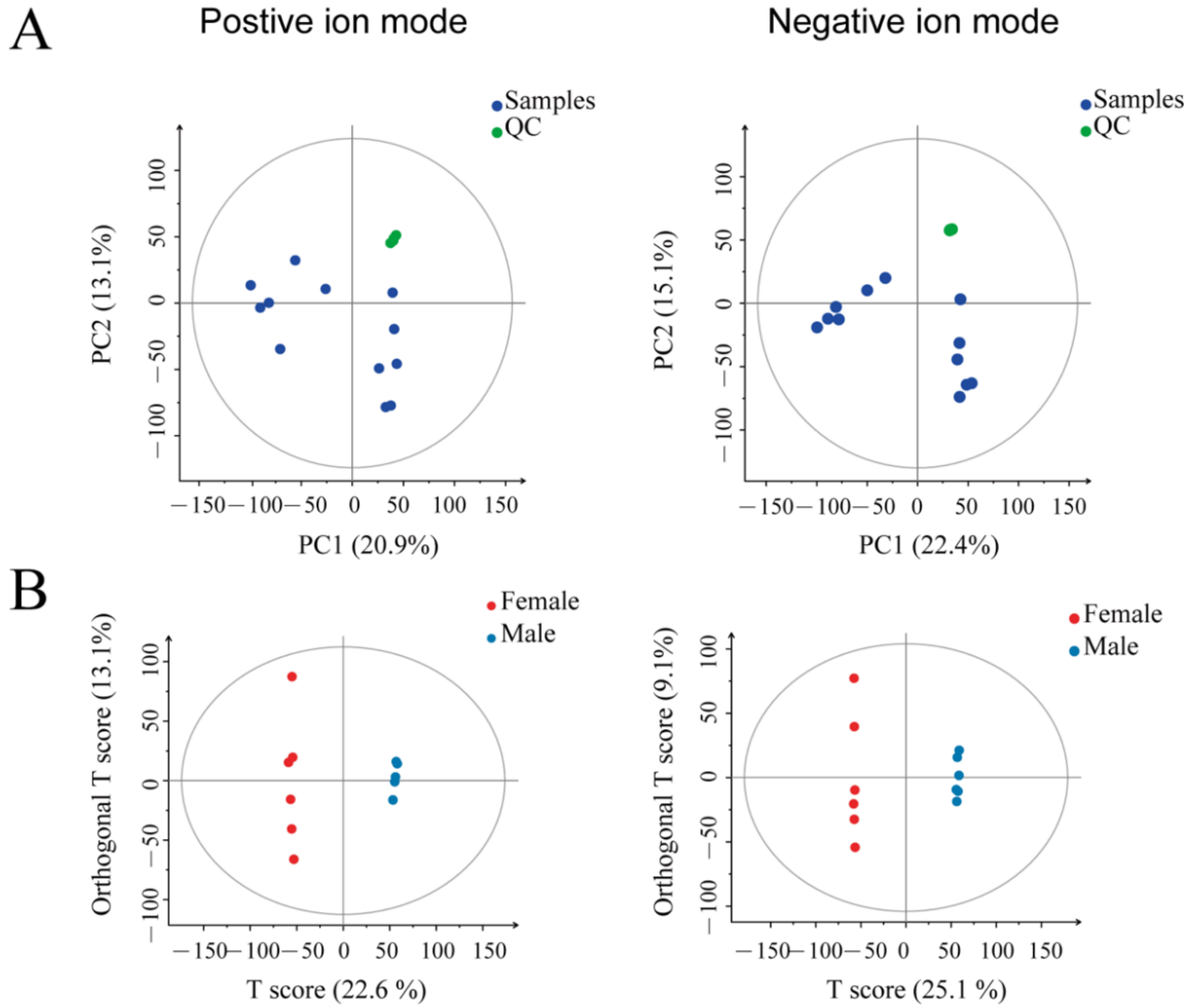
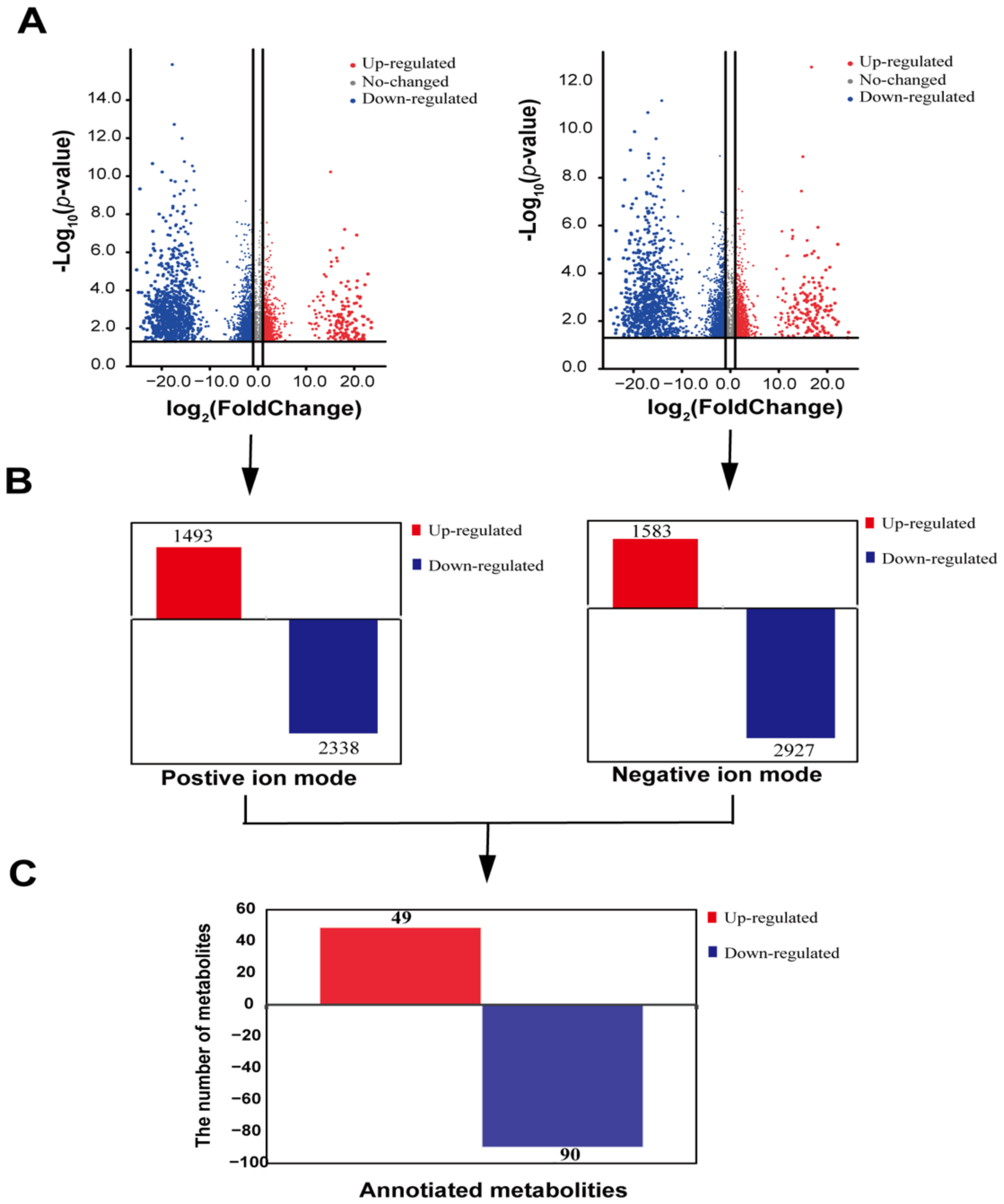


| Mode | Pre | R2X (cum) | R2Y (cum) | Q2 (cum) |
|---|---|---|---|---|
| ESI+ | 2 | 0.38 | 0.999 | 0.886 |
| ESI− | 2 | 0.342 | 1 | 0.862 |
| Compound’s Name | VIP | Fold Change (Female/Male) | p-Value | FDR |
|---|---|---|---|---|
| Fomepizole | 1.32246 | 2.9797 × 10−7 | 0.00278 | 0.02494 |
| L-Lactic acid | 1.64043 | 1.5124 × 10−6 | 0.00278 | 0.02494 |
| Choline | 1.69649 | 5.0626 × 10−7 | 0.00278 | 0.02494 |
| epsilon-Caprolactone | 1.90125 | 4.6829 × 10−6 | 0.00278 | 0.02494 |
| Pyrrolidonecarboxylic acid | 1.61869 | 202,830 | 0.00278 | 0.02494 |
| Pyroglutamic acid | 1.01192 | 480,360 | 0.00278 | 0.02494 |
| Myosmine | 1.37260 | 1.0198 × 10−6 | 0.00278 | 0.02494 |
| Vanillin | 1.61543 | 2.6944 × 10−7 | 0.00278 | 0.02494 |
| Methyl jasmonate | 1.38237 | 88,632 | 0.00278 | 0.02494 |
| Picolinic acid | 1.41010 | 0.49169 | 0.00508 | 0.02494 |
| Thymine | 1.72226 | 2.1228 | 0.00508 | 0.02494 |
| Ketoleucine | 1.38514 | 0.43895 | 0.00507 | 0.02494 |
| Heptanoic acid | 1.89661 | 1.7569 | 0.00507 | 0.02494 |
| 3-Hydroxypicolinic acid | 1.39658 | 0.31057 | 0.00507 | 0.02494 |
| Dimethylbenzimidazole | 1.22882 | 20.875 | 0.00507 | 0.02494 |
| 2-Oxo-4-methylthiobutanoic acid | 1.16255 | 0.07529 | 0.00507 | 0.02494 |
| 3,4-Methylenedioxyamphetamine | 1.83562 | 0.30120 | 0.00507 | 0.02494 |
| Dodecanoic acid | 1.38491 | 0.15975 | 0.00507 | 0.02494 |
| Cerulenin | 1.25354 | 0.10284 | 0.00507 | 0.02494 |
| 5-Hydroxypentanoic acid | 1.55774 | 1.08900 | 0.00824 | 0.03531 |
| Indoleacetaldehyde | 1.59745 | 0.07987 | 0.00824 | 0.03531 |
| myo-Inositol | 1.51714 | 4236500 | 0.00962 | 0.04094 |
| 3-Hydroxymethylglutaric acid | 1.78003 | 2.5674 × 10−6 | 0.00278 | 0.02911 |
| 3-Methyl-2-oxovaleric acid | 1.58333 | 0.28013 | 0.00507 | 0.02911 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yang, Z.; He, Y.; Yang, Q.; Tang, Q.; Yang, Z.; Qi, J.; Hu, Q.; Bai, L.; Li, L. Metabolic Profiling Reveals That the Olfactory Cues in the Duck Uropygial Gland Potentially Act as Sex Pheromones. Animals 2022, 12, 413. https://doi.org/10.3390/ani12040413
Liu H, Yang Z, He Y, Yang Q, Tang Q, Yang Z, Qi J, Hu Q, Bai L, Li L. Metabolic Profiling Reveals That the Olfactory Cues in the Duck Uropygial Gland Potentially Act as Sex Pheromones. Animals. 2022; 12(4):413. https://doi.org/10.3390/ani12040413
Chicago/Turabian StyleLiu, Hehe, Zhao Yang, Yifa He, Qinglan Yang, Qian Tang, Zhenghui Yang, Jingjing Qi, Qian Hu, Lili Bai, and Liang Li. 2022. "Metabolic Profiling Reveals That the Olfactory Cues in the Duck Uropygial Gland Potentially Act as Sex Pheromones" Animals 12, no. 4: 413. https://doi.org/10.3390/ani12040413





