Aggression, Erection, and Masturbation in Feral Pottoka Ponies and Implications for Equine Welfare
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Observation of Behavior
2.3. Data Management and Analysis
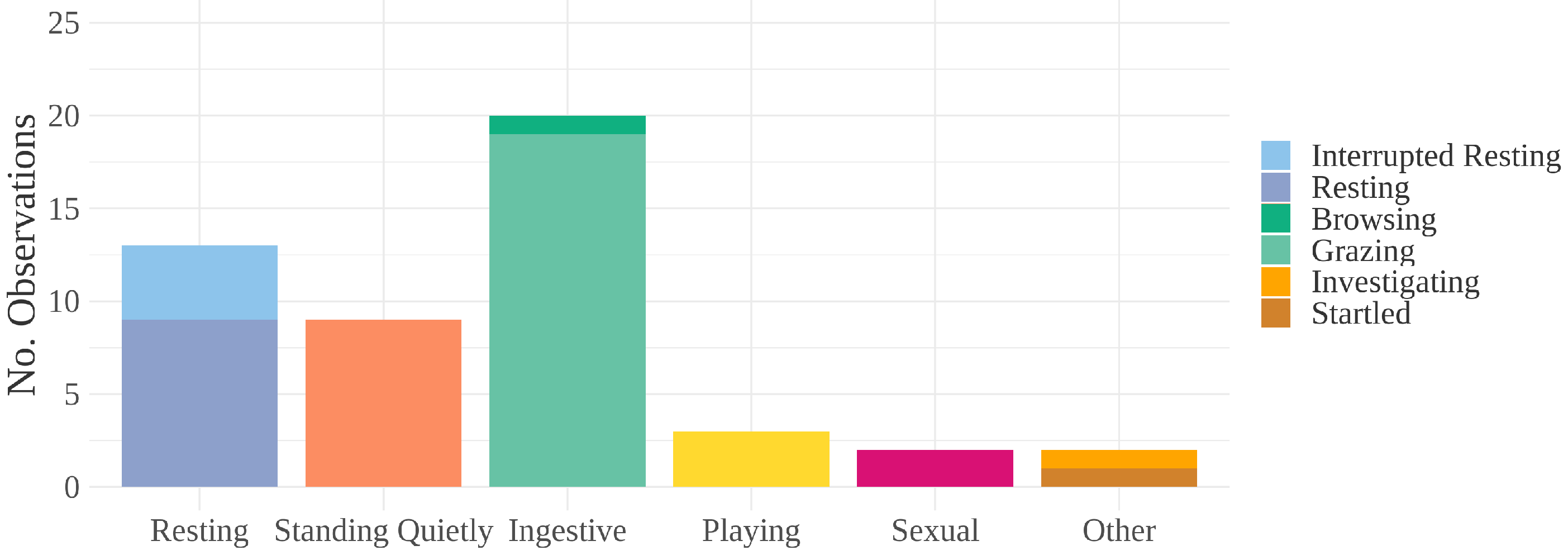
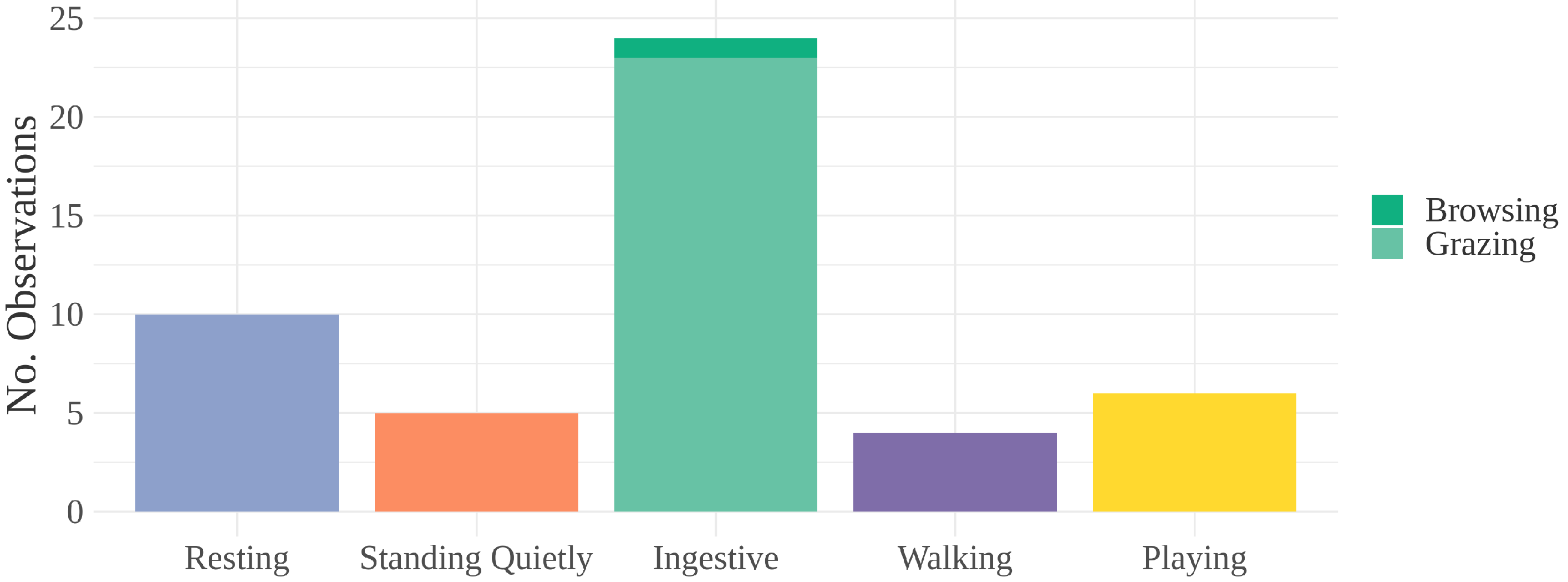
3. Results
4. Discussion and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| E/M | Erection and Masturbation |
| SEAM | Spontaneous Erection and Masturbation |
References
- McDonnell, S.M.; Henry, M.; Bristol, F. Spontaneous erection and masturbation in equids. J. Reprod. Fertil. 1991, 44, 664–665. [Google Scholar]
- Andersson, K.E.; Wagner, G. Physiology of penile erection. Physiol. Rev. 1995, 75, 191–236. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, S.; Dusza, K.; Houpt, K. The relationship between recumbent rest and masturbation in stallions. J. Equine Vet. Sci. 1991, 11, 23–26. [Google Scholar] [CrossRef]
- McDonnell, S.M. Ejaculation: Physiology and dysfunction. Vet. Clin. N. Am. Equine Pract. 1992, 8, 57–70. [Google Scholar] [CrossRef]
- McDonnell, S.M. Reproductive behavior of donkeys (Equus asinus). Appl. Anim. Behav. Sci. 1998, 60, 277–282. [Google Scholar] [CrossRef]
- Perumal, P.; Vupru, K.; Khate, K.; Veeraselvam, M.; Verma, A.K.; Nahak, A.; Rajkhowa, C. Spontaneous erection and masturbation in mithun (Bos frontalis) bulls. Int. J. Bio-Resour. Stress Manag. 2013, 4, 645–647. [Google Scholar]
- Jainudeen, M.; Hafez, E. Reproductive cycles. In Reproduction in Farm Animals, 5th ed.; Lea & Febiger: Philadelphia, PA, USA, 1987. [Google Scholar]
- Marchinton, R.L.; Moore, W.G. Auto-erotic behavior in male white-tailed deer. J. Mammal. 1971, 52, 616–617. [Google Scholar] [CrossRef]
- Aronson, L.R. Behavior resembling spontaneous emissions in the domestic cat. J. Comp. Physiol. Psychol. 1949, 42, 226. [Google Scholar] [CrossRef]
- Morisaka, T.; Sakai, M.; Kogi, K.; Nakasuji, A.; Sakakibara, K.; Kasanuki, Y.; Yoshioka, M. Spontaneous ejaculation in a wild Indo-Pacific bottlenose dolphin (Tursiops aduncus). PLoS ONE 2013, 8, e72879. [Google Scholar] [CrossRef] [Green Version]
- Jainudeen, M.; Katongole, C.; Short, R. Plasma testosterone levels in relation to musth and sexual activity in the male Asiatic elephant, Elephas maximus. Reproduction 1972, 29, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Buechner, H.K.; Mackler, S. Breeding behaviour in captive Indian rhinoceros. Der Zool. Gart. 1978, 48, 177–186. [Google Scholar]
- Michael, R.P.; Wilson, M. Effects of castration and hormone replacement in fully adult male rhesus monkeys (Macaca mulatto). Endocrinology 1974, 95, 150–159. [Google Scholar] [CrossRef]
- Holmgren, B.; Urbá-Holmgren, R.; Trucios, N.; Zermeno, M.; Eguibar, J. Association of spontaneous and dopaminergic-induced yawning and penile erections in the rat. Pharmacol. Biochem. Behav. 1985, 22, 31–35. [Google Scholar] [CrossRef]
- Beach, F.A. A review of physiological and psychological studies of sexual behavior in mammals. Physiol. Rev. 1947, 27, 240–307. [Google Scholar] [CrossRef]
- Schiavi, R.C.; White, D. Androgens and male sexual function: A review of human studies. J. Sex Marital. Ther. 1976, 2, 214–228. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.M. Reproductive behavior of stallions and mares: Comparison of free-running and domestic in-hand breeding. Anim. Reprod. Sci. 2000, 60, 211–219. [Google Scholar] [CrossRef]
- Mountjoy, P.T. Some early attempts to modify penile erection in horse and human: An historical analysis. Psychol. Rec. 1974, 24, 291–308. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Hinze, A.L. Aversive conditioning of periodic spontaneous erection adversely affects sexual behavior and semen in stallions. Anim. Reprod. Sci. 2005, 89, 77–92. [Google Scholar] [CrossRef]
- Jackson, J. Citizen Science—Why Do Clicker Trained Horses Drop? 2014. Available online: http://bookendsfarm.blogspot.com/2014/04/citizen-science-why-do-clicker-trained.html (accessed on 27 October 2021).
- Anonymous. Training Not to..Ahem…Drop. 2016. Available online: https://www.horseforum.com/threads/training-not-to-ahem-drop.678938 (accessed on 27 October 2021).
- Waring, G.H. Horse Behavior. The Behavioral Traits and Adaptations of Domestic and Wild Horses, Including Ponies; Noyes Publications: Mill Road, UK, 1983. [Google Scholar]
- Tischner, M. Patterns of stallion sexual behaviour in the absence of mares. J. Reprod. Fertil. 1982, 32, 65–70. [Google Scholar]
- Feist, J.D. Behavior of Feral Horses in the Pryor Mountain Wild Horse Range. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 1971. [Google Scholar]
- Ransom, J.I.; Cade, B.S. Quantifying Equid Behavior—A Research Ethogram for Free-Roaming Feral Horses: U.S. Geological Survey Techniques and Methods 2-A9; Publications of the US Geological Survey: Reston, VA, USA, 2009.
- Boyd, L.; Houpt, K.A. Przewalski’s Horse: The History and Biology of An Endangered Species; Suny Press: Albany, NY, USA, 1994. [Google Scholar]
- Gygax, L.; Hillmann, E. “Naturalness” and its relation to animal welfare from an ethological perspective. Agriculture 2018, 8, 136. [Google Scholar] [CrossRef]
- Bracke, M.B.; Hopster, H. Assessing the importance of natural behavior for animal welfare. J. Agric. Environ. Ethics 2006, 19, 77–89. [Google Scholar] [CrossRef]
- Luescher, U.A.; McKeown, D.; Halip, J. Reviewing the causes of obsessive-compulsive disorders in horses. Vet. Med. 1991, 86, 527–530. [Google Scholar]
- Houpt, K.A.; McDonnell, S.M. Equine stereotypies. Comp. Cont. Educ. Pract. Vet. 1993, 15, 1265–1271. [Google Scholar]
- Marsden, D. A new perspective on stereotypic behaviour problems in horses. Practice 2002, 24, 558–569. [Google Scholar] [CrossRef]
- Henderson, A.J. Rising to the Occasion: Why Is My Gelding Always “Dropping”? 2021. Available online: https://horsesport.com/magazine/behaviour/rising-occasion-why-gelding-dropping/ (accessed on 27 October 2021).
- Weston, H. Why Do Some Horses Drop Their Penis in Training? 2019. Available online: https://connectiontraining.com/2019/06/why-do-some-horses-drop-their-penis-in-training/ (accessed on 27 October 2021).
- Waran, N.; McGreevy, P.; Casey, R. Training methods and horse welfare. In The Welfare of Horses; Springer: Dordrecht, The Netherlands, 2007; pp. 151–180. [Google Scholar]
- McGreevy, P.D.; McLean, A.N. Punishment in horse-training and the concept of ethical equitation. J. Vet. Behav. 2009, 4, 193–197. [Google Scholar] [CrossRef]
- Dai, F.; Dalla Costa, A.; Bonfanti, L.; Caucci, C.; Di Martino, G.; Lucarelli, R.; Padalino, B.; Minero, M. Positive reinforcement-based training for self-loading of meat horses reduces loading time and stress-related behavior. Front. Vet. Sci. 2019, 6, 350. [Google Scholar] [CrossRef]
- Freymond, S.B.; Briefer, E.F.; Zollinger, A.; Gindrat-von Allmen, Y.; Wyss, C.; Bachmann, I. Behaviour of horses in a judgment bias test associated with positive or negative reinforcement. Appl. Anim. Behav. Sci. 2014, 158, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Innes, L.; McBride, S. Negative versus positive reinforcement: An evaluation of training strategies for rehabilitated horses. Appl. Anim. Behav. Sci. 2008, 112, 357–368. [Google Scholar] [CrossRef]
- Hockenhull, J.; Creighton, E. Training horses: Positive reinforcement, positive punishment, and ridden behavior problems. J. Vet. Behav. 2013, 8, 245–252. [Google Scholar] [CrossRef]
- Lesimple, C. Indicators of horse Welfare: State-of-the-Art. Animals 2020, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Mellor, D.J. Moving beyond the “five freedoms” by updating the “five provisions” and introducing aligned “animal welfare aims”. Animals 2016, 6, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, D. The importance of ethology in understanding the behaviour of the horse. Equine Vet. J. 1999, 31, 15–19. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.; Oddie, C.; Burton, F.; McLean, A. The horse–human dyad: Can we align horse training and handling activities with the equid social ethogram? Vet. J. 2009, 181, 12–18. [Google Scholar] [CrossRef]
- Gonyou, H.W. Why the study of animal behavior is associated with the animal welfare issue. J. Anim. Sci. 1994, 72, 2171–2177. [Google Scholar] [CrossRef]
- Rendo, F.; Iriondo, M.; Manzano, C.; Estonba, A. Effects of a 10-year conservation programme on the genetic diversity of the Pottoka pony–new clues regarding their origin. J. Anim. Breed. Genet. 2012, 129, 234–243. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.M. The Equid Ethogram: A Practical Field Guide to Horse Behavior; Eclipse Press: Washington, DC, USA, 2003. [Google Scholar]
- McDonnell, S.M.; Poulin, A. Equid play ethogram. Appl. Anim. Behav. Sci. 2002, 78, 263–290. [Google Scholar] [CrossRef]
- Goulden, M.C.; Gronda, E.; Yang, Y.; Zhang, Z.; Tao, J.; Wang, C.; Duan, X.; Ambrose, G.A.; Abbott, K.; Miller, P. CCVis: Visual analytics of student online learning behaviors using course clickstream data. Electron. Imaging 2019, 2019, 681-1–681-12. [Google Scholar] [CrossRef] [Green Version]
- Lamer, A.; Laurent, G.; Pelayo, S.; El Amrani, M.; Chazard, E.; Marcilly, R. Exploring patient path through Sankey diagram: A proof of concept. In Digital Personalized Health and Medicine; EFMI: Lausanne, Switzerland, 2020; Volume 270, pp. 218–222. [Google Scholar]
- McDonnell, S.; Diehl, N.; Garcia, M.; Kenney, R. Gonadotropin releasing hormone (GnRH) affects precopulatory behavior in testosterone-treated geldings. Physiol. Behav. 1989, 45, 145–149. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Murray, S.C. Bachelor and harem stallion behavior and endocrinology. Biol. Reprod. 1995, 52, 577–590. [Google Scholar] [CrossRef] [Green Version]
- McCallum, L.; Dumbell, L. Social play and its initiation in an established group of young domestic horses. In Proceedings of the British Society of Animal Science; Cambridge University Press: Cambridge, UK, 2017; Volume 2009, 8p. [Google Scholar]
- Rees, L. Horses in Company. The Crowood Press: Marlborough, UK, 2017. [Google Scholar]
- McDonnell, S.; Haviland, J. Agonistic ethogram of the equid bachelor band. Appl. Anim. Behav. Sci. 1995, 43, 147–188. [Google Scholar] [CrossRef]
- King, S.R.; Asa, C.; Pluhacek, J.; Houpt, K.; Ransom, J.I. Behavior of horses, zebras, and asses. Wild Equids Ecol. Manag. Conserv. 2016, 23, 40. [Google Scholar]
- Fureix, C.; Bourjade, M.; Henry, S.; Sankey, C.; Hausberger, M. Exploring aggression regulation in managed groups of horses Equus caballus. Appl. Anim. Behav. Sci. 2012, 138, 216–228. [Google Scholar] [CrossRef] [Green Version]
- Amann, R. A review of anatomy and physiology of the stallion. J. Equine Vet. Sci. 1981, 1, 83–105. [Google Scholar] [CrossRef]
- Proops, L.; Grounds, K.; Smith, A.V.; McComb, K. Animals remember previous facial expressions that specific humans have exhibited. Curr. Biol. 2018, 28, 1428–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, L. The Horse’s Mind; Arco Pub: New York, NY, USA, 1985. [Google Scholar]
- Draaisma, R. Language Signs and Calming Signals of Horses: Recognition and Application; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Recordati, G. A thermodynamic model of the sympathetic and parasympathetic nervous systems. Auton. Neurosci. 2003, 103, 1–12. [Google Scholar] [CrossRef]
- Foster, R.; CAAB, C. Understanding and implementing principles of learning in the equine veterinary practice. In Proceedings of the AAEP Annual Convention, San Antonio, TX, USA, 18–21 November 2017; Volume 63, pp. 255–261. [Google Scholar]






| Subject | Band | Age |
|---|---|---|
| Basoa | Bachelor Band | 2 |
| Beltz | Bachelor Band | 2 |
| Erbi | Bachelor Band | 2 |
| Oihan | Bachelor Band | 4 |
| Indartxu | Pintxo’s Band | 1 |
| Pintxo | Pintxo’s Band | 10 |
| Ibai | Ibai’s Band | 4 |
| Gabiri | Gabiri’s Band | 10 |
| Ethogram Category | Description |
|---|---|
| Aggressive | Biting, kicking, striking, or chasing another horse, with |
| ears pinned and muscles tense [47] | |
| Erection | Penis fully extended and tumescent [47] |
| Masturbation | Bouncing or pressing of erect penis against belly [47] |
| Ingestive | Grazing, browsing, or drinking [47] |
| Play | Conspecific interactions involving locomotion and play |
| fighting; including nipping, biting, pushing, bucking, | |
| rearing, mounting, grasping, kicking, neck wrestle, chase [25] | |
| Walking | Four-beat locomotion [25] |
| Resting | Standing with eyes closed or one hind leg rested, |
| lateral or sternal lying [25] | |
| Sexual | Behavior involving a stallion and a mare that may include |
| teasing, odor or pheromone detection, nudging, biting, | |
| mounting or attempted mounting, and copulation [25] | |
| Standing Quietly | Standing without resting a leg, muscles relaxed, ears, head, |
| and neck showing little movement * | |
| Other | Any other behaviors; one observation of investigative |
| behavior (sniffing a backpack) and one startle |
| Subject | Status a | No. Obs. E | No. Obs. M | Total Hrs Obs. | E/hour | M/hour |
|---|---|---|---|---|---|---|
| Gabiri | HS | 1 | 3 | >0.5 b | ||
| Ibai | HS | 1 | 4 | >1.3 b | ||
| Pintxo | HS | 1 | 1 | >0.9 b | ||
| Basoa | B | 5 | 7 | 7.8 c | 0.6 | 0.9 |
| Beltz | B | 5 | 6 | 7.8 c | 0.6 | 0.8 |
| Erbi | B | 5 | 3 | 7.8 c | 0.6 | 0.4 |
| Oihan | B | 3 | 2 | 7.8 c | 0.4 | 0.3 |
| Indartxu | Y | 1 | 1 | >0.9 b | ||
| Mean | 0.6 | 0.6 |
| Behavior | No. of Obs. | Duration (Range) | Duration (Average) |
|---|---|---|---|
| Erection | 22 | <1–4 min (22 obs.) | 1.7 min ± 0.8 |
| Masturbation | 27 | <1–5 min (25 obs.) | 1.8 min ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillaert, K. Aggression, Erection, and Masturbation in Feral Pottoka Ponies and Implications for Equine Welfare. Animals 2022, 12, 421. https://doi.org/10.3390/ani12040421
Grillaert K. Aggression, Erection, and Masturbation in Feral Pottoka Ponies and Implications for Equine Welfare. Animals. 2022; 12(4):421. https://doi.org/10.3390/ani12040421
Chicago/Turabian StyleGrillaert, Katherine. 2022. "Aggression, Erection, and Masturbation in Feral Pottoka Ponies and Implications for Equine Welfare" Animals 12, no. 4: 421. https://doi.org/10.3390/ani12040421





