Advantages, Factors, Obstacles, Potential Solutions, and Recent Advances of Fish Germ Cell Transplantation for Aquaculture—A Practical Review
Abstract
:Simple Summary
Abstract
1. Fish Germ Cell Transplantation in Aquaculture
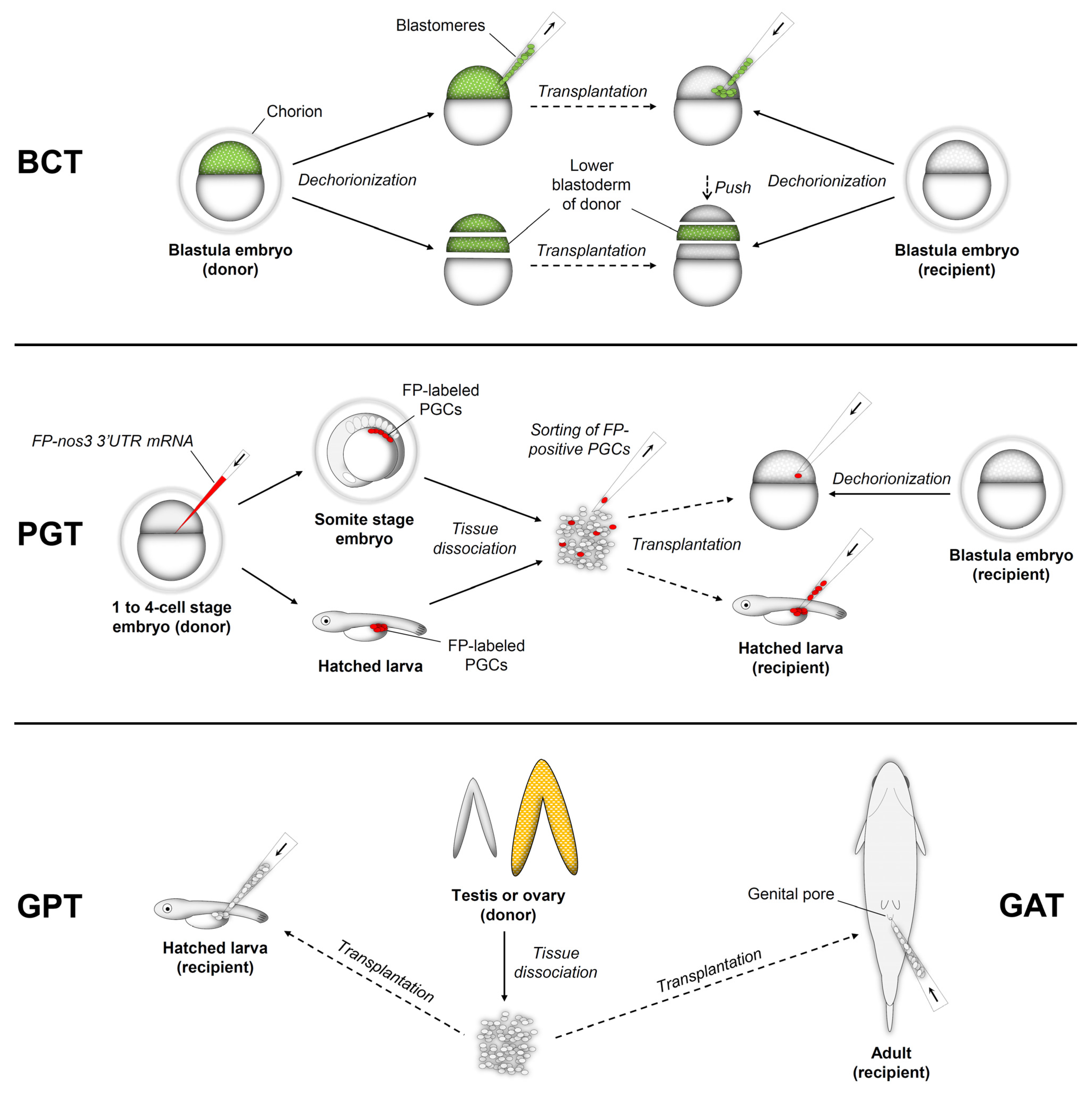
2. Germ Cell Transplantation Methods in Fish
2.1. Blastula Cell Transplantation (BCT)
2.2. Primordial Germ Cell Transplantation (PGT)
2.3. Gonadal Germ Cell Transplantation into the Peritoneal Cavity of Larvae (GPT)
2.4. Gonadal Germ Cell Transplantation into Adult Recipients (GAT)
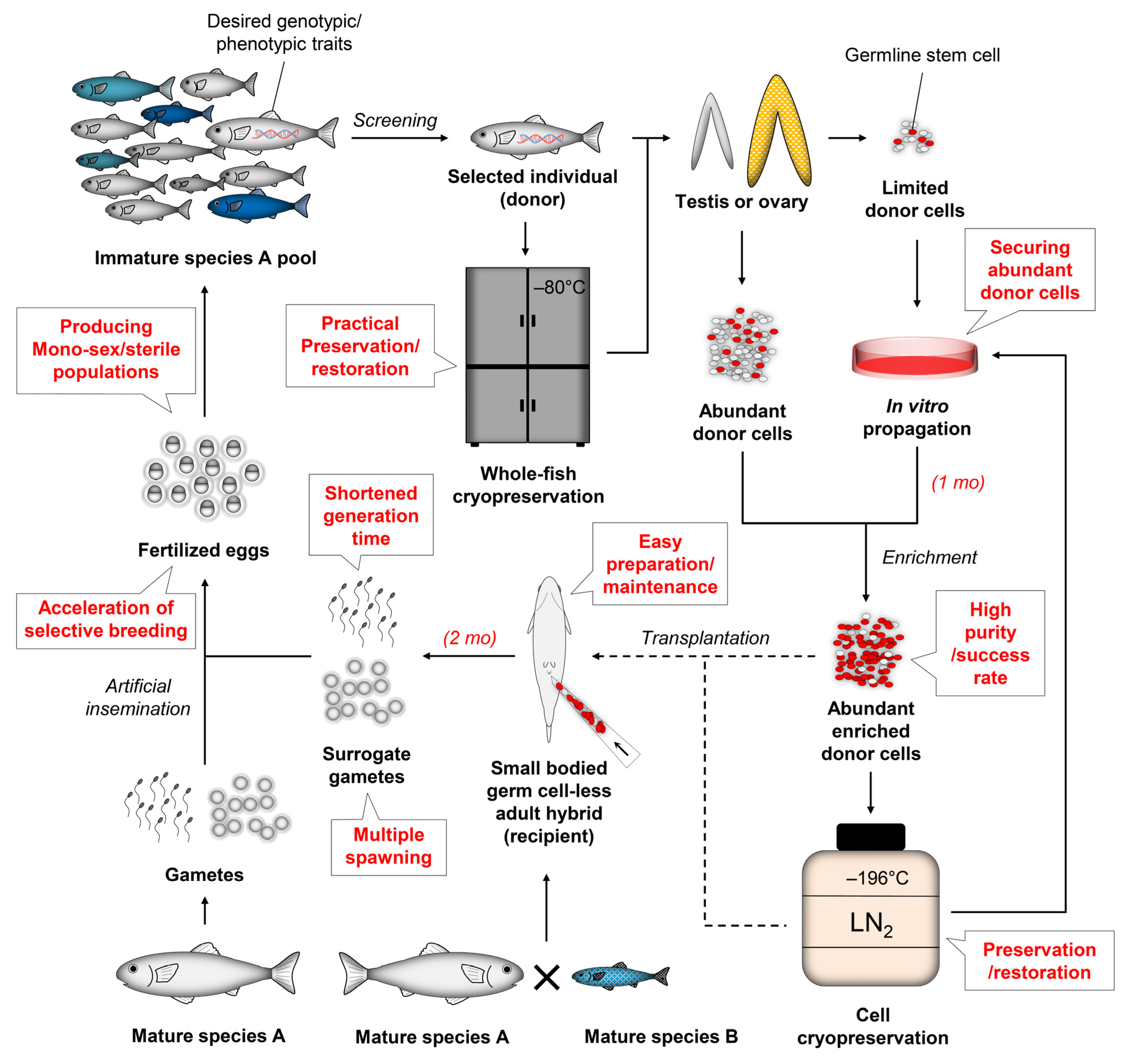
3. Advantages of Germ Cell Transplantation in Aquaculture
3.1. Shortening Generation Time
3.2. Achieving Gamete Production of Semelparous Fish Recurrently through Multiple Seasons
3.3. Solving Bottlenecks of Broodstock Maintenance
3.4. Preservation and Restoration of Superior Strains Using Cryopreserved Cells
4. Factors Affecting the Success of Germ Cell Transplantation
4.1. The Number of Donor Cells
4.2. Purity of Donor Cells (Enrichment)
4.2.1. Percoll Density Gradient Centrifugation (PDGC)
4.2.2. Differential Plating (DP)
4.2.3. Centrifugal Elutriation (CE)
4.2.4. Fluorescence-Activated Cell Sorting (FACS)
4.2.5. Magnetic-Activated Cell Sorting (MACS)
4.2.6. Applications
4.3. Age of Recipients
4.4. Prevention of Endogenous Germ Cell Development in Recipient Gonads
4.4.1. Interspecific Hybridization
4.4.2. Triploidization
4.4.3. Dnd-Knockdown
4.4.4. Dnd-Knockout
4.4.5. Co-Treatment of High Temperature and Busulfan
4.4.6. Other Aspects
4.4.7. Applications
5. Obstacles and Potential Solutions of Germ Cell Transplantation in Aquaculture
5.1. Practical Acceleration of Selective Breeding Process
5.2. Securing Enough Donor Cells for Transplantation via In Vivo or In Vitro Propagation
5.3. Enhancing Transplantation Efficiency with Vulnerable Recipients during Early Development
5.4. Choice of Compatible Recipient Species for Xenotransplantation
6. Advanced Applications of Germ Cell Transplantation
6.1. Production of Mono-Sex Populations
6.2. Production of Sterile Fish through Transplantation of Germ Cells Carrying Mutated Somatic Genes
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Donor | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells | % of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) | % of Donor-Derived Offspring | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Cells | Species | Fish | Total | Male | Female | ||||
| C. auratus | All-female (XX) | A lower part of BD | Hybrid | ♂ C. carpio × ♀ C. auratus, all-male | N/A | N/A | 100 at SM | N/A | Con: 79.9 TP: 72.3 at 4 d | N/A | [11] |
| WT | BMs | C. auratus langsdorfii | 3N | 3.1 at 4 d | N/A | N/A | N/A | Con: 65.8 CP + TP: 35.6 at 4 d | N/A | [9] | |
| WT | 50–100 BMs | D. rerio | WT | 7.3 at 24 h | N/A | N/A | N/A | 73.3 at 24 h | N/A | [13] | |
| C. auratus langsdorfii | 3N | A lower part of BD | C. auratus | WT | N/A | N/A | N/A | 100 at 3–4 y | Con: 91.4 TP: 98.4 at 3 d | 3.1–89.3 | [10] |
| D. albolineatus | WT | 50–100 BMs | D. rerio | WT | 6.6 at 24 h | N/A | N/A | N/A | 96.8 at 24 h | N/A | [13] |
| D. rerio | WT or TG:pRSV-LacZ (LacZ/-) | 20–100 BMs | D. rerio | Albino | N/A | 17.9 at 2–3 mo | N/A | N/A | 16.7 at SM | 1.0–40.0 | [6] |
| WT | 50–100 BMs | D. rerio | WT | 9.0 at 24 h | N/A | N/A | N/A | 86.7 at 24 h | N/A | [13] | |
| TG:pvasa-DsRed2-vasa;pβact-EGFP | A whole BD | D. rerio | Dnd-KD | 94.7 at prim-5 St | N/A | N/A | N/A | Con: 62.9 Dnd-KD: 50.3 TP: 26.1 at adult | N/A | [8] | |
| M. anguillicaudatus | WT | 50–100 BMs | D. rerio | WT | 1.5 at 24 h | N/A | N/A | N/A | 75.8 at 24 h | N/A | [13] |
| O. mykiss | WT, mid-blastula (2.5 d) | ~80 BMs | O. mykiss | WT, early blastula (1.5 d) | N/A | 31.6 at 2 y | 55.6 at 2 y | 10.0 at 2 y | 8.0 at 2 y | 0.3–14.6 | [7] |
| O. latipes | TG:pLFABP-rfp;pvasa-GFP | 30 BMs | O. latipes | WT | 47.2 at St 18–23 | N/A | N/A | N/A | N/A | 4.3 | [64] |
| TG:pLFABP-rfp;pvasa-GFP, dnd mRNA injected | WT | 81.2 at St 18–23 | N/A | N/A | N/A | N/A | 31.4 | ||||
| TG:pLFABP-rfp;pvasa-GFP, dnd mRNA injected | Dnd-KD | 81.1 at St 18–23 | 91.4 at SM | N/A | N/A | N/A | 96.5 | ||||
| TG:pLFABP-rfp;pvasa-GFP, dnd mRNA injected | γ-irradiated | 79.2 at St 18–23 | N/A | N/A | N/A | N/A | N/A | ||||
| Donor | Enrichment | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells | % of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) | % of Donor-Derived Offspring | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Age | Cells | Method | Purity | Species | Fish | Age | Total | Male | Female | ||||
| A. japonica | WT | Somito-genesis | 1 PGC | MC | N/A | D. rerio | WT | Blastula | 42.7 at 2 d | N/A | N/A | N/A | Con: 92.7 TP: 92.6 at 2 d | N/A | [161] |
| C. auratus | WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | N/A | N/A | Y at 10–12 mo | N | N/A | N/A | [16] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 63.5 at 2 d | N/A | N/A | N/A | 69.0 at 2 d | N/A | [13] | |
| Albino | 10–15 somite St | 1 PGC | MC | N/A | C. auratus | Dnd-KD | Blastula | 42.7 at 3 d | 42.9 at 5 mo–2 y | 62.5 at 5 mo–2 y | 30.1 at 5 mo–2 y | Dnd-KD: 88.3 TP: 84.9 | 92.5–100 | [65] | |
| D. albolineatus | WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 45.1 at 2 d | 89.5 at SM | 93.8 at SM | 66.7 at SM | Dnd-KD: 59.2 TP: 74.7 at 2 d | 100 | [16] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 40.2 at 2 d | N/A | N/A | N/A | 76.8 at 2 d | N/A | [13] | |
| D. rerio | WT | 10–15 somite St | 1 PGC | FACS | 87.8–100 | D. rerio | Dnd-KD | Blastula | 31.5 at 1 dpt | N/A | N/A | N/A | Dnd-KD: 92.9 Dnd-KD + TP: 81.4 at 1 dpt | N/A | [17] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 30.0 at 2 d | N/A | N/A | N/A | 89.7 at 2 d | N/A | [13] | |
| 21–25 somite St | 10.1 at 2 d | N/A | N/A | N/A | 82.4 at 2 d | N/A | |||||||||
| Prim-5 St | 5.3 at 2 d | N/A | N/A | N/A | 78.4 at 2 d | N/A | |||||||||
| Prim-15 St | 4.5 at 2 d | N/A | N/A | N/A | 87.3 at 2 d | N/A | |||||||||
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 20.7 at prim-5 St | N/A | N/A | N/A | Con: 74.1 Dnd-KD: 59.4 TP: 66.7 | N/A | [8] | |
| M. anguillicaudatus | WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | N/A | N/A | Y at 10–12 mo | N | N/A | N/A | [16] |
| WT | 10–15 somite St | 1 PGC | MC | N/A | D. rerio | Dnd-KD | Blastula | 43.1 at 2 d | N/A | N/A | N/A | 85.7 at 2 d | N/A | [13] | |
| O. masou | WT | 40 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | Newly hatched | 24.4 at 10 dpt | N/A | N/A | N/A | N/A | N/A | [66] |
| O. mykiss | TG:pvasa-GFP | 35 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | 2.5 d | 0 at 30 dpt | N/A | N/A | N/A | 26 at 30 dpt | ♂: 3.9 ♀: 4.2–6.1 | [15] |
| 35 d | 6 d | 0 at 30 dpt | N/A | N/A | N/A | 62 at 30 dpt | |||||||||
| 35 d | 35 d | 21.6 at 30 dpt | 15.4 at 1–2 y | 16.7 at 1 y | 14.3 at 2 y | 94 at 30 dpt | |||||||||
| 35 d | 35 d | 10–20 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 40 d | 35 d | 10–20 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 45 d | 35 d | 0–10 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 35 d | 40 d | 10–20 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 40 d | 40 d | 0–10 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 45 d | 40 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 35 d | 45 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 40 d | 45 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| 45 d | 45 d | 0 * at 30 dpt | N/A | N/A | N/A | N/A | |||||||||
| TG:pvasa-GFP | 35 d | 20 PGCs | MC | N/A | O. masou | WT | Newly hatched | 16.7 at 30 dpt | N/A | 13.5 at 1 y | Y at 17 mo | N/A | ♂: 0.4 | [154] | |
| WT | 32 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | Newly hatched | 12.3 at 10 dpt | N/A | N/A | N/A | N/A | N/A | [66] | |
| TG:pvasa-GFP | 30 dpf | 15–20 PGCs | MC | N/A | O. mykiss | WT | 32–34 dpf | Fresh: 12.5 CP (d1): 10.1 at 30 dpt | Fresh: 10.9 CP (d1): 8.3 at 3 y | Fresh: 10.3 CP (d1): 7.8 at 3 y | Fresh: 12.1 CP (d1): 9.1 at 3 y | Fresh: 98.5 CP (d1): 86.5 at 30 dpt | Fresh ♂: 0.2–16.3 Fresh ♀: 0.2–7.6 CP (d1) ♂: 2.0–13.5 CP (d1) ♀: 0.1–3.3 | [53] | |
| S. trutta | WT | 40 d | 5–10 PGCs | MC | N/A | O. mykiss | WT | Newly hatched | 9.4 at 10 dpt | N/A | N/A | N/A | N/A | N/A | [66] |
| Hybrid | H. huso × A. ruthenus | Late-neural St | 1 PGC | MC | N/A | C. auratus | WT | Blastula | 9.1 at 3 d | N/A | N/A | N/A | 95.7 at 3 d | N/A | [162] |
| Donor | Enrichment | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells | % of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) | % of Donor-Derived Offspring | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Age (Size) | Cells | Method | Purity | Species | Fish | Age (Size) | Total | Male | Female | ||||
| A. baerii | WT | 4 y | TCs | PDGC | 79.4 | A. ruthenus | WT | 1 wph | 60.0 at 90 dpt | N/A | N/A | N/A | Con: 96.7 TP: 95.8 | N/A | [39] |
| WT | 4 y | TCs | N/C | N/A | A. ruthenus | WT | Newly hatched | Fresh: 55.0 CP: 65.0 at 90 dpt | N/A | N/A | N/A | N/A | N/A | [195] | |
| WT | 4 y | OCs | PDGC | 70.8 | A. ruthenus | WT | 1 wph | 60.0 at 90 dpt | N/A | N/A | N/A | Con: 96.7 TP: 95.8 | N/A | [39] | |
| WT | 4 y | OCs | N/C | N/A | A. ruthenus | WT | Newly hatched | Fresh: 70.0 CP: 55.0 at 90 dpt | N/A | N/A | N/A | N/A | N/A | [195] | |
| A. dabryanus | WT | 2 y | 50,000 TCs | N/C | N/A | A. dabryanus | WT | 7–8 dph | 70.0 at 51 dpt | N/A | N/A | N/A | Con: 86.2 TP: 76.7 at 51 dpt | N/A | [196] |
| A. sinensis | WT | 11.5 y | 50,000 OCs | N/C | N/A | A. dabryanus | WT | 7–8 dph | 20.0 at 51 dpt | N/A | N/A | N/A | Con: 86.2 TP: 68.6 at 51 dpt | N/A | [196] |
| C. carpio | WT | 2 y | 30,000–50,000 TCs | PDGC | N/A | C. auratus | Dnd-KD | 7 d | 62.5 at 1 mo | 43.7 at 3 y | 32.4 at 3 y | 11.2 at 3 y | Con: 74 Dnd-KD: 79 Dnd-KD + TP: 72 at 18 mpf | 100 | [29] |
| D. rerio | TG:ppiwil1-DsRed | 2–3 mo | 10–60 SG | PDGC | N/A | D. rerio | Dnd-KD | 2 w | N/A | N/A | Cult (3 w): 19 Cult (6 w): 18 at SM | N/A | Cult (3 w): 72.2 Cult (6 w): 64.9 at SM | 100 | [126] |
| TG:pvasa-EGFP | Adult | 3000 GFP+ TCs | N/C | N/A | D. rerio | WT | 9–10 d | 6.7 at 50 dpt | N/A | 6.7 at SM | N/A | N/A | 3.9–7.3 | [101] | |
| Dnd-KO | 5.0 at 50 dpt | N/A | 5.0 at SM | N/A | N/A | 100 | |||||||||
| TG:pvasa-EGFP | 2 mo | 3000–5000 TCs | N/C | N/A | D. rerio | 3N | 7 d | 53.2 at adult | N/A | 43.5 at adult | N/A | 2N Con: 83.3 3N Con: 75.6 3N TP: 68.9 at adult | 100 | [145] | |
| TG:pvasa-EGFP or TG:pβact-YFP | 3–6 mo | 3000 GFP+ TCs | N/C | N/A | D. rerio | WT | 7 d | Fresh: 31.1 CP: 24.4 VF: 22.2 at 50 dpt | N/A | N/A | N/A | Contro: 80 TP: 85 at 50 dpt | N/A | [135] | |
| Dnd-KD | Fresh:58.3 CP: 47.4 VF: 50.0 at 50 dpt | N/A | 43 at 6 mo | N/A | N/A | 100 | |||||||||
| TG:pvasa-DsRed2- vasa;pβact-EGFP | 3 mo | OCs | PDGC | N/A | Hybrid | ♂ D. albolineatus × ♀ D. rerio | 2 w | 20.0 at 8 w | 17.9 at 6 mo | 17.9 at 6 mo | 0 at 6 mo | 52.7 at 6 mo | 100 | [69] | |
| TG:pziwi-Neo;pziwi-DsRed | 10–12 w | 20–40 FGSCs | PDGC | N/A | D. rerio | Dnd-KD | 2 w | N/A | N/A | Cult (3 w): 20.0 Cult (6 w): 16.0 at SM | N/A | Cult (3 w): 73.5 Cult (6 w): 71.7 at SM | 100 | [123] | |
| TG:pvasa-EGFP | 2 mo | 500 OCs | N/C | N/A | D. rerio | 3N | 7 d | 43.6 at adult | N/A | 34.5 at adult | N/A | 2N Con: 83.3 3N Con: 75.6 3N TP: 61.1 at adult | 100 | [145] | |
| N. mitsukurii | WT | 3 mo | 10,000 TCs | N/C | N/A | S. japonicus | WT | 5 d | 33.5 at 21 dpt | N/A | N/A | N/A | 20.3 at 21 dpt | N/A | [99] |
| 7 d | 70.0 at 21 dpt | N/A | N/A | N/A | 20.6 at 21 dpt | N/A | |||||||||
| 9 d | 4.4 at 21 dpt | N/A | N/A | N/A | 27.6 at 21 dpt | N/A | |||||||||
| WT | 3 mo | 3000 TCs | FACS | Unsort: 18.8 Sort: 94.0 | N. mitsukurii | WT | 12 d | Unsort: 7.6 Sort: 60.0 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [19] | |
| TG:pHSC-GFP | 3 mo | 10,000 TCs | N/C | N/A | N. mitsukurii | 3N (<100% incidence) | 12 dph | 2N: 63.3 3N: 56.0 at 18 dpt | 2N: 6.1 3N: 31.4 at 6 mo | 2N: 5.3 3N: 36.8 at 6 mo | 2N: 6.8 3N: 28.9 at 6 mo | 41.1 at 18 dpt | 2N ♂: 6.8–89.2 3N ♂: 100 2N ♀: 2.2–25.0 3N ♀: 100 | [63] | |
| TG:pHSC-GFP | 3 mo | 10,000 TCs | N/C | N/A | N. mitsukurii | WT | 12 d | 58.3 at 2 wpt | N/A | N/A | N/A | 68.1 at 2 wpt | N/A | [27] | |
| Hybrid | ♂ P. argentata × ♀ N. mitsukurii, 3N | 12 d | 58.6 at 2 wpt | 34.4 at 6 mo | 34.4 at 6 mo | 0 at 6 mo | 34.7 at 2 wpt | ♂: 100 | |||||||
| O. masou | WT | 12–13 mo | 3000 TCs | FACS | Unsort: 16.4 Sort: 75.6 | O. mykiss | WT | Newly hatched | Unsort: 0–25 * Sort: 80.0 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [19] |
| WT, black | 8 mo | 50,000 TCs | N/C | N/A | O. masou | 3N | 40–42 d | Fresh: 50–60 * CP: 58.2 at 50 dpt | CP: 39.1 at 2 y | CP: 43.5 at 2 y | CP: 34.8 at 2 y | N/A | 100 | [28] | |
| WT, black | 8 mo | 50,000 OCs | N/C | N/A | O. masou | 3N | 40–42 d | Fresh: 40–60 * CP: 41.2 at 50 dpt | CP: 25.8 at 2 y | CP: 29.3 at 2 y | CP: 23.1 at 2 y | N/A | 100 | [28] | |
| O. mykiss | Albino, TG:pvasa-GFP | 9 mo | 18,000 TCs (10,000 GFP+ cells) | FACS | N/A | O. mykiss | WT | Newly hatched | ♂: 38.8 ♀: 57.8 at 2 mpt | N/A | 50 at 1 y | 40 at 2 y | N/A | ♂: 5.5 (0.2–40.5) ♀: 2.1 (0.1–9.9) | [37] |
| TG:pvasa-GFP | Adult | TCs | N/C | N/A | O. masou | 3N | Newly hatched | N/A | N/A | 34.5 at 2 y | 50.0 at 17 mo; 10.0 at 2–3 y | N/A | ♂: 100 ♀: 100 | [146] | |
| TG:pvasa-GFP | 8–10 mo | 10,000 TCs | DP | 95–99 | O. mykiss | WT | Newly hatched | Sort: 77.7 Sort + Cult (1 mo): 52.2 at 30 dpt | N/A | N/A | N/A | N/A | N/A | [75] | |
| TG:pvasa-GFP | 16 mo | 3000 TCs | FACS | N/A | O. mykiss | WT | Newly hatched | Sort-A: 51.0 Sort-B: 0 at 60 dpt | N/A | N/A | N/A | N/A | N/A | [67] | |
| 7 mo | 3000 ASG | N/C | N/A | 53.6 at 60 dpt | N/A | N/A | N/A | N/A | N/A | ||||||
| WT | 8–12 mo | 3000 TCs | FACS | N/A | O. mykiss | WT | Newly hatched | Unsort: 23.0 Sort: 78.3 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [19] | |
| TG:pvasa-GFP | 8–12 mo | Unsort: 35.9 Sort: 93.2 | Unsort: 29.0 Sort: 82.0 at 20 dpt | N/A | N/A | N/A | N/A | N/A | |||||||
| TG:pvasa-GFP | 16 or 24 mo | Sort: 90.0–96.2 | N/A | N/A | N/A | N/A | N/A | N/A | |||||||
| TG:pvasa-GFP | 11–12 mo | 5000 TCs | DP | >90 | O. mykiss | WT | 25–27 d | Fresh: 59.7 Cult + Sort: 94.5 Cult + Sort + Tryp-2 h: 43.9 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [73] | |
| TG:pMlc2-GFP | 6 mo | 10,000 ASG | PDGC + CE | >90 | O. mykiss | WT | Peri-hatching | 90 at 6 mo | N/A | 90 at 1–2 y | N/A | N/A | 1–56 | [23] | |
| 1 y | >90 | 78 at 6 mo | N/A | N/A | N/A | N/A | N/A | ||||||||
| Mature | >70 | 42 at 6 mo | N/A | N/A | N/A | N/A | N/A | ||||||||
| TG:pvasa-GFP | 11 mo | 500 GFP+ TCs | N/C | N/A | O. mykiss | 3N | 32 d | Fresh: 30–40 * CP (F371): 30–40 * at 50 dpt | Fresh: 32.4 CP (F371): 28.6 at 2 y | Fresh: 35.0 CP (F371): 40.0 at 2 y | Fresh: 29.4 CP (F371): 15.4 at 2 y | N/A | 100 | [61] | |
| 18 mo | O. mykiss | 32 d | Fresh: 40–60 * CP (F371): 40–60 * at 30 dpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 11 mo | O. masou | 37 d | Fresh: 20–30 * CP (F371): 20–30 * at 50 dpt | Fresh: 27.6 CP (F371): 24.0 at 2 y | Fresh: 33.3 CP (F371): 30.8 at 2 y | Fresh: 23.5 CP (F371): 16.7 at 2 y | N/A | 100 | |||||||
| TG:pvasa-GFP | 8 mo | 30,000 TCs | N/C | N/A | O. masou | WT | 38–40 d | 63.2 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [124] | |
| Dnd-KD | 70 at 20 dpt | 54.5 at 2 y | 46.7 at 2 y | 71.4 at 2 y | N/A | ♂: 100 (6 Inds); 72.3 (1 Ind) ♀: 100 (2 Inds); 21.1 (3 Inds) | |||||||||
| TG:pvasa-GFP | 9–11 mo | 1000 TCs | FACS (AB No. 80 or No. 95) | Unsort: <20 * Sort (No. 80): 70.7 Sort (No. 95): 80.9 | O. mykiss | WT | Newly hatched | Unsort: 1.1 Sort (No. 80): 19.2 Sort (No. 95): 18.4 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [20] | |
| TG:pvasa-GFP | 13–14 mo | <2000 TCs | MACS (AB No. 172) | Unsort: 49.8 Sort: 81.7 | O. mykiss | WT | Newly hatched | Unsort: 10–20 * Sort: 30–40 * at 20 dpt | N/A | N/A | N/A | N/A | N/A | [62] | |
| TG:pvasa-GFP | 12–15 mo | 15,000 SG | DP | N/A | O. mykiss | 3N | 30 d | Fresh: 60–80 * Cult: 60–80 * at 20 dpt | Fresh: 89.2 Cult: 65.0 at 1 y | Fresh: 88.0 Cult: 77.6 at 1 y | Fresh: 73.3 Cult: 36.8 at 2 y | N/A | ♂: 100 ♀: 100 | [147] | |
| TG:pvasa-GFP | 6–9 mo | 15,000 OCs | N/C | N/A | O. mykiss | 3N | 25 d | ♂: 50.0 ♀: 47.1 at 5 mo | 10.0 at 2 y | 15.0 at 2 y | 5.0 at 2 y | N/A | ♂: 100 ♀: 100 | [21] | |
| TG:pvasa-GFP | 9 mo | <10,000 GPF+ OCs | N/C | N/A | O. mykiss | 3N | 33 d | Fresh: 60–80 * CP: 57.1 at 50 dpt | Fresh: 25.0 CP: 24.0 at 2.5 y | Fresh: 30.8 CP: 28.0 at 2.5 y | Fresh: 18.2 CP: 20.0 at 2.5 y | N/A | Fresh: 100 CP: 100 | [56] | |
| TG:pvasa-GFP | 12–14 mo | <10,000 OCs | MACS (AB No. 172) | Unsort: 5.2 Sort: 54.8 | O. mykiss | WT | Newly hatched | Unsort: 0–20 * Sort: 60–80 * at 20 dpt | N/A | N/A | N/A | N/A | N/A | [62] | |
| O. nerka | WT | 15 mo | <5000 TCs | MACS (AB No. 172) | Unsort: 16.4 Sort: 68.6 | O. mykiss | WT | Newly hatched | Unsort: 0–20 * Sort: 40–60 * at 20 dpt | N/A | N/A | N/A | N/A | N/A | [62] |
| O. niloticus | TG:pβact-EGFP | 5–12 mo | 20,000 TCs | N/C | N/A | O. niloticus | WT | 6 d | 0 at 5 mo | N/A | N/A | N/A | N/A | N/A | [137] |
| 7 d | 6.7 at 5 mo | 37.5 at 1 y | 40.0 at 1 y | 33.3 at 1 y | N/A | 2.4 | |||||||||
| 8 d | 0 at 5 mo | N/A | N/A | N/A | N/A | N/A | |||||||||
| O. latipes | TG:pvasa-GFP | 2–4 mo | 15,000 TCs | N/C | N/A | O. latipes | WT | 7 d | >50 * at 1 mpt | N/A | N/A | N/A | 20.0 at 1 mpt | N/A | [55] |
| 15,000 TCs | WT | 11 d | >50 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 15,000 TCs | WT | 14 d | <50 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 15,000 TCs | WT | 19 d | 0 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 15,000 TCs | WT | 23 d | 0 * at 1 mpt | N/A | N/A | N/A | 90 at 1 mpt | N/A | |||||||
| 300 TCs | WT | 11 d | 10 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 1000 TCs | WT | 11 d | 10 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 3000 TCs | WT | 11 d | 60 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 10,000 TCs | WT | 11 d | 60 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 30,000 TCs | WT | 11 d | 60 at 1 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| 15,000 TCs | 3N | 11 d | N/A | Fresh: 63.6 CP: 70.0 at 2–3 mo | N/A | N/A | N/A | 100 | |||||||
| Wnt4b (-/-) mutant | - | 15,000 TCs | N/C | N/A | O. latipes | 3N | 11 d | N/A | CP: 83.3 at 2–3 mo | N/A | N/A | N/A | 100 | [55] | |
| Tokyo-medaka (endangered) | 1 y | TG:pvasa-GFP, 3N | N/A | CP: 63.9 at 2–3 mo | N/A | N/A | N/A | 100 | |||||||
| Kaga (inbred) | - | TG:pvasa-GFP, 3N | N/A | CP: 58.6 at 2–3 mo | N/A | N/A | N/A | 100 | |||||||
| Fshr mutant (-/-), XX male | 3 mo | 3000–9000 TCs | N/C | N/A | Hybrid | ♂ O. curvinotus × ♀ O. latipes or ♂ O. latipes × ♀ O. curvinotus | Newly hatched | N/A | N/A | N/A | 35.3 at 3 mo | N/A | 100 | [144] | |
| TG:pvasa-GFP | 2 mo | 12,000 TCs | N/A | N/A | N/A | 48.3 at 3 mo | N/A | N/A | |||||||
| WT | Adult | 1000 OCs | PDGC + DP | N/A | O. latipes | WT | 11 d | [Cult (10 d)] Con: 15.0 On pDA: 25.0 On pDA/PLL: 0 at 20 d | N/A | N/A | N/A | 50.0–80.0 at 20 d | N/A | [197] | |
| WT | Adult | 1000 OCs | PDGC + DP | N/A | O. latipes | WT | 11 d | Con: 14.3 Sort: 32.1 at 20 d | N/A | N/A | N/A | 57.1–72.4 at 20 d | N/A | [70] | |
| P. dentatus | WT | N/A | TCs | PDGC | N/A | P. olivaceus | 3N | 17 dph | 100 at 14 dpt | N/A | Merged: 100 at 2 y | N/A | [Merged] Con: 27.1 at 3 y TP: 4.0 at 2 y | Merged: Y | [148] |
| 20 dph | 90.0 at 14 dpt | N/A | N/A | ||||||||||||
| P. olivaceus | WT | N/A | TCs | PDGC | N/A | P. olivaceus | 3N | 16 dph | 100 at 14 dpt | N/A | Merged: 100 at 2 y | N/A | [Merged] Con: 27.1 at 3 y TP: 6.8 at 2 y | Merged: Y | [148] |
| 19 dph | 100 at 14 dpt | N/A | N/A | ||||||||||||
| 22 dph | 100 at 14 dpt | N/A | N/A | ||||||||||||
| P. spathula | WT | 2.5 y | 50,000 TCs | PDGC | N/A | A. dabryanus | WT | 7–8 dph | 80.6 at 2 mo | N/A | N/A | N/A | Con: 76.5 TP: 68.5 at 2 mo | N/A | [198] |
| S. salar | WT | 1 y | 2000–5000 TCs | N/C | N/A | O. mykiss | 3N (Shasta/ Shasta/albino) | Newly hatched | 61.1 at 4 wpt | 11.0 at 1–2 y | 10.0 at 1–2 y | 12.1 at 2 y | N/A | ♂: 100 ♀: N/A | [150] |
| S. trutta | WT | 9–12 mo | 4000–5000 TCs | FACS (AB No. 95) | Unsort: 21.2 Sort: 80.6 | O. mykiss | WT | Newly hatched | Con: 15.8 Sort: 34.7 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [86] |
| S. leucomaenis | WT | 12–13 mo | 3000 TCs | FACS | Unsort: 10.1 Sort: 79.9 | O. mykiss | WT | Newly hatched | Unsort: 0 Sort: 48.8 at 20 dpt | N/A | N/A | N/A | N/A | N/A | [19] |
| S. maximus | WT | N/A | TCs | PDGC | N/A | P. olivaceus | 3N | 15 dph | 60.0 at 14 dpt | N/A | Merged: 40.0 at 2 y | N/A | [Merged] Con: 27.1 at 3 y TP: 10.0 at 2 y | Merged: Y | [148] |
| 18 dph | 40.0 at 14 dpt | N/A | N/A | ||||||||||||
| 21 dph | 30.0 at 14 dpt | N/A | N/A | ||||||||||||
| S. quinqueradiata | WT | 10 mo | 20,000 TCs | N/C | N/A | S. quinqueradiata | WT | 6 dph | 100 at 28 dpt | N/A | N/A | N/A | 8.0 at 28 dpt | N/A | [155] |
| 8 dph | 100 at 28 dpt | N/A | N/A | N/A | 10.9 at 28 dpt | N/A | |||||||||
| 10 dph | 85.7 at 28 dpt | N/A | N/A | N/A | 15.9 at 28 dpt | N/A | |||||||||
| 12 dph | 85.7 at 28 dpt | N/A | N/A | N/A | 29.6 at 28 dpt | N/A | |||||||||
| Merged | N/A | N/A | 100 at 1.5–2.5 y | 100 at 2.5 y | 5.08 at SM | ♂: 66.6 (19.6–98.8) ♀: 63.2 (17.0–97.5) | |||||||||
| T. rubripes | WT | 1 y | 4000–6000 TCs | N/C | N/A | T. alboplumbeus | 3N (<100% incidence) | 1 dph | 50 at 4 wpt | N/A | 38.3 at 11 mo | 31.3 at 2 y | 47 at 72 wpt | ♂: 100 ♀: 5–95 | [138] |
| 5 dph | 33.3 at 4 wpt | N/A | 23.7 at 11 mo | 23.1 at 2 y | 41.4 at 72 wpt | ||||||||||
| WT | 10–12 mo | 5000 TCs | N/C | N/A | T. alboplumbeus | 3N (<100% incidence) | 1 dph | Fresh: 39.1 CP: 38.9 at 40 dph | N/A | [CP] 2N: 36.4 3N: 64.2 at 10 mph | [CP] 2N: 0 3N: 1.6 at 2 y | Con: 41.4 Fresh: 21.8 CP: 14.5 at 40 dph | 2N ♂: 3.2–87.5 (3 Inds) 2N ♀: 0 (10 Inds) 3N ♂: 100 (11 Inds); 62.5 (1 Ind) 3N ♀: 100 (1 Ind) | [120] | |
| WT | 1 y | 5000 TCs | N/C | N/A | T. alboplumbeus | WT | 1 dph | 40.0 at 40 dph | N/A | N/A | N/A | Con: 23.8 at 40 dph; 45.3 at 12 mph TP: 14.8 at 40 dph | N/A | [127] | |
| Dnd-KD | 40.5 at 40 dph | N/A | 91.7 at 10 mo | 26.7 at 2 y | Dnd-KD: 20.3 and 35.0 Dnd-KD + TP: 15.8 and 16.9 at 40 dph and 12 mph | ♂: 100 (7 Inds); 12.5–87.5 (2 Inds) ♀: 100 (3 Inds) | |||||||||
| T. maccoyii | WT | 57.0 kg | TCs | PDGC | N/A | S. lalandi | WT | 6–7 dph | 33.3 at 18 dpt | N/A | N/A | N/A | 9.1 at 64 dph | N/A | [47] |
| 9–10 dph | 37.5 at 18 dpt | N/A | N/A | N/A | 9.2 at 64 dph | N/A | |||||||||
| T. orientalis | WT | 0 y | TCs | N/C | N/A | S. japonicus | WT | 7 d | 0 at 21 dpt | N/A | N/A | N/A | N/A | N/A | [22] |
| 1 y | 5.4 at 21 dpt | N/A | N/A | N/A | N/A | N/A | |||||||||
| 3 y | 0 at 21 dpt | N/A | N/A | N/A | N/A | N/A | |||||||||
| WT | 2–4 y | >10,000 TCs | FACS (AB No. 152) | Unsort: 16.6 Sort: 77.3 | N. mitsukurii | WT | 12 dph | PDGC Sort (PKH26 labled): 33.3 PDGC Sort (No. 152 AB labeled): 63.3 at 14 dpt | N/A | N/A | N/A | N/A | N/A | [45] | |
| WT | 3 y | >10,000 TCs | PDGC | N/A | Hybrid | ♂ S. japonicus × ♀ S. australasicus | 10 d | 100 at 14 dpt | N/A | N/A | N/A | N/A | N/A | [48] | |
| WT | 2 y | >30,000 TCs | N/C | N/A | S. japonicus | WT | 7 dph | Y at 14 dpt and 4 mpt | N/A | N/A | N/A | N/A | N/A | [46] | |
| E. affinis | WT | 10 dph | Y at 14 dpt and 3 mpt | N/A | N/A | N/A | N/A | N/A | |||||||
| Donor | Enrichment | Recipient | % of Recipients with Gonadal Incorporation of Donor Cells | % of Recipients Producing Donor-Derived Gametes | Survival of Recipients (%) | % of Donor-Derived Offspring | Ref | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Fish | Age (Size) | Cells | Method | Purity | Species | Fish | Sex | Age (Size) | Total | Male | Female | ||||
| D. rerio | TG:pvasa -EGFP | Adult | TCs | FACS | Unsort: 10–20 * Sort: 60–70 * | D. rerio | WT, HT + B treated | ♂ | Adult | 30 at 3 wpt | N/A | N/A | N/A | N/A | N/A | [128] |
| ♀ | Adult | Y at 3 wpt | N/A | N/A | N/A | N/A | N/A | |||||||||
| I. furcatus | WT | 2 y | 2 × 104–1.43 × 106 TCs | PDGC | N/A | I. punctatus | 3N | ♂ | 2 y | 87.5 at 10 mpt | N/A | Y at 2 ypt | N/A | N/A | Y | [199] |
| N. mitsukurii | TG:pHSC -GFP | 3 mo | 2 × 106 TCs | N/C | N/A | Hybrid | ♂ P. argentata × ♀ N. mitsukurii | ♂ | 6 mo | 37.5 at 6 wpt | N/A | 10 at 25 wpt | N/A | 77.0 at 1 dpt | 100 | [31] |
| TG:pHSC -GFP | 3 mo | 2 × 106 OCs | N/C | N/A | Hybrid | ♂ P. argentata × ♀ N. mitsukurii, 3N | ♀ | 6 mo | N/A | 33.3 at 9 mpt | 16.6 at 9 mpt | 16.6 at 9 mpt | 75 at mpt | 100 | [104] | |
| O. bonariensis | WT | 4–5 mo | 4 × 104 TCs (for each lobe, surgery) | PDGC | N/A | O. hatcheri | WT, HT + B treated | ♂ | 1 y | 33.3 at 6 wpt | N/A | 20.0 at 6 mpt | N/A | N/A | 1.2–13.3 | [149] |
| WT | 4–5 mo | 3.75 × 106 TCs | PDGC | N/A | O. hatcheri | WT, HT + B treated | ♂ | 1 y | 80.0 at 8 wpt | N/A | 17.6 at 7 mpt | N/A | N/A | 12.6–39.7 | [100] | |
| WT | 4–5 mo | 3.75 × 106 Ocs | PDGC | N/A | O. hatcheri | WT, HT + B treated | ♀ | 1 y | 60.0 at 8 wpt | N/A | N/A | 5.0 at 7 mpt | N/A | 39.7–52.2 | [100] | |
| O. niloticus | WT | Adult | 1 × 107 TCs | PDGC + DP | N/A | O. niloticus | WT, HT + B treated | ♂ | Adult | 89.5 at 8–9 wpt | N/A | Y at 9 wpt | N/A | N/A | 6.3 | [32] |
| P. olivaceus | WT | 1 y | OCs | PDGC | Unsort: 37.8 Sort: 83.6 | P. olivaceus | WT, HT + B treated | ♂ | 2 y | Y at 123 dpt | N/A | 11.7 at 10 mpt | N/A | 100 at 10 mpt | 43.0–45.5 | [72] |
| R. quelen | WT | 289 g | 1 × 107 TCs | PDGC + DP | N/A | O. niloticus | WT, HT + B treated | ♂ | 208 g | 100 at 2 mpt | N/A | N/A | N/A | N/A | N/A | [163] |
References
- Goto, R.; Saito, T. A State-of-the-Art Review of Surrogate Propagation in Fish. Theriogenology 2019, 133, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Yazawa, R. Application of Surrogate Broodstock Technology in Aquaculture. Fish. Sci. 2019, 85, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Braat, A.K.; Speksnijder, J.E.; Zivkovic, D. Germ Line Development in Fishes. Int. J. Dev. Biol. 1999, 43, 745–760. [Google Scholar]
- Raz, E. Primordial Germ-Cell Development: The Zebrafish Perspective. Nat. Rev. Genet. 2003, 4, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Joly, J.-S.; Kress, C.; Vandeputte, M.; Bourrat, F.; Chourrout, D. Irradiation of Fish Embryos Prior to Blastomere Transfer Boosts the Colonisation of Their Gonads by Donor-Derived Gametes. Mol. Reprod. Dev. 1999, 53, 394–397. [Google Scholar] [CrossRef]
- Lin, S.; Long, W.; Chen, J.; Hopkins, N. Production of Germ-Line Chimeras in Zebrafish by Cell Transplants from Genetically Pigmented to Albino Embryos. Proc. Natl. Acad. Sci. USA 1992, 89, 4519–4523. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, Y.; Yoshizaki, G.; Takeuchi, T. Production of Germ-Line Chimeras in Rainbow Trout by Blastomere Transplantation. Mol. Reprod. Dev. 2001, 59, 380–389. [Google Scholar] [CrossRef]
- Tzung, K.W.; Goto, R.; Saju, J.M.; Sreenivasan, R.; Saito, T.; Arai, K.; Yamaha, E.; Hossain, M.S.; Calvert, M.E.K.; Orbán, L. Early Depletion of Primordial Germ Cells in Zebrafish Promotes Testis Formation. Stem Cell Rep. 2015, 4, 61–73. [Google Scholar] [CrossRef]
- Kusuda, S.; Teranishi, T.; Koide, N.; Nagai, T.; Arai, K.; Yamaha, E. Pluripotency of Cryopreserved Blastomeres of the Goldfish. J. Exp. Zool. 2004, 301A, 131–138. [Google Scholar] [CrossRef]
- Yamaha, E.; Kazama-Wakabayashi, M.; Otani, S.; Fujimoto, T.; Arai, K. Germ-Line Chimera by Lower-Part Blastoderm Transplantation between Diploid Goldfish and Triploid Crucian Carp. Genetica 2001, 111, 227–236. [Google Scholar] [CrossRef]
- Yamaha, E.; Murakami, M.; Hada, K.; Otani, S.; Fujimoto, T.; Tanaka, M.; Sakao, S.; Kimura, S.; Sato, S.; Arai, K. Recovery of Fertility in Male Hybrids of a Cross between Goldfish and Common Carp by Transplantation of PGC (Primordial Germ Cell)-Containing Graft. Genetica 2003, 119, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Robles, V.; Riesco, M.F.; Psenicka, M.; Saito, T.; Valcarce, D.G.; Cabrita, E.; Herráez, P. Biology of Teleost Primordial Germ Cells (PGCs) and Spermatogonia: Biotechnological Applications. Aquaculture 2017, 472, 4–20. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Fujimoto, T.; Kawakami, Y.; Arai, K.; Yamaha, E. Inter-Species Transplantation and Migration of Primordial Germ Cells in Cyprinid Fish. Int. J. Dev. Biol. 2010, 54, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Psenicka, M. Novel Technique for Visualizing Primordial Germ Cells in Sturgeons (Acipenser ruthenus, A. gueldenstaedtii, A. baerii, and Huso huso). Biol. Reprod. 2015, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Yoshizaki, G.; Takeuchi, T. Generation of Live Fry from Intraperitoneally Transplanted Primordial Germ Cells in Rainbow Trout. Biol. Reprod. 2003, 69, 1142–1149. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Arai, K.; Yamaha, E. Xenogenesis in Teleost Fish through Generation of Germ-Line Chimeras by Single Primordial Germ Cell Transplantation. Biol. Reprod. 2008, 78, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Goto-Kazeto, R.; Saito, T.; Takagi, M.; Arai, K.; Yamaha, E. Isolation of Teleost Primordial Germ Cells Using Flow Cytometry. Int. J. Dev. Biol. 2010, 54, 1487–1492. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, Y.; Yoshizaki, G.; Kobayashi, T.; Takeuchi, T. Mass Isolation of Primordial Germ Cells from Transgenic Rainbow Trout Carrying the Green Fluorescent Protein Gene Driven by the vasa Gene Promoter. Biol. Reprod. 2002, 67, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Kise, K.; Yoshikawa, H.; Sato, M.; Tashiro, M.; Yazawa, R.; Nagasaka, Y.; Takeuchi, Y.; Yoshizaki, G. Flow-Cytometric Isolation and Enrichment of Teleost Type A Spermatogonia Based on Light-Scattering Properties. Biol. Reprod. 2012, 86, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Ichida, K.; Sadaie, S.; Miwa, M.; Fujihara, R.; Nagasaka, Y.; Yoshizaki, G. Establishment of Novel Monoclonal Antibodies for Identification of Type A Spermatogonia in Teleosts. Biol. Reprod. 2019, 101, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Ichikawa, M.; Hayashi, M.; Iwasaki, Y.; Miwa, M.; Shikina, S.; Okutsu, T. Sexual Plasticity of Ovarian Germ Cells in Rainbow Trout. Development 2010, 137, 1227–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazawa, R.; Takeuchi, Y.; Morita, T.; Ishida, M.; Yoshizaki, G. The Pacific Bluefin Tuna (Thunnus orientalis) dead end Gene Is Suitable as a Specific Molecular Marker of Type A Spermatogonia. Mol. Reprod. Dev. 2013, 80, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Bellaiche, J.; Lareyre, J.J.; Cauty, C.; Yano, A.; Allemand, I.; Le Gac, F. Spermatogonial Stem Cell Quest: nanos2, Marker of a Subpopulation of Undifferentiated a Spermatogonia in Trout Testis. Biol. Reprod. 2014, 90, 1–14. [Google Scholar] [CrossRef]
- Nakamura, S.; Kobayashi, K.; Nishimura, T.; Higashijima, S.I.; Tanaka, M. Identification of Germline Stem Cells in the Ovary of the Teleost Medaka. Science 2010, 328, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Shikina, S.; Takeuchi, Y.; Yoshizaki, G. Lymphocyte Antigen 75 (Ly75/CD205) Is a Surface Marker on Mitotic Germ Cells in Rainbow Trout. Biol. Reprod. 2010, 83, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Hayashi, M.; Yoshizaki, G. Stem Cell Activity of Type A Spermatogonia Is Seasonally Regulated in Rainbow Trout. Biol. Reprod. 2017, 96, 1303–1316. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Xu, D.; Ino, Y.; Yoshino, T.; Hayashida, T.; Wang, J.; Yazawa, R.; Yoshizaki, G.; Takeuchi, Y. Hybrid Sterility in Fish Caused by Mitotic Arrest of Primordial Germ Cells. Genetics 2018, 209, 507–521. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Bang, W.Y.; Yang, H.S.; Lee, D.S.; Song, H.Y. Production of Juvenile Masu Salmon (Oncorhynchus masou) from Spermatogonia-Derived Sperm and Oogonia-Derived Eggs via Intraperitoneal Transplantation of Immature Germ Cells. Biochem. Biophys. Res. Commun. 2021, 535, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Franěk, R.; Kašpar, V.; Shah, M.A.; Gela, D.; Pšenička, M. Production of Common Carp Donor-Derived Offspring from Goldfish Surrogate Broodstock. Aquaculture 2021, 534, 736252. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in Fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Xu, D.; Yoshino, T.; Konishi, J.; Yoshikawa, H.; Ino, Y.; Yazawa, R.; Dos Santos Nassif Lacerda, S.M.; De França, L.R.; Takeuchi, Y. Germ Cell-Less Hybrid Fish: Ideal Recipient for Spermatogonial Transplantation for the Rapid Production of Donor-Derived Sperm. Biol. Reprod. 2019, 101, 492–500. [Google Scholar] [CrossRef]
- Lacerda, S.M.S.N.; Batlouni, S.R.; Costa, G.M.J.; Segatelli, T.M.; Quirino, B.R.; Queiroz, B.M.; Kalapothakis, E.; França, L.R. A New and Fast Technique to Generate Offspring after Germ Cells Transplantation in Adult Fish: The Nile Tilapia (Oreochromis niloticus) Model. PLoS ONE 2010, 5, e10740. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M. Selective Breeding of Quantitative Traits in the Common Carp (Cyprinus carpio): A Review. Aquat. Living Resour. 2003, 16, 399–407. [Google Scholar] [CrossRef]
- Janssen, K.; Chavanne, H.; Berentsen, P.; Komen, H. Impact of Selective Breeding on European Aquaculture. Aquaculture 2017, 472, 8–16. [Google Scholar] [CrossRef]
- Stevens, C.H.; Croft, D.P.; Paull, G.C.; Tyler, C.R. Stress and Welfare in Ornamental Fishes: What Can Be Learned from Aquaculture? J. Fish Biol. 2017, 91, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Tattam, I.A.; Ruzycki, J.R.; McCormick, J.L.; Carmichael, R.W. Length and Condition of Wild Chinook Salmon Smolts Influence Age at Maturity. Trans. Am. Fish. Soc. 2015, 144, 1237–1248. [Google Scholar] [CrossRef]
- Okutsu, T.; Suzuki, K.; Takeuchi, Y.; Takeuchi, T.; Yoshizaki, G. Testicular Germ Cells Can Colonize Sexually Undifferentiated Embryonic Gonad and Produce Functional Eggs in Fish. Proc. Natl. Acad. Sci. USA 2006, 103, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, G. Germ Cell Transplantation in Fish: Mutant dnd Rainbow Trout Can Produce Chinook Salmon Gametes. In Proceedings of the 2021 Exotic Species Webinar Series Recordings, 10 February 2021; Society for the Study of Reproduction: Reston, VA, USA, 2021. [Google Scholar]
- Pšenička, M.; Saito, T.; Linhartová, Z.; Gazo, I. Isolation and Transplantation of Sturgeon Early-Stage Germ Cells. Theriogenology 2015, 83, 1085–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederholm, C.J.; Kunze, M.D.; Murota, T.; Sibatani, A. Pacific Salmon Carcasses: Essential Contributions of Nutrients and Energy for Aquatic and Terrestrial Ecosystems. Fisheries 1999, 24, 6–15. [Google Scholar] [CrossRef]
- Unwin, M.J.; Kinnison, M.T.; Quinn, T.P. Exceptions to Semelparity: Postmaturation Survival, Morphology, and Energetics of Male Chinook Salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. 1999, 56, 1172–1181. [Google Scholar] [CrossRef]
- Zohar, Y.; Mylonas, C.C.; Rosenfeld, H.; de la Gándara, F.; Corriero, A. Chapter 7—Reproduction, Broodstock Management, and Spawning in Captive Atlantic Bluefin Tuna. In Advances in Tuna Aquaculture; Benetti, D.D., Partridge, G.J., Buentello, A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 159–188. [Google Scholar]
- Bluefin Tuna Fish Farming. Available online: https://factsanddetails.com/world/cat53/sub340/item2188.html (accessed on 6 January 2022).
- Sawada, Y.; Okada, T.; Miyashita, S.; Murata, O.; Kumai, H. Completion of the Pacific Bluefin Tuna Thunnus orientalis (Temminck et Schlegel) Life Cycle. Aquac. Res. 2005, 36, 413–421. [Google Scholar] [CrossRef]
- Ichida, K.; Kawamura, W.; Miwa, M.; Iwasaki, Y.; Kubokawa, T.; Hayashi, M.; Yazawa, R.; Yoshizaki, G. Specific Visualization of Live Type A Spermatogonia of Pacific Bluefin Tuna Using Fluorescent Dye-Conjugated Antibodies. Biol. Reprod. 2019, 100, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, R.; Kubokawa, T.; Ichida, K.; Kawamura, W.; Tani, R.; Kamio, S.; Morita, T.; Yoshizaki, G. Establishment of a Tracing Technique for Transplanted Bluefin Tuna Germ Cells in Recipient’s Gonads Using Monoclonal Antibodies Specifically Recognizing Bluefin Tuna Spermatogenic Cells. Fish. Sci. 2021, 87, 105–112. [Google Scholar] [CrossRef]
- Bar, I.; Smith, A.; Bubner, E.; Yoshizaki, G.; Takeuchi, Y.; Yazawa, R.; Chen, B.N.; Cummins, S.; Elizur, A. Assessment of Yellowtail Kingfish (Seriola lalandi) as a Surrogate Host for the Production of Southern Bluefin Tuna (Thunnus maccoyii) Seed via Spermatogonial Germ Cell Transplantation. Reprod. Fertil. Dev. 2016, 28, 2051–2064. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, W.; Tani, R.; Yahagi, H.; Kamio, S.; Morita, T.; Takeuchi, Y.; Yazawa, R.; Yoshizaki, G. Suitability of Hybrid Mackerel (Scomber australasicus × S. japonicus) with Germ Cell-Less Sterile Gonads as a Recipient for Transplantation of Bluefin Tuna Germ Cells. Gen. Comp. Endocrinol. 2020, 295, 113525. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, E.; Sarasquete, C.; Martínez-Páramo, S.; Robles, V.; Beirão, J.; Pérez-Cerezales, S.; Herráez, M.P. Cryopreservation of Fish Sperm: Applications and Perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- Chao, N.H.; Liao, I.C. Cryopreservation of Finfish and Shellfish Gametes and Embryos. Aquaculture 2001, 197, 161–189. [Google Scholar] [CrossRef]
- Leveroni Calvi, S.; Maisse, G. Cryopreservation of Rainbow Trout (Oncorhynchus mykiss) Blastomeres: Influence of Embryo Stage on Postthaw Survival Rate. Cryobiology 1998, 36, 255–262. [Google Scholar] [CrossRef]
- Kawakami, Y.; Goto-Kazeto, R.; Saito, T.; Fujimoto, T.; Higaki, S.; Takahashi, Y.; Arai, K.; Yamaha, E. Generation of Germ-Line Chimera Zebrafish Using Primordial Germ Cells Isolated from Cultured Blastomeres and Cryopreserved Embryoids. Int. J. Dev. Biol. 2010, 54, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Takeuchi, Y.; Takeuchi, T.; Yoshizaki, G. Generation of Viable Fish from Cryopreserved Primordial Germ Cells. Mol. Reprod. Dev. 2007, 74, 207–213. [Google Scholar] [CrossRef]
- Lee, S.; Iwasaki, Y.; Shikina, S.; Yoshizaki, G. Generation of Functional Eggs and Sperm from Cryopreserved Whole Testes. Proc. Natl. Acad. Sci. USA 2013, 110, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Seki, S.; Kusano, K.; Lee, S.; Iwasaki, Y.; Yagisawa, M.; Ishida, M.; Hiratsuka, T.; Sasado, T.; Naruse, K.; Yoshizaki, G. Production of the Medaka Derived from Vitrified Whole Testes by Germ Cell Transplantation. Sci. Rep. 2017, 7, 43185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Katayama, N.; Yoshizaki, G. Generation of Juvenile Rainbow Trout Derived from Cryopreserved Whole Ovaries by Intraperitoneal Transplantation of Ovarian Germ Cells. Biochem. Biophys. Res. Commun. 2016, 478, 1478–1483. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, G.; Lee, S. Production of Live Fish Derived from Frozen Germ Cells via Germ Cell Transplantation. Stem Cell Res. 2018, 29, 103–110. [Google Scholar] [CrossRef]
- Chen, S.L.; Tian, Y.S.; Yang, J.F.; Shao, C.W.; Ji, X.S.; Zhai, J.M.; Liao, X.L.; Zhuang, Z.M.; Su, P.Z.; Xu, J.Y.; et al. Artificial Gynogenesis and Sex Determination in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2009, 11, 243–251. [Google Scholar] [CrossRef]
- Geffroy, B.; Bardonnet, A. Sex Differentiation and Sex Determination in Eels: Consequences for Management. Fish Fish. 2016, 17, 375–398. [Google Scholar] [CrossRef]
- Baroiller, J.F.; D’Cotta, H.; Bezault, E.; Wessels, S.; Hoerstgen-Schwark, G. Tilapia sex determination: Where temperature and genetics meet. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Seki, S.; Katayama, N.; Yoshizaki, G. Production of Viable Trout Offspring Derived from Frozen Whole Fish. Sci. Rep. 2015, 5, 16045. [Google Scholar] [CrossRef]
- Ichida, K.; Hayashi, M.; Miwa, M.; Kitada, R.; Takahashi, M.; Fujihara, R.; Boonanuntanasarn, S.; Yoshizaki, G. Enrichment of Transplantable Germ Cells in Salmonids Using a Novel Monoclonal Antibody by Magnetic-Activated Cell Sorting. Mol. Reprod. Dev. 2019, 86, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Takeuchi, Y.; Ino, Y.; Wang, J.; Iwata, G.; Kabeya, N.; Yazawa, R.; Yoshizaki, G. Efficient Production of Donor-Derived Gametes from Triploid Recipients Following Intra-Peritoneal Germ Cell Transplantation into a Marine Teleost, Nibe Croaker (Nibea mitsukurii). Aquaculture 2017, 478, 35–47. [Google Scholar] [CrossRef]
- Li, M.; Hong, N.; Xu, H.; Song, J.; Hong, Y. Germline Replacement by Blastula Cell Transplantation in the Fish Medaka. Sci. Rep. 2016, 6, 29658. [Google Scholar] [CrossRef] [Green Version]
- Goto, R.; Saito, T.; Takeda, T.; Fujimoto, T.; Takagi, M.; Arai, K.; Yamaha, E. Germ Cells Are Not the Primary Factor for Sexual Fate Determination in Goldfish. Dev. Biol. 2012, 370, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Tago, Y.; Takeuchi, Y.; Sawatari, E.; Kobayashi, T.; Takeuchi, T. Green Fluorescent Protein Labeling of Primordial Germ Cells Using a Nontransgenic Method and Its Application for Germ Cell Transplantation in Salmonidae. Biol. Reprod. 2005, 73, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Suzuki, K.; Yoshizaki, G. Flow-Cytometric Isolation of Testicular Germ Cells from Rainbow Trout (Oncorhynchus mykiss) Carrying the Green Fluorescent Protein Gene Driven by Trout vasa Regulatory Regions. Biol. Reprod. 2008, 78, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Pertoft, H.; Laurent, T.C.; Låås, T.; Kågedal, L. Density Gradients Prepared from Colloidal Silica Particles Coated by Polyvinylpyrrolidone (Percoll). Anal. Biochem. 1978, 88, 271–282. [Google Scholar] [CrossRef]
- Wong, T.T.; Saito, T.; Crodian, J.; Collodi, P. Zebrafish Germline Chimeras Produced by Transplantation of Ovarian Germ Cells into Sterile Host Larvae. Biol. Reprod. 2011, 84, 1190–1197. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.H.; Gong, S.P. Enhanced Enrichment of Medaka Ovarian Germline Stem Cells by a Combination of Density Gradient Centrifugation and Differential Plating. Biomolecules 2020, 10, 1477. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Morishima, K.; Fujimoto, T.; Saito, T.; Kobayashi, T.; Yamaha, E.; Arai, K. Chromosome Doubling in Early Spermatogonia Produces Diploid Spermatozoa in a Natural Clonal Fish. Biol. Reprod. 2009, 80, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Sun, Z.; Wang, Y.; Yu, Q.; Wang, G.; He, Z.; Liu, Y.; Jiang, X.; Kang, X.; Hou, J. Production of Donor-Derived Offsprings by Allogeneic Transplantation of Oogonia in the Adult Japanese Flounder (Paralichthys olivaceus). Aquaculture 2021, 543, 736977. [Google Scholar] [CrossRef]
- Shikina, S.; Nagasawa, K.; Hayashi, M.; Furuya, M.; Iwasaki, Y.; Yoshizaki, G. Short-Term in Vitro Culturing Improves Transplantability of Type A Spermatogonia in Rainbow Trout (Oncorhynchus mykiss). Mol. Reprod. Dev. 2013, 80, 763–773. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Kubota, Y.; Ogawa, T. In Vitro Sperm Production from Mouse Spermatogonial Stem Cell Lines Using an Organ Culture Method. Nat. Protoc. 2013, 8, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Shikina, S.; Ihara, S.; Yoshizaki, G. Culture Conditions for Maintaining the Survival and Mitotic Activity of Rainbow Trout Transplantable Type A Spermatogonia. Mol. Reprod. Dev. 2008, 75, 529–537. [Google Scholar] [CrossRef]
- Shikina, S.; Yoshizaki, G. Improved In Vitro Culture Conditions to Enhance the Survival, Mitotic Activity, and Transplantability of Rainbow Trout Type A Spermatogonia. Biol. Reprod. 2010, 83, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, N.; Li, Z.; Hong, Y. Fish Stem Cell Cultures. Int. J. Biol. Sci. 2011, 7, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, Z.; Hosseini, S.M.; Hajian, M.; Abedi, P.; Bahadorani, M.; Baharvand, H.; Nasr-Esfahani, M.H. Effects of Different Feeder Layers on Short-Term Culture of Prepubertal Bovine Testicular Germ Cells In-Vitro. Theriogenology 2012, 77, 1519–1528. [Google Scholar] [CrossRef]
- Shinohara, T.; Avarbock, M.R.; Brinster, R.L. β1- and α6-Integrin Are Surface Markers on Mouse Spermatogonial Stem Cells. Proc. Natl. Acad. Sci. USA 1999, 96, 5504–5509. [Google Scholar] [CrossRef] [Green Version]
- Marraccino, R.L.; Keng, P.C. Centrifugal Elutriation. In Cell Cycle—Materials and Methods; Pagano, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 53–62. [Google Scholar]
- Liu, Y.; Nan, B.; Niu, J.; Kapler, G.M.; Gao, S. An Optimized and Versatile Counter-Flow Centrifugal Elutriation Workflow to Obtain Synchronized Eukaryotic Cells. Front. Cell Dev. Biol. 2021, 9, 905. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xin, H.; Deng, G. Single Cell Isolation and Analysis. Front. Cell Dev. Biol. 2016, 4, 116. [Google Scholar] [CrossRef] [Green Version]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow Cytometry: Basic Principles and Applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Pšenička, M.; Saito, T. Chapter 16 Specificity of Germ Cell Technologies in Sturgeons. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J.F., Eds.; Springer: Singapore, 2020; pp. 335–356. [Google Scholar]
- Kobayashi, T.; Yoshizaki, G.; Takeuchi, Y.; Takeuchi, T. Isolation of Highly Pure and Viable Primordial Germ Cells from Rainbow Trout by GFP-Dependent Flow Cytometry. Mol. Reprod. Dev. 2004, 67, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Matsushita, Y.; Amano, Y.; Miwa, M.; Nagasawa, K.; Hayashi, M.; Mizutani, H.; Takahashi, M.; Boonanuntanasarn, S.; Yoshizaki, G. Visualization and Tracking of Live Type a Spermatogonia Using a Fluorescence-Conjugated Antibody in Salmo Species. Aquaculture 2021, 533, 736096. [Google Scholar] [CrossRef]
- Xie, X.; Tichopád, T.; Kislik, G.; Langerová, L.; Abaffy, P.; Šindelka, R.; Franěk, R.; Fučíková, M.; Steinbach, C.; Shah, M.A.; et al. Isolation and Characterization of Highly Pure Type A Spermatogonia From Sterlet (Acipenser ruthenus) Using Flow-Cytometric Cell Sorting. Front. Cell Dev. Biol. 2021, 9, 772625. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Sato, M.; Nagasaka, Y.; Sadaie, S.; Kobayashi, S.; Yoshizaki, G. Enrichment of Spermatogonial Stem Cells Using Side Population in Teleost1. Biol. Reprod. 2014, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, B.D.; Murthy, S.K.; Lewis, L.H. Fundamentals and Application of Magnetic Particles in Cell Isolation and Enrichment: A Review. Rep. Prog. Phys. 2015, 78, 016601. [Google Scholar] [CrossRef] [PubMed]
- Osmond, A.T.Y.; Colombo, S.M. The Future of Genetic Engineering to Provide Essential Dietary Nutrients and Improve Growth Performance in Aquaculture: Advantages and Challenges. J. World Aquac. Soc. 2019, 50, 490–509. [Google Scholar] [CrossRef]
- Altman, E.; Yango, P.; Moustafa, R.; Smith, J.F.; Klatsky, P.C.; Tran, N.D. Characterization of Human Spermatogonial Stem Cell Markers in Fetal, Pediatric, and Adult Testicular Tissues. Reproduction 2014, 148, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Falciatori, I.; Borsellino, G.; Haliassos, N.; Boitani, C.; Corallini, S.; Battistini, L.; Bernardi, G.; Stefanini, M.; Vicini, E. Identification and Enrichment of Spermatogonial Stem Cells Displaying Side-Population Phenotype in Immature Mouse Testis. FASEB J. 2004, 18, 376–378. [Google Scholar] [CrossRef]
- Dovey, S.L.; Valli, H.; Hermann, B.P.; Sukhwani, M.; Donohue, J.; Castro, C.A.; Chu, T.; Sanfilippo, J.S.; Orwig, K.E. Eliminating Malignant Contamination from Therapeutic Human Spermatogonial Stem Cells. J. Clin. Investig. 2013, 123, 1833–1843. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Du, X.; Zhao, F.; Xu, B. Magnetic Nanoparticles for the Manipulation of Proteins and Cells. Chem. Soc. Rev. 2012, 41, 2912–2942. [Google Scholar] [CrossRef]
- Kokkinaki, M.; Lee, T.L.; He, Z.; Jiang, J.; Golestaneh, N.; Hofmann, M.C.; Chan, W.Y.; Dym, M. The Molecular Signature of Spermatogonial Stem/Progenitor Cells in the 6-Day-Old Mouse Testis. Biol. Reprod. 2009, 80, 707–717. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Kokkinaki, M.; Jiang, J.; Dobrinski, I.; Dym, M. Isolation, Characterization, and Culture of Human Spermatogonia. Biol. Reprod. 2010, 82, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.Y.; Lufkin, T. Development of the “Three-Step Macs”: A Novel Strategy for Isolating Rare Cell Populations in the Absence of Known Cell Surface Markers from Complex Animal Tissue. J. Biomol. Tech. 2012, 23, 69–77. [Google Scholar] [CrossRef]
- Zhu, B.; Murthy, S.K. Stem Cell Separation Technologies. Curr. Opin. Chem. Eng. 2013, 2, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Yazawa, R.; Takeuchi, Y.; Higuchi, K.; Yatabe, T.; Kabeya, N.; Yoshizaki, G. Chub Mackerel Gonads Support Colonization, Survival, and Proliferation of Intraperitoneally Transplanted Xenogenic Germ Cells. Biol. Reprod. 2010, 82, 896–904. [Google Scholar] [CrossRef]
- Majhi, S.K.; Hattori, R.S.; Rahman, S.M.; Strüssmann, C.A. Surrogate Production of Eggs and Sperm by Intrapapillary Transplantation of Germ Cells in Cytoablated Adult Fish. PLoS ONE 2014, 9, e95294. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Fujii, W.; Naito, K.; Yoshizaki, G. Application of dead end-Knockout Zebrafish as Recipients of Germ Cell Transplantation. Mol. Reprod. Dev. 2017, 84, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.T.; Zohar, Y. Production of Reproductively Sterile Fish: A Mini-Review of Germ Cell Elimination Technologies. Gen. Comp. Endocrinol. 2015, 221, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Shibata, N.; Sakaizumi, M.; Yamashita, M. Production of Diploid Eggs through Premeiotic Endomitosis in the Hybrid Medaka between Oryzias latipes and O. curvinotus. Zool. Sci. 2000, 17, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Yoshino, T.; De Bello Cioffi, M.; Yoshikawa, H.; Ino, Y.; Yazawa, R.; Lacerda, S.M.D.S.N.; Takeuchi, Y. Production of Donor-Derived Eggs after Ovarian Germ Cell Transplantation into the Gonads of Adult, Germ Cell-Less, Triploid Hybrid Fish. Biol. Reprod. 2020, 103, 1289–1299. [Google Scholar] [CrossRef]
- Golpour, A.; Siddique, M.A.M.; Siqueira-Silva, D.H.; Pšenička, M. Induced Sterility in Fish and Its Potential and Challenges for Aquaculture and Germ Cell Transplantation Technology: A Review. Biologia 2016, 71, 853–864. [Google Scholar] [CrossRef]
- Nam, Y.K.; Choi, G.C.; Park, D.J.; Kim, D.S. Survival and Growth of Induced Tetraploid Mud Loach. Aquac. Int. 2001, 9, 61–71. [Google Scholar] [CrossRef]
- Refstie, T.; Vassvik, V.; Gjedrem, T. Induction of Polyploidy in Salmonids by Cytochalasin B. Aquaculture 1977, 10, 65–74. [Google Scholar] [CrossRef]
- Benfey, T.J.; Sutterlin, A.M. Triploidy Induced by Heat Shock and Hydrostatic Pressure in Landlocked Atlantic Salmon (Salmo salar L.). Aquaculture 1984, 36, 359–367. [Google Scholar] [CrossRef]
- Okomoda, V.T.; Aminath, L.; Oladimeji, S.A.; Abol-Munafi, A.B.; Korede, A.I.; Ikhwanuddin, M.; Umaru, J.A.; Hassan, A.; Martins, C.O.; Shahreza, S.M. First Report on Successful Triploidy Induction in Clarias gariepinus (Burchell, 1822) Using Electroporation. Sci. Rep. 2020, 10, 2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamasaki, M.; Takeuchi, Y.; Miyaki, K.; Yoshizaki, G. Gonadal Development and Fertility of Triploid Grass Puffer Takifugu niphobles Induced by Cold Shock Treatment. Mar. Biotechnol. 2013, 15, 133–144. [Google Scholar] [CrossRef]
- Tiwary, B.K.; Kirubagaran, R.; Ray, A.K. The Biology of Triploid Fish. Rev. Fish Biol. Fish. 2004, 14, 391–402. [Google Scholar] [CrossRef]
- Wolters, W.R.; Libey, G.S.; Chrisman, C.L. Effect of Triploidy on Growth and Gonad Development of Channel Catfish. Trans. Am. Fish. Soc. 1982, 111, 102–105. [Google Scholar] [CrossRef]
- Wolters, W.R.; Libey, G.S.; Chrisman, C.L. Induction of Triploidy in Channel Catfish. Trans. Am. Fish. Soc. 1981, 110, 310–312. [Google Scholar] [CrossRef]
- Lincoln, R.F.; Scott, A.P. Production of All-Female Triploid Rainbow Trout. Aquaculture 1983, 30, 375–380. [Google Scholar] [CrossRef]
- Otterå, H.; Thorsen, A.; Karlsen, Ø.; Fjelldal, P.G.; Morton, H.C.; Taranger, G.L. Performance of Triploid Atlantic Cod (Gadus morhua L.) in Commercial Aquaculture. Aquaculture 2016, 464, 699–709. [Google Scholar] [CrossRef]
- Chourrout, D.; Chevassus, B.; Krieg, F.; Happe, A.; Burger, G.; Renard, P. Production of Second Generation Triploid and Tetraploid Rainbow Trout by Mating Tetraploid Males and Diploid Females—Potential of Tetraploid Fish. Theor. Appl. Genet. 1986, 72, 193–206. [Google Scholar] [CrossRef]
- Arai, K.; Mukaino, M. Clonal Nature of Gynogenetically Induced Progeny of Triploid (Diploid x Tetraploid) Loach, Misgurnus anguillicaudatus (Pisces: Cobitididae). J. Exp. Zool. 1997, 278, 193–206. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Morishima, K.; Kusuda, S.; Yamaha, E.; Arai, K. Diploid Sperm Produced by Artificially Sex-Reversed Clone Loaches. J. Exp. Zool. 2007, 307A, 75–83. [Google Scholar] [CrossRef]
- Nam, Y.K.; Kim, D.S. Ploidy Status of Progeny from the Crosses between Tetraploid Males and Diploid Females in Mud Loach (Misgurnus mizolepis). Aquaculture 2004, 236, 575–582. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Ino, Y.; Shigenaga, K.; Katayama, T.; Kuroyanagi, M.; Yoshiura, Y. Production of Tiger Puffer Takifugu rubripes from Cryopreserved Testicular Germ Cells Using Surrogate Broodstock Technology. Aquaculture 2018, 493, 302–313. [Google Scholar] [CrossRef]
- Baloch, A.R.; Franěk, R.; Saito, T.; Pšenička, M. Dead-end (Dnd) Protein in Fish—A Review. Fish Physiol. Biochem. 2021, 47, 777–784. [Google Scholar] [CrossRef]
- Weidinger, G.; Stebler, J.; Slanchev, K.; Dumstrei, K.; Wise, C.; Lovell-Badge, R.; Thisse, C.; Thisse, B.; Raz, E. Dead end, a Novel Vertebrate Germ Plasm Component, Is Required for Zebrafish Primordial Germ Cell Migration and Survival. Curr. Biol. 2003, 13, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.T.; Tesfamichael, A.; Collodi, P. Production of Zebrafish Offspring from Cultured Female Germline Stem Cells. PLoS ONE 2013, 8, e62660. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, G.; Takashiba, K.; Shimamori, S.; Fujinuma, K.; Shikina, S.; Okutsu, T.; Kume, S.; Hayashi, M. Production of Germ Cell-Deficient Salmonids by dead end Gene Knockdown, and Their Use as Recipients for Germ Cell Transplantation. Mol. Reprod. Dev. 2016, 83, 298–311. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.T.; Zohar, Y. Production of Reproductively Sterile Fish by a Non-Transgenic Gene Silencing Technology. Sci. Rep. 2015, 5, 15822. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.T.; Collodi, P. Dorsomorphin Promotes Survival and Germline Competence of Zebrafish Spermatogonial Stem Cells in Culture. PLoS ONE 2013, 8, e71332. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, H.; Ino, Y.; Kishimoto, K.; Koyakumaru, H.; Saito, T.; Kinoshita, M.; Yoshiura, Y. Induction of Germ Cell-Deficiency in Grass Puffer by dead end 1 Gene Knockdown for Use as a Recipient in Surrogate Production of Tiger Puffer. Aquaculture 2020, 526, 735385. [Google Scholar] [CrossRef]
- Nóbrega, R.H.; Greebe, C.D.; van de Kant, H.; Bogerd, J.; de França, L.R.; Schulz, R.W. Spermatogonial Stem Cell Niche and Spermatogonial Stem Cell Transplantation in Zebrafish. PLoS ONE 2010, 5, e12808. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, T.; Hiraku, Y.; Oikawa, S.; Mizutani, H.; Kojima, M.; Kawanishi, S. DNA Intrastrand Cross-Link at the 5′-GA-3′ Sequence Formed by Busulfan and Its Role in the Cytotoxic Effect. Cancer Sci. 2004, 95, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Brinster, R.L.; Avarbock, M.R. Germline Transmission of Donor Haplotype Following Spermatogonial Transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef] [Green Version]
- Nozu, R.; Nakamura, M. Influence of Prolonged Cultivation on Sexual Characteristics of Sterilized Female Tilapia, Oreochromis mossambicus, Induced by High-Temperature Treatment. Aquaculture 2020, 524, 735245. [Google Scholar] [CrossRef]
- Nakamura, M.; Nozu, R.; Ijiri, S.; Kobayashi, T.; Hirai, T.; Yamaguchi, Y.; Seale, A.; Lerner, D.T.; Grau, G.E. Sexual Characteristics of High-Temperature Sterilized Male Mozambique Tilapia, Oreochromis mossambicus. Zool. Lett. 2015, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Pandit, N.P.; Bhandari, R.K.; Kobayashi, Y.; Nakamura, M. High Temperature-Induced Sterility in the Female Nile Tilapia, Oreochromis niloticus. Gen. Comp. Endocrinol. 2015, 213, 110–117. [Google Scholar] [CrossRef]
- Jin, Y.H.; Davie, A.; Migaud, H. Temperature-Induced Testicular Germ Cell Loss and Recovery in Nile Tilapia Oreochromis niloticus. Gen. Comp. Endocrinol. 2019, 283, 113227. [Google Scholar] [CrossRef]
- Marinović, Z.; Li, Q.; Lujić, J.; Iwasaki, Y.; Csenki, Z.; Urbányi, B.; Yoshizaki, G.; Horváth, Á. Preservation of Zebrafish Genetic Resources through Testis Cryopreservation and Spermatogonia Transplantation. Sci. Rep. 2019, 9, 13861. [Google Scholar] [CrossRef] [Green Version]
- Franěk, R.; Cheng, Y.; Fučíková, M.; Kašpar, V.; Xie, X.; Shah, M.A.; Linhart, O.; Šauman, I.; Pšenička, M. Who Is the Best Surrogate for Germ Stem Cell Transplantation in Fish? Aquaculture 2022, 549, 737759. [Google Scholar] [CrossRef]
- Farlora, R.; Hattori-Ihara, S.; Takeuchi, Y.; Hayashi, M.; Octavera, A.; Alimuddin; Yoshizaki, G. Intraperitoneal Germ Cell Transplantation in the Nile Tilapia Oreochromis niloticus. Mar. Biotechnol. 2014, 16, 309–320. [Google Scholar] [CrossRef]
- Hamasaki, M.; Takeuchi, Y.; Yazawa, R.; Yoshikawa, S.; Kadomura, K.; Yamada, T.; Miyaki, K.; Kikuchi, K.; Yoshizaki, G. Production of Tiger Puffer Takifugu rubripes Offspring from Triploid Grass Puffer Takifugu niphobles Parents. Mar. Biotechnol. 2017, 19, 579–591. [Google Scholar] [CrossRef]
- Pradhan, A.; Olsson, P.E. Germ Cell Depletion in Zebrafish Leads to Incomplete Masculinization of the Brain. Gen. Comp. Endocrinol. 2018, 265, 15–21. [Google Scholar] [CrossRef]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis Following Male Germ-Cell Transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.T.; Tesfamichael, A.; Collodi, P. Identification of Promoter Elements Responsible for Gonad-Specific Expression of Zebrafish Deadend and Its Application to Ovarian Germ Cell Derivation. Int. J. Dev. Biol. 2013, 57, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, H.; Saito, D.; Nakamura, S.; Katoh-Fukui, Y.; Ohta, K.; Baba, T.; Morohashi, K.I.; Tanaka, M. Germ Cells Are Essential for Sexual Dimorphism in the Medaka Gonad. Proc. Natl. Acad. Sci. USA 2007, 104, 16958–16963. [Google Scholar] [CrossRef] [Green Version]
- Siegfried, K.R.; Nüsslein-Volhard, C. Germ Line Control of Female Sex Determination in Zebrafish. Dev. Biol. 2008, 324, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, K.; Ishida, M.; Octavera, A.; Kusano, K.; Kezuka, F.; Kitano, T.; Yoshiura, Y.; Yoshizaki, G. Novel Method for Mass Producing Genetically Sterile Fish from Surrogate Broodstock via Spermatogonial Transplantation. Biol. Reprod. 2019, 100, 535–546. [Google Scholar] [CrossRef]
- Franěk, R.; Tichopád, T.; Fučíková, M.; Steinbach, C.; Pšenička, M. Production and Use of Triploid Zebrafish for Surrogate Reproduction. Theriogenology 2019, 140, 33–43. [Google Scholar] [CrossRef]
- Okutsu, T.; Shikina, S.; Kanno, M.; Takeuchi, Y.; Yoshizaki, G. Production of Trout Offspring from Triploid Salmon Parents. Science 2007, 317, 1517. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki-Takahashi, Y.; Shikina, S.; Watanabe, M.; Banba, A.; Yagisawa, M.; Takahashi, K.; Fujihara, R.; Okabe, T.; Valdez, D.M.; Yamauchi, A.; et al. Production of Functional Eggs and Sperm from in Vitro-Expanded Type A Spermatogonia in Rainbow Trout. Commun. Biol. 2020, 3, 308. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Liu, Q.; Yang, J.; Xu, S.; Wu, Z.; Wang, Y.; You, F.; Song, Z.; Li, J. Successful Spermatogonial Stem Cells Transplantation within Pleuronectiformes: First Breakthrough at inter-family Level in Marine Fish. Int. J. Biol. Sci. 2021, 17, 4426–4441. [Google Scholar] [CrossRef]
- Majhi, S.K.; Hattori, R.S.; Yokota, M.; Watanabe, S.; Strüssmann, C.A. Germ Cell Transplantation Using Sexually Competent Fish: An Approach for Rapid Propagation of Endangered and Valuable Germlines. PLoS ONE 2009, 4, e6132. [Google Scholar] [CrossRef] [Green Version]
- Hattori, R.S.; Yoshinaga, T.T.; Katayama, N.; Hattori-Ihara, S.; Tsukamoto, R.Y.; Takahashi, N.S.; Tabata, Y.A. Surrogate Production of Salmo salar Oocytes and Sperm in Triploid Oncorhynchus mykiss by Germ Cell Transplantation Technology. Aquaculture 2019, 506, 238–245. [Google Scholar] [CrossRef]
- OECD. Atlantic salmon (Salmo salar). In Safety Assessment of Transgenic Organisms in the Environment; OECD Publishing: Paris, France, 2017; Volume 7. [Google Scholar] [CrossRef]
- Moore, G.A. The Germ Cells of the Trout (Salmo irideus Gibbons). Trans. Am. Microsc. Soc. 1937, 56, 105–112. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Sakatani, S.; Tominaga, H.; Takeuchi, T. Cloning and Characterization of a vasa-like Gene in Rainbow Trout and Its Expression in the Germ Cell Lineage. Mol. Reprod. Dev. 2000, 55, 364–371. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yoshizaki, G.; Takeuchi, T. Biotechnology: Surrogate Broodstock Produces Salmonids. Nature 2004, 430, 629–630. [Google Scholar] [CrossRef]
- Morita, T.; Kumakura, N.; Morishima, K.; Mitsuboshi, T.; Ishida, M.; Hara, T.; Kudo, S.; Miwa, M.; Ihara, S.; Higuchi, K.; et al. Production of Donor-Derived Offspring by Allogeneic Transplantation of Spermatogonia in the Yellowtail (Seriola quinqueradiata). Biol. Reprod. 2012, 86, 176. [Google Scholar] [CrossRef]
- Kawasaki, T.; Siegfried, K.R.; Sakai, N. Differentiation of Zebrafish Spermatogonial Stem Cells to Functional Sperm in Culture. Development 2016, 143, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Sakuma, D.; Yoshizaki, G. Production of Functional Sperm by Subcutaneous Auto-Grafting of Immature Testes in Rainbow Trout. Mol. Reprod. Dev. 2018, 85, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Saito, K.; Sakai, C.; Shinya, M.; Sakai, N. Production of Zebrafish Offspring from Cultured Spermatogonial Stem Cells. Genes Cells 2012, 17, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, T.T.; Júnior, J.R.K.; Butzge, A.J.; Olio, R.L.; Hernandez-Blazquez, F.J.; Carreira, A.C.O.; Gomes, C.D.O.M.S.; Bianchi, P.K.F.D.C.; Tabata, Y.A.; Hattori, R.S. Testicular Subcutaneous Allografting Followed by Immunosuppressive Treatment Promotes Maintenance of Spermatogonial Cells in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2021, 112, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Nóbrega, R.; Pšenička, M. Spermatogonial Stem Cells in Fish: Characterization, Isolation, Enrichment, and Recent Advances of In Vitro Culture Systems. Biomolecules 2020, 10, 644. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Goto-Kazeto, R.; Kawakami, Y.; Nomura, K.; Tanaka, H.; Adachi, S.; Arai, K.; Yamaha, E. The Mechanism for Primordial Germ-Cell Migration Is Conserved between Japanese Eel and Zebrafish. PLoS ONE 2011, 6, e24460. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Psěnička, M.; Goto, R.; Adachi, S.; Inoue, K.; Arai, K.; Yamaha, E. The Origin and Migration of Primordial Germ Cells in Sturgeons. PLoS ONE 2014, 9, e86861. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.A.; Costa, G.M.J.; Lacerda, S.M.S.N.; Brandão-Dias, P.F.P.; Kalapothakis, E.; Silva Júnior, A.F.; Alvarenga, E.R.; França, L.R. Successful Xenogeneic Germ Cell Transplantation from Jundia Catfish (Rhamdia quelen) into Adult Nile Tilapia (Oreochromis niloticus) Testes. Gen. Comp. Endocrinol. 2016, 230–231, 48–56. [Google Scholar] [CrossRef]
- Crouse, C.; Davidson, J.; May, T.; Summerfelt, S.; Good, C. Production of Market-Size European Strain Atlantic Salmon (Salmo salar) in Land-Based Freshwater Closed Containment Aquaculture Systems. Aquac. Eng. 2021, 92, 102138. [Google Scholar] [CrossRef]
- Yamamoto, E. Studies on Sex-Manipulation and Production of Cloned Populations in Hirame, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture 1999, 173, 235–246. [Google Scholar] [CrossRef]
- Sheehan, R.J.; Shasteen, S.P.; Suresh, A.V.; Kapuscinski, A.R.; Seeb, J.E. Better Growth in All-Female Diploid and Triploid Rainbow Trout. Trans. Am. Fish. Soc. 1999, 128, 491–498. [Google Scholar] [CrossRef]
- Bye, V.J.; Lincoln, R.F. Commercial Methods for the Control of Sexual Maturation in Rainbow Trout (Salmo gairdneri R.). Aquaculture 1986, 57, 299–309. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Monosex Male Production in Finfish as Exemplified by Tilapia: Applications, Problems, and Prospects. Aquaculture 2001, 197, 283–301. [Google Scholar] [CrossRef]
- Piferrer, F.; Lim, L.C. Application of Sex Reversal Technology in Ornamental Fish Culture. Aquar. Sci. Conserv. 1997, 1, 113–118. [Google Scholar] [CrossRef]
- Shen, Z.G.; Wang, H.P. Environmental Sex Determination and Sex Differentiation in Teleosts—How Sex Is Established. In Sex Control in Aquaculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 85–115. [Google Scholar]
- Baroiller, J.F.; D’Cotta, H.; Saillant, E. Environmental Effects on Fish Sex Determination and Differentiation. Sex. Dev. 2009, 3, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Magerhans, A.; Müller-Belecke, A.; Hörstgen-Schwark, G. Effect of Rearing Temperatures Post Hatching on Sex Ratios of Rainbow Trout (Oncorhynchus mykiss) Populations. Aquaculture 2009, 294, 25–29. [Google Scholar] [CrossRef]
- Maria Poli, B. Farmed Fish Welfare-Suffering Assessment and Impact on Product Quality. Ital. J. Anim. Sci. 2009, 8, 139–160. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.P.; Shen, Z.G. Sex Control in Aquaculture. In Sex Control in Aquaculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–34. [Google Scholar]
- Weber, G.M.; Leeds, T.D.; Schneider, R.P. Sex Reversal of Female Rainbow Trout by Immersion in 17α-Methyltestosterone. Aquaculture 2020, 528, 735535. [Google Scholar] [CrossRef]
- Okutsu, T.; Shikina, S.; Sakamoto, T.; Mochizuki, M.; Yoshizaki, G. Successful Production of Functional Y Eggs Derived from Spermatogonia Transplanted into Female Recipients and Subsequent Production of YY Supermales in Rainbow Trout, Oncorhynchus mykiss. Aquaculture 2015, 446, 298–302. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Yazawa, R.; Yoshizaki, G. Chapter 17 Intraperitoneal Germ Cell Transplantation Technique in Marine Teleosts. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J.F., Eds.; Springer: Singapore, 2020; pp. 357–379. [Google Scholar]
- Weber, G.M.; Hostuttler, M.A.; Cleveland, B.M.; Leeds, T.D. Growth Performance Comparison of Intercross-Triploid, Induced Triploid, and Diploid Rainbow Trout. Aquaculture 2014, 433, 85–93. [Google Scholar] [CrossRef]
- Norberg, B.; Weltzien, F.A.; Karlsen, Ø.; Holm, J.C. Effects of Photoperiod on Sexual Maturation and Somatic Growth in Male Atlantic Halibut (Hippoglossus hippoglossus L.). Comp. Biochem. Physiol. 2001, 129B, 357–365. [Google Scholar] [CrossRef]
- Good, C.; Davidson, J. A Review of Factors Influencing Maturation of Atlantic Salmon, Salmo salar, with Focus on Water Recirculation Aquaculture System Environments. J. World Aquac. Soc. 2016, 47, 605–632. [Google Scholar] [CrossRef]
- Oppedal, F.; Taranger, G.L.; Hansen, T. Growth Performance and Sexual Maturation in Diploid and Triploid Atlantic Salmon (Salmo salar L.) in Seawater Tanks Exposed to Continuous Light or Simulated Natural Photoperiod. Aquaculture 2003, 215, 145–162. [Google Scholar] [CrossRef]
- Iversen, M.; Myhr, A.I.; Wargelius, A. Approaches for Delaying Sexual Maturation in Salmon and Their Possible Ecological and Ethical Implications. J. Appl. Aquac. 2016, 28, 330–369. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Duston, J. Preventing Sexual Maturation in Arctic Charr by 24 h Light Overwinter and Suppressing Somatic Growth. Aquaculture 2016, 464, 537–544. [Google Scholar] [CrossRef]
- Bailey, C. Transgenic Salmon: Science, Politics, and Flawed Policy. Soc. Nat. Resour. 2015, 28, 1249–1260. [Google Scholar] [CrossRef]
- Okoli, A.S.; Blix, T.; Myhr, A.I.; Xu, W.; Xu, X. Sustainable Use of CRISPR/Cas in Fish Aquaculture: The Biosafety Perspective. Transgenic Res. 2021. [Google Scholar] [CrossRef]
- Scheerer, P.D.; Thorgaard, G.H. Increased Survival in Salmonid Hybrids by Induced Triploidy. Can. J. Fish. Aquat. 1983, 40, 2040–2044. [Google Scholar] [CrossRef]
- Egami, N.; Hama-Furukawa, A. Late Effects of Continuous Gamma-Irradiation of the Developmental Stage on the Gonads in Oryzias Latipes. In Radiation Effects on Aquatic Organisms; Egami, N., Ed.; Japan Scientific Societies Press: Tokyo, Japan, 1980; pp. 105–117. [Google Scholar]
- Saito, T.; Güralp, H.; Iegorova, V.; Rodina, M.; Pšenička, M. Elimination of Primordial Germ Cells in Sturgeon Embryos by Ultraviolet Irradiation†. Biol. Reprod. 2018, 99, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Wargelius, A.; Leininger, S.; Skaftnesmo, K.O.; Kleppe, L.; Andersson, E.; Taranger, G.L.; Schulz, R.W.; Edvardsen, R.B. Dnd Knockout Ablates Germ Cells and Demonstrates Germ Cell Independent Sex Differentiation in Atlantic Salmon. Sci. Rep. 2016, 6, 21284. [Google Scholar] [CrossRef] [Green Version]
- Piferrer, F.; Beaumont, A.; Falguière, J.-C.; Flajšhans, M.; Haffray, P.; Colombo, L. Polyploid Fish and Shellfish: Production, Biology and Applications to Aquaculture for Performance Improvement and Genetic Containment. Aquaculture 2009, 293, 125–156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Lau, S.W.; Zhang, L.; Ge, W. Disruption of Zebrafish Follicle-Stimulating Hormone Receptor (fshr) but Not Luteinizing Hormone Receptor (lhcgr) Gene by TALEN Leads to Failed Follicle Activation in Females Followed by Sexual Reversal to Males. Endocrinology 2015, 156, 3747–3762. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.L.; Desvignes, T.; Bremiller, R.; Wilson, C.; Dillon, D.; High, S.; Draper, B.; Buck, C.L.; Postlethwait, J. Gonadal Soma Controls Ovarian Follicle Proliferation through Gsdf in Zebrafish. Dev. Dyn. 2017, 246, 925–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Cui, Y.; Jiang, L.; Ge, W. Functional Analysis of Nuclear Estrogen Receptors in Zebrafish Reproduction by Genome Editing Approach. Endocrinology 2017, 158, 2292–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhu, B.; Ge, W. Genetic Analysis of Zebrafish Gonadotropin (FSH and LH) Functions by TALEN-Mediated Gene Disruption. Mol. Endocrinol. 2015, 29, 76–98. [Google Scholar] [CrossRef] [Green Version]
- Pšenička, M.; Saito, T.; Rodina, M.; Dzyuba, B. Cryopreservation of Early Stage Siberian Sturgeon Acipenser baerii Germ Cells, Comparison of Whole Tissue and Dissociated Cells. Cryobiology 2016, 72, 119–122. [Google Scholar] [CrossRef]
- Ye, H.; Li, C.J.; Yue, H.M.; Du, H.; Yang, X.G.; Yoshino, T.; Hayashida, T.; Takeuchi, Y.; Wei, Q.W. Establishment of Intraperitoneal Germ Cell Transplantation for Critically Endangered Chinese Sturgeon Acipenser sinensis. Theriogenology 2017, 94, 37–47. [Google Scholar] [CrossRef]
- Jeong, Y.; Ryu, J.H.; Nam, Y.K.; Gong, S.P.; Kang, S.M. Enhanced Adhesion of Fish Ovarian Germline Stem Cells on Solid Surfaces by Mussel-Inspired Polymer Coating. Mar. Drugs 2019, 17, 11. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Takeuchi, Y.; Wu, M.; Yue, H.; Ruan, R.; Du, H.; Zhou, C.; Xiang, H.; Li, C.; Wei, Q. Assessment of Yangtze Sturgeon as Recipient for the Production of American Paddlefish Gametes through Spermatogonia Transplantation. Theriogenology 2020, 158, 168–179. [Google Scholar] [CrossRef]
- Perera, D.A.; Alsaqufi, A.; Shang, M.; Wade, D.C.; Su, B.; Elaswad, A.; Fobes, M.; Beam, R.; Garcia, G.; Dunn, D.A.; et al. Xenogenesis-Production of Channel Catfish × Blue Catfish Hybrid Progeny by Fertilization of Channel Catfish Eggs with Sperm from Triploid Channel Catfish Males with Transplanted Blue Catfish Germ Cells. N. Am. J. Aquac. 2017, 79, 61–74. [Google Scholar] [CrossRef]
| Method | Principle | Advantage | Disadvantage | Enrichment of Fish GSCs |
|---|---|---|---|---|
| PDGC | Fractionating cells by their density with centrifugation | Simple procedure No need for special materials, techniques, and equipment | Low purity | The most frequently used method 60–83.6% purity [39,71,72] |
| DP | Positive or negative selection based on adherence of cells by in vitro culture | Simple procedure No need for special materials, techniques, and equipment | Low purity Relatively long procedure Potential risks of spontaneous differentiation during in vitro culture | Frequently used >90% purity by serial DPs [73,75] No available commercial molecules for positive selection |
| CE | Aligning and eluting cells by their physical characteristics with centrifugation | High purity expected Not requiring TGs or ABs | Requiring special sorting conditions and equipment | >90% purity by combining with PDGC [23] |
| FACS | Isolation of cells based on light-scattering properties | High purity expected Flexible sorting condition by customizing gates based on size, granularity, and fluorescent intensity of cells | Requiring special skills and equipment Requiring specific ABs against the surface protein of target cells, TGs carrying germ cells expressing FPs, or other specific sorting conditions Not suitable for large scales | Up to 100% purity of PGCs labeled with mRNA-nanos3 3′ UTR encoded FP [17] 93.2–99% purity with TGs [18,19,85] 70.7–80.9% purity using ABs [20,45,86] 75.6–94.9% purity without using TGs or ABs [19,87] Limitation of using TG fish for commercial application No available commercial fish ABs |
| MACS | Affinity based cell sorting with magnetic particles-conjugated ABs against cell surface proteins | High purity expected No need for special techniques or equipment Simpler than FACS Applicable to large scales | Requiring specific ABs against the surface protein of target cells | 54.8–81.7% purity [62] No available commercial fish ABs |
| Method | Principle | Advantage | Disadvantage | Recipient Case |
|---|---|---|---|---|
| Interspecific hybridization | Preventing normal meiosis by a chromosomal mismatch or arresting mitosis of PGCs | Simple preparation by breeding without any treatment Complete germ cell elimination (in germ cell-less hybrids) | Unpredictable reproductive phenotypes depending on species/sex combination | ♂ D. albolineatus × ♀ D. rerio [69], O. curvinotus × O. latipes [144], ♂ P. argentata × ♀ N. mitsukurii [27,31,104] |
| Triploidization | Preventing normal meiosis by a chromosomal mismatch | Relatively simple procedure | Potential risks of endogenous germ cell-derived gamete production Unstable efficiency depending on species and method | D. rerio [145], N. mitsukurii [63], O. masou [28,146], O. mykiss [21,56,147], O. latipes [55], P. olivaceus [148], T. alboplumbeus [120,138] |
| Dnd-knockdown | Disrupting PGC development by MO-mediated inhibition of Dnd expression | High efficiency expected Possibility of complete germ cell elimination | Requiring special techniques, apparatus, or materials Not guaranteed 100% sterility | C. auratus [29,65], D. rerio [16,123,126], O. masou [124], O. latipes [64], T. alboplumbeus [127] |
| Dnd-knockout | Disrupting germ cell development by blocking Dnd expression through gene editing | Guaranteed 100% sterility in homozygous mutants Complete germ cell elimination | Requiring genetic knowledge, techniques, apparatus, and time to establish mutant lines Only 25% sterile fish of total | D. rerio [101], O. mykiss [38] |
| High temperature + busulfan co-treatment | Arresting meiosis and inducing apoptosis of germ cells | Preparable in a few weeks with wild-type adults | Incomplete germ cell ablation Requiring optimized conditions to achieve low mortality and successful germ cell ablation | O. hatcheri [100,149], O. niloticus [32], P. olivaceus [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.H.; Xu, L.; Wong, T.-T. Advantages, Factors, Obstacles, Potential Solutions, and Recent Advances of Fish Germ Cell Transplantation for Aquaculture—A Practical Review. Animals 2022, 12, 423. https://doi.org/10.3390/ani12040423
Ryu JH, Xu L, Wong T-T. Advantages, Factors, Obstacles, Potential Solutions, and Recent Advances of Fish Germ Cell Transplantation for Aquaculture—A Practical Review. Animals. 2022; 12(4):423. https://doi.org/10.3390/ani12040423
Chicago/Turabian StyleRyu, Jun Hyung, Lan Xu, and Ten-Tsao Wong. 2022. "Advantages, Factors, Obstacles, Potential Solutions, and Recent Advances of Fish Germ Cell Transplantation for Aquaculture—A Practical Review" Animals 12, no. 4: 423. https://doi.org/10.3390/ani12040423






