Neuropathological Characterization of Dolphin Morbillivirus Infection in Cetaceans Stranded in Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Neuropathological Investigation
2.3. PCR and Sequence Analysis in CNS
2.4. Microbiological Analysis of the CNS: Standard and Specific for Brucella Isolation
2.5. Statistical Analysis
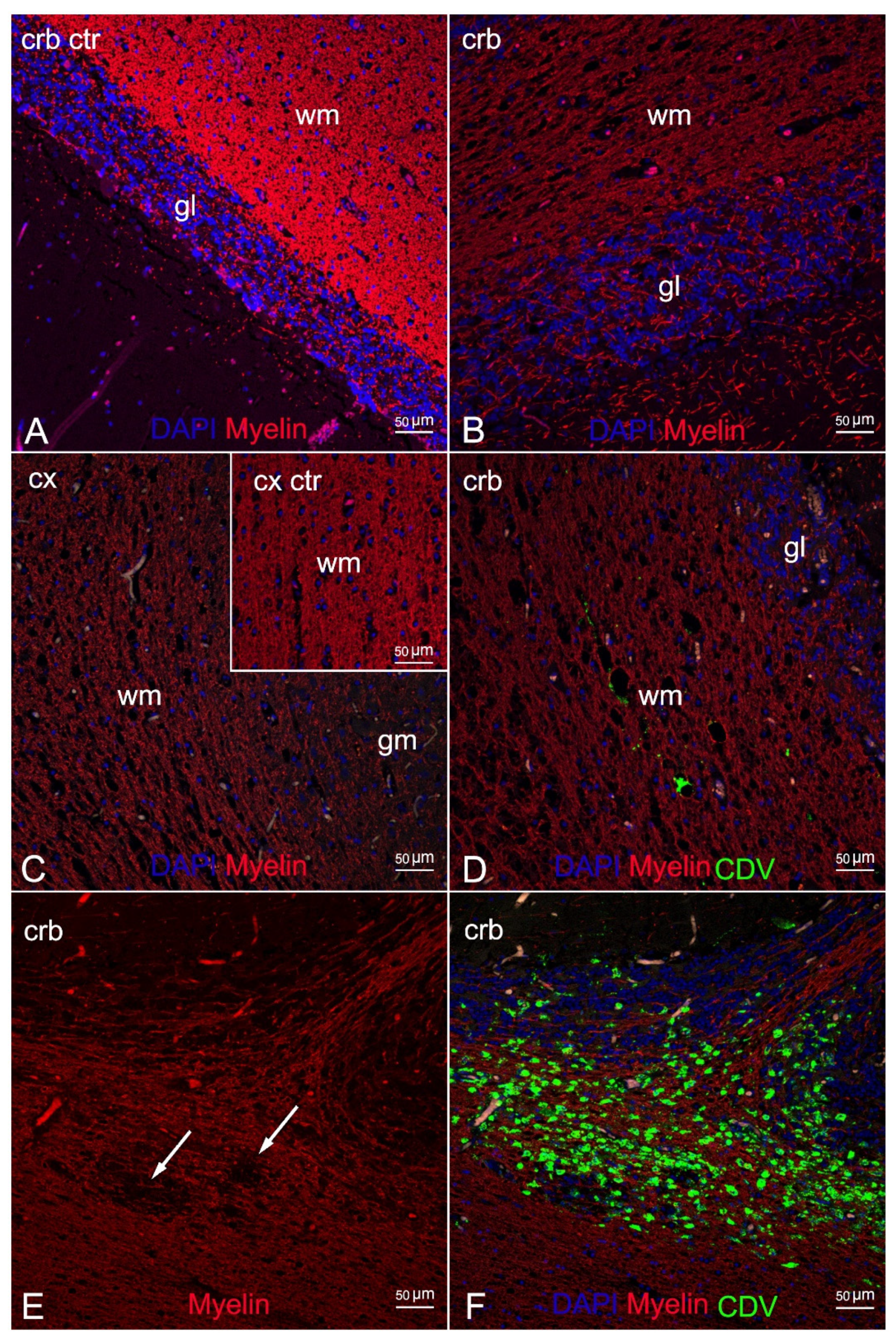
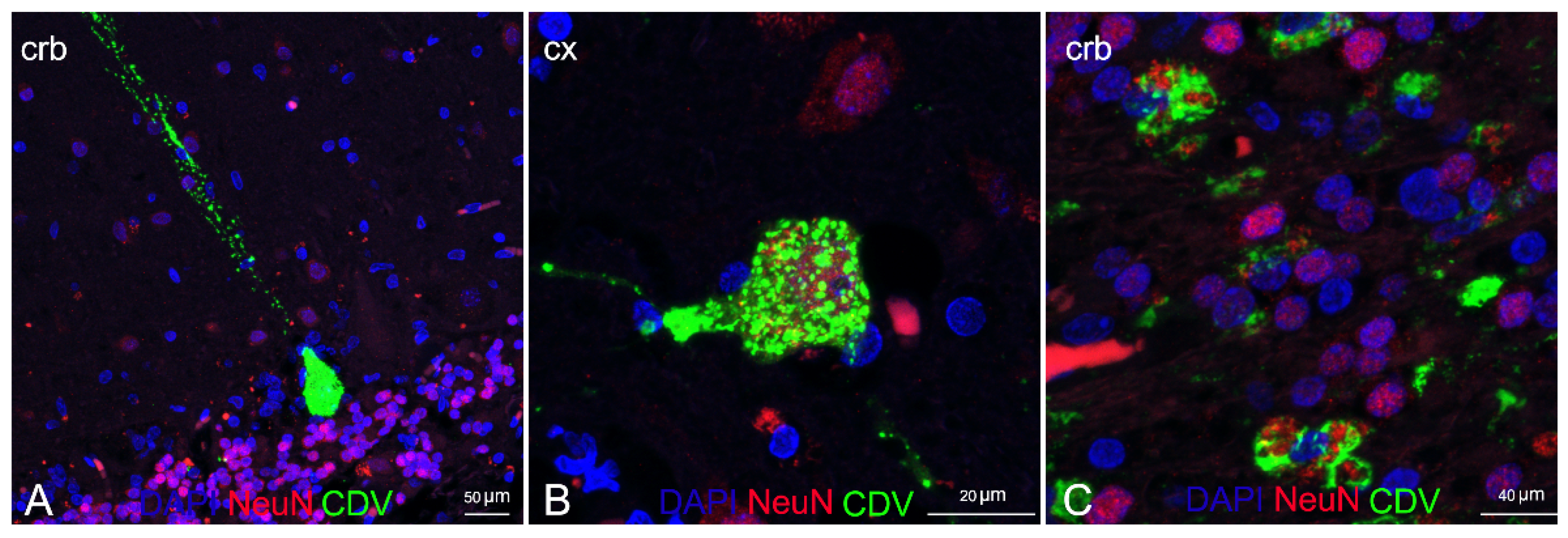
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pintore, M.D.; Mignone, W.; Di Guardo, G.; Mazzariol, S.; Ballardini, M.; Florio, C.L.; Goria, M.; Romano, A.; Caracappa, S.; Giorda, F.; et al. Neuropathologic findings in cetaceans stranded in Italy (2002–14). J. Wildl. Dis. 2018, 54, 295–303. [Google Scholar] [CrossRef]
- Sierra, E.; Fernández, A.; Felipe-Jiménez, I.; Zucca, D.; Díaz-Delgado, J.; Puig-Lozano, R.; Câmara, N.; Consoli, F.; Díaz-Santana, P.; Suárez-Santana, C.; et al. Histopathological Differential Diagnosis of Meningoencephalitis in Cetaceans: Morbillivirus, Herpesvirus, Toxoplasma gondii, Brucella sp., and Nasitrema sp. Front. Vet. Sci. 2020, 7, 650. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.E.; Deaville, R.; Perkins, M.W.; Jepson, P.D.; Penrose, R.; Rocchi, M.S.; Maley, M.; Ballingall, K.T.; Dagleish, M.P. Novel Presentation of DMV-Associated Encephalitis in a Long-Finned Pilot Whale (Globicephala melas). J. Comp. Pathol. 2021, 183, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Zinzula, L.; Mazzariol, S.; Di Guardo, G. Molecular signatures in cetacean morbillivirus and host species proteomes: Unveiling the evolutionary dynamics of an enigmatic pathogen? Microbiol. Immunol. 2021, 66, 52–58. [Google Scholar] [CrossRef]
- Groch, K.R.; Colosio, A.C.; Marcondes, M.C.C.; Zucca, D.; Díaz-Delgado, J.; Niemeyer, C.; Marigo, J.; Brandão, P.E.; Fernández, A.; Catão-Dias, J.L. Novel cetacean morbillivirus in Guiana Dolphin, Brazil. Emerg. Infect. Dis. 2014, 20, 511–513. [Google Scholar] [CrossRef] [PubMed]
- West, K.L.; Sanchez, S.; Rotstein, D.; Robertson, K.M.; Dennison, S.; Levine, G.; Davis, N.; Schofield, D.; Potter, C.W.; Jensen, B. A Longman’s beaked whale (Indopacetus pacificus) strands in Maui, Hawaii, with first case of morbillivirus in the central Pacific. Mar. Mammal Sci. 2013, 29, 767–776. [Google Scholar] [CrossRef]
- Stephens, N.; Duignan, P.J.; Wang, J.; Bingham, J.; Finn, H.; Bejder, L.; Patterson, I.A.P.; Holyoake, C. Cetacean morbillivirus in coastal indo-pacific bottlenose dolphins, Western Australia. Emerg. Infect. Dis. 2014, 20, 666–670. [Google Scholar] [CrossRef] [PubMed]
- West, K.L.; Silva-Krott, I.; Landrau-Giovannetti, N.; Rotstein, D.; Saliki, J.; Raverty, S.; Nielsen, O.; Popov, V.L.; Davis, N.; Walker, W.A.; et al. Novel cetacean morbillivirus in a rare Fraser’s dolphin (Lagenodelphis hosei) stranding from Maui, Hawai‘i. Sci. Rep. 2021, 11, 15986. [Google Scholar] [CrossRef]
- Peletto, S.; Caruso, C.; Cerutti, F.; Modesto, P.; Biolatti, C.; Pautasso, A.; Grattarola, C.; Giorda, F.; Mazzariol, S.; Mignone, W.; et al. Efficient isolation on Vero.DogSLAMtag cells and full genome characterization of Dolphin Morbillivirus (DMV) by next generation sequencing. Sci. Rep. 2018, 8, 860. [Google Scholar] [CrossRef]
- Cerutti, F.; Giorda, F.; Grattarola, C.; Mignone, W.; Beltramo, C.; Keck, N.; Lorusso, A.; Di Francesco, G.; Di Renzo, L.; Di Guardo, G.; et al. Specific capture and whole-genome phylogeography of Dolphin morbillivirus. Sci. Rep. 2020, 10, 20831. [Google Scholar] [CrossRef]
- Mira, F.; Rubio-Guerri, C.; Purpari, G.; Puleio, R.; Caracappa, G.; Gucciardi, F.; Russotto, L.; Loria, G.R.; Guercio, A. Circulation of a novel strain of dolphin morbillivirus (DMV) in stranded cetaceans in the Mediterranean Sea. Sci. Rep. 2019, 9, 9792. [Google Scholar] [CrossRef]
- Pautasso, A.; Iulini, B.; Grattarola, C.; Giorda, F.; Goria, M.; Peletto, S.; Masoero, L.; Mignone, W.; Varello, K.; Petrella, A.; et al. Novel dolphin morbillivirus (DMV) outbreak among Mediterranean striped dolphins Stenella coeruleoalba in Italian waters. Dis. Aquat. Organ. 2019, 132, 215–220. [Google Scholar] [CrossRef]
- Vandevelde, M.; Zurbriggen, A. Demyelination in canine distemper virus infection: A review. Acta Neuropathol. 2005, 109, 56–68. [Google Scholar] [CrossRef]
- Ulrich, R.; Puff, C.; Wewetzer, K.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W. Transcriptional changes in canine distemper virus-induced demyelinating leukoencephalitis favor a biphasic mode of demyelination. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, S.; Peletto, S.; Mondin, A.; Centelleghe, C.; Di Guardo, G.; Di Francesco, C.E.; Casalone, C.; Acutis, P.L. Dolphin morbillivirus infection in a captive harbor seal (phoca vitulina). J. Clin. Microbiol. 2013, 51, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Duignan, P.J.; Van Bressem, M.F.; Baker, J.D.; Barbieri, M.; Colegrove, K.M.; de Guise, S.; de Swart, R.L.; di Guardo, G.; Dobson, A.; Duprex, W.P.; et al. Phocine distemper Virus: Current knowledge and future directions. Viruses 2014, 6, 5093–5134. [Google Scholar] [CrossRef]
- Duignan, P.J.; Geraci, J.R.; Raga, J.A.; Calzada, N. Pathology of morbillivirus infection in striped dolphins (Stenella coeruleoalba) from Valencia and Murcia, Spain. Can. J. Vet. Res. 1992, 56, 242–248. [Google Scholar] [PubMed]
- Domingo, M.; Vilafranca, M.; Visa, J.; Prats, N.; Trudgett, A.; Visser, I. Evidence for chronic morbillivirus infection in the Mediterranean striped dolphin (Stenella coeruleoalba). Vet. Microbiol. 1995, 44, 229–239. [Google Scholar] [CrossRef]
- Soto, S.; Alba, A.; Ganges, L.; Vidal, E.; Raga, J.A.; Alegre, F.; González, B.; Medina, P.; Zorrilla, I.; Martínez, J.; et al. Post-epizootic chronic dolphin morbillivirus infection in Mediterranean striped dolphins Stenella coeruleoalba. Dis. Aquat. Organ. 2011, 96, 187–194. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Groch, K.R.; Sierra, E.; Sacchini, S.; Zucca, D.; Quesada-Canales, Ó.; Arbelo, M.; Fernández, A.; Santos, E.; Ikeda, J.; et al. Comparative histopathologic and viral immunohistochemical studies on CeMV infection among Western Mediterranean, Northeast-Central, and Southwestern Atlantic cetaceans. PLoS ONE 2019, 14, e0213363. [Google Scholar] [CrossRef]
- Geraci, J.R.; Lounsbury, V.J.; Texas A & M University. Sea Grant College Program. In Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005; Volume 486, ISBN 9780977460908. [Google Scholar]
- Carlini, R.; de Francesco, M.C.; Libera, S. Della Biometric measures indicating sexual dimorphism in Stenella coeruleoalba (Meyen, 1833) (Delphinidae) in the north-central Tyrrhenian sea. Aquat. Mamm. 2014, 40, 59–68. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. (Eds.) Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling. ACCOBAMS ASCOBANS, Ed. In Proceedings of the 25th Meeting of the Advisory Committee, Stralsund, Germany, 17–19 September 2019; p. 73. [Google Scholar]
- Hernández-Mora, G.; González-Barrientos, R.; Morales, J.A.; Chaves-Olarte, E.; Guzmán-Verri, C.; Baquero-Calvo, E.; De-Miguel, M.J.; Marín, C.M.; Blasco, J.M.; Moreno, E. Neurobrucellosis in stranded dolphins, Costa Rica. Emerg. Infect. Dis. 2008, 14, 1430–1433. [Google Scholar] [CrossRef]
- Di Guardo, G.; Proietto, U.; Di Francesco, C.E.; Marsilio, F.; Zaccaroni, A.; Scaravelli, D.; Mignone, W.; Garibaldi, F.; Kennedy, S.; Forster, F.; et al. Cerebral toxoplasmosis in striped dolphins (stenella coeruleoalba) stranded along the ligurian sea coast of Italy. Vet. Pathol. 2010, 47, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Verna, F.; Giorda, F.; Miceli, I.; Rizzo, G.; Pautasso, A.; Romano, A.; Iulini, B.; Pintore, M.D.; Mignone, W.; Grattarola, C.; et al. Detection of morbillivirus infection by RT-PCR RFLP analysis in cetaceans and carnivores. J. Virol. Methods 2017, 247, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Grattarola, C.; Gallina, S.; Giorda, F.; Pautasso, A.; Ballardini, M.; Iulini, B.; Varello, K.; Goria, M.; Peletto, S.; Masoero, L.; et al. First report of Salmonella 1,4,[5],12:i:- in free-ranging striped dolphins (Stenella coeruleoalba), Italy. Sci. Rep. 2019, 9, 6061. [Google Scholar] [CrossRef] [PubMed]
- Garofolo, G.; Petrella, A.; Lucifora, G.; Di Francesco, G.; Di Guardo, G.; Pautasso, A.; Iulini, B.; Varello, K.; Giorda, F.; Goria, M.; et al. Occurrence of Brucella ceti in striped dolphins from Italian Seas. PLoS ONE 2020, 15, e0240178. [Google Scholar] [CrossRef] [PubMed]
- Giorda, F.; Romani-Cremaschi, U.; Marsh, A.E.; Grattarola, C.; Iulini, B.; Pautasso, A.; Varello, K.; Berio, E.; Gazzuola, P.; Marsili, L.; et al. Evidence for Unknown Sarcocystis-Like Infection in Stranded Striped Dolphins (Stenella coeruleoalba) from the Ligurian Sea, Italy. Animals 2021, 11, 1201. [Google Scholar] [CrossRef]
- Giorda, F.; Di Guardo, G.; Varello, K.; Pautasso, A.; Sierra, E.; Pintore, M.D.; Grattarola, C.; Colella, E.M.; Berio, E.; Goria, M.; et al. Retrospective immunohistochemical investigation on dolphin morbillivirus infection by comparing the performance of heterologous monoclonal and polyclonal antibodies—Short communication. Acta Vet. Hung. 2021, 69, 204–210. [Google Scholar] [CrossRef]
- Alldinger, S.; Baumgärtner, W.; Örvell, C. Restricted expression of viral surface proteins in canine distemper encephalitis. Acta Neuropathol. 1993, 85, 635–645. [Google Scholar] [CrossRef]
- Van Bressem, M.F.; Duignan, P.J.; Banyard, A.; Barbieri, M.; Colegrove, K.M.; de Guise, S.; di Guardo, G.; Dobson, A.; Domingo, M.; Fauquier, D.; et al. Cetacean morbillivirus: Current knowledge and future directions. Viruses 2014, 6, 5145–5181. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef]
- Vitale, M.; Galluzzo, P.; Currò, V.; Gozdzik, K.; Schillaci, D.; Di Marco Lo Presti, V. A high sensitive nested PCR for Toxoplasma gondii detection in animal and food samples. J. Microb. Biochem. Technol. 2013, 5, 39–41. [Google Scholar] [CrossRef]
- Bellière, E.N.; Esperón, F.; Fernández, A.; Arbelo, M.; Muñoz, M.J.; Sánchez-Vizcaíno, J.M. Phylogenetic analysis of a new Cetacean morbillivirus from a short-finned pilot whale stranded in the Canary Islands. Res. Vet. Sci. 2011, 90, 324–328. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; World Organisation for Animal Health (OIE), Ed.; World Organisation for Animal Health: Paris, France, 2018; ISBN 978-92-95108-18-9. [Google Scholar]
- Giorda, F.; Ballardini, M.; Di Guardo, G.; Pintore, M.D.; Grattarola, C.; Iulini, B.; Mignone, W.; Goria, M.; Serracca, L.; Varello, K.; et al. Postmortem findings in cetaceans found stranded in the pelagos sanctuary, Italy, 2007–2014. J. Wildl. Dis. 2017, 53. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Guerri, C.; Melero, M.; Esperón, F.; Bellière, E.N.; Arbelo, M.; Crespo, J.L.; Sierra, E.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. Unusual striped dolphin mass mortality episode related to cetacean morbillivirus in the Spanish Mediterranean sea. BMC Vet. Res. 2013, 9, 106. [Google Scholar] [CrossRef]
- Casalone, C.; Mazzariol, S.; Pautasso, A.; Di Guardo, G.; Di Nocera, F.; Lucifora, G.; Ligios, C.; Franco, A.; Fichi, G.; Cocumelli, C.; et al. Cetacean strandings in Italy: An unusual mortality event along the Tyrrhenian Sea coast in 2013. Dis. Aquat. Organ. 2014, 109, 81–86. [Google Scholar] [CrossRef] [PubMed]
- C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2016). Available online: https://www.izsplv.it/components/com_publiccompetitions/includes/download.php?id=863:report_diagnostica_spiaggiamenti_2016.pdf (accessed on 17 December 2021).
- C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2017). Available online: https://www.izsplv.it/components/com_publiccompetitions/includes/download.php?id=864:report_spiaggiamenti_2017.pdf (accessed on 17 December 2021).
- C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2018). Available online: https://www.izsplv.it/components/com_publiccompetitions/includes/download.php?id=865:report-senza-tabella-2018-vers-3-ottobre-2019.pdf (accessed on 17 December 2021).
- C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2019). Available online: https://www.izsplv.it/components/com_publiccompetitions/includes/download.php?id=866:report-credima-2019.pdf (accessed on 17 December 2021).
- C.Re.Di.Ma. Italian Diagnostic Report on Stranded Cetaceans (2020). Available online: https://www.izsplv.it/components/com_publiccompetitions/includes/download.php?id=1955:report-2020_merged.pdf (accessed on 17 December 2021).
- Di Guardo, G.; Mazzariol, S. Cetacean Morbillivirus—Associated Pathology: Knowns and Unknowns. Front. Microbiol. 2016, 7, 112. [Google Scholar] [CrossRef]
- Mazzariol, S.; Centelleghe, C.; Cozzi, B.; Povinelli, M.; Marcer, F.; Ferri, N.; Di Francesco, G.; Badagliacca, P.; Profeta, F.; Olivieri, V.; et al. Multidisciplinary studies on a sick-leader syndrome-associated mass stranding of sperm whales (Physeter macrocephalus) along the Adriatic coast of Italy. Sci. Rep. 2018, 8, 11577. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Osterhaus, A.D.; Ludlow, M. Transmission of morbilliviruses within and among marine mammal species. Curr. Opin. Virol. 2018, 28, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.A.; Cattaneo, R.; von Messling, V. Canine Distemper Virus Uses both the Anterograde and the Hematogenous Pathway for Neuroinvasion. J. Virol. 2006, 80, 9361–9370. [Google Scholar] [CrossRef]
- Costa-Silva, S.; Sacristán, C.; Gonzales-Viera, O.; Díaz-Delgado, J.; Sánchez-Sarmiento, A.M.; Marigo, J.; Groch, K.R.; Carvalho, V.L.; Ewbank, A.C.; Colosio, A.C.; et al. Toxoplasma gondii in cetaceans of Brazil: A histopathological and immunohistochemical survey. Rev. Bras. Parasitol. Vet. 2019, 28, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Antoniassi, N.A.B.; Boabaid, F.M.; Souza, R.L.; Nakazato, L.; Pimentel, M.F.A.; Filho, J.O.X.; Pescador, C.A.; Driemeier, D.; Colodel, E.M. Granulomatous meningoencephalitis due to Toxoplasma gondii in a black-headed night monkey (Aotus nigriceps). J. Zoo Wildl. Med. 2011, 42, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mora, G.; Palacios-Alfaro, J.D.; González-Barrientes, R. Wildlife reservoirs of brucellosis: Brucella in aquatic environments. Rev. Sci. Tech. 2013, 32, 89–103. [Google Scholar] [CrossRef]
- Esperón, F.; Fernández, A.; Sánchez-Vizcaíno, J.M. Herpes simplex-like infection in a bottlenose dolphin stranded in the Canary Islands. Dis. Aquat. Organ. 2008, 81, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Van Elk, C.E.; Van De Bildt, M.W.G.; De Jong, A.A.W.; Osterhaus, A.D.M.E.; Kuiken, T. Genital herpesvirus in bottlenose dolphins (Tursiops truncatus): Cultivation, epidemiology, and associated pathology. J. Wildl. Dis. 2009, 45, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Sierra, E.; Sánchez, S.; Saliki, J.T.; Blas-Machado, U.; Arbelo, M.; Zucca, D.; Fernández, A. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the canary Islands. J. Clin. Microbiol. 2014, 52, 2390–2397. [Google Scholar] [CrossRef]
- Vargas-Castro, I.; Melero, M.; Crespo-Picazo, J.L.; Jiménez, M.d.l.Á.; Sierra, E.; Rubio-Guerri, C.; Arbelo, M.; Fernández, A.; García-Párraga, D.; Sánchez-Vizcaíno, J.M. Systematic determination of herpesvirus in free-ranging cetaceans stranded in the western mediterranean: Tissue tropism and associated lesions. Viruses 2021, 13, 2180. [Google Scholar] [CrossRef]
- Di Francesco, G.; Cammà, C.; Curini, V.; Mazzariol, S.; Proietto, U.; Di Francesco, C.E.; Ferri, N.; Di Provvido, A.; Di Guardo, G. Coinfection by Ureaplasma spp., Photobacterium damselae and an Actinomyces-like microorganism in a bottlenose dolphin (Tursiops truncatus) with pleuropneumonia stranded along the Adriatic coast of Italy. Res. Vet. Sci. 2016, 105, 111–114. [Google Scholar] [CrossRef]
- Gan, L.; Mao, P.; Jiang, H.; Zhang, L.; Liu, D.; Cao, X.; Wang, Y.; Wang, Y.; Sun, H.; Huang, Y.; et al. Two Prevalent Listeria ivanovii subsp. ivanovii Clonal Strains With Different Virulence Exist in Wild Rodents and Pikas of China. Front. Vet. Sci. 2020, 7, 88. [Google Scholar] [CrossRef]
- Disson, O.; Lecuit, M. Targeting of the central nervous system by Listeria monocytogenes. Virulence 2012, 3, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.K.; Morgan, J.H.; McLauchlin, J.; Grant, K.A.; Shallcross, J.A. Listeria innocua isolated from a case of ovine meningoencephalitis. Vet. Microbiol. 1994, 42, 245–253. [Google Scholar] [CrossRef]
- Rocha, P.R.D.A.; Dalmasso, A.; Grattarola, C.; Casalone, C.; Del Piero, F.; Bottero, M.T.; Capucchio, M.T. Atypical cerebral listeriosis associated with Listeria innocua in a beef bull. Res. Vet. Sci. 2013, 94, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Lucá, R.; Giacominelli-Stuffler, R.; Mazzariol, S.; Roperto, S.; Cocumelli, C.; Di Guardo, G. Neuronal and astrocytic involvement in striped dolphins (Stenella coeruleoalba) with morbilliviral encephalitis. Acta Virol. 2017, 61, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Da Fontoura Budaszewski, R.; von Messling, V. Morbillivirus experimental animal models: Measles virus pathogenesis insights from canine distemper virus. Viruses 2016, 8, 274. [Google Scholar] [CrossRef] [PubMed]





| Antigen | Target | Antibody/Antiserum | Host | Dilution | Source | Technique(s) |
|---|---|---|---|---|---|---|
| CDV-NP | Infected cells | Mono | Mouse | 1:500 | VMRD | IF, IHC |
| GFAP | Astrocyte | Poly | Rabbit | 1:1000 | Millipore | IF |
| Iba-1 | Microglia | Poly | Rabbit | 1:1000 | Wako | IF |
| Myelin-PLP | Myelin | Poly | Rabbit | 1:500 | Abcam | IF |
| NeuN | Neuron | Poly | Rabbit | 1:1000 | Abcam | IF |
| Olig2 | Oligodendrocyte | Poly | Rabbit | 1:250 | Millipore | IF, IHC |
| Olig2 | Oligodendrocyte | Mono | Rabbit | 1:100 | Abcam | IHC |
| Case No. | Cerebral and Cerebellar Cortex | Lesions in Other Regions | Associated Lesions | SI | Co-Infections | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | PC | Astro-Mg | Malacia | NN | S | INCIBs | H | ||||||
| 1 | ++ | ++ | + | - | +++ | + | - | + | Diffuse and mild NS meningoencephalitis associated with focal and minimal NS plexus choroiditis | Protozoan tissue cysts and syncytia | S | T. gondii | [1,9,10,30,37] |
| 2 | +++ | +++ | +++ | + | +++ | ++ | - | + | - | Syncytia | S | - | [1,10] |
| 3 | - | + | - | - | - | +++ | - | - | Mild NS myelitis | - | A | - | [1,37] |
| 4 | - | - | - | - | ++ | +++ | - | + | Multifocal and mild NS plexus choroiditis | A | - | [1] | |
| 5 | - | + | - | - | - | ++ | - | - | - | - | A | T. gondii | [1] |
| 6 | + | ++ | ++++ | - | +++ | ++ | - | + | Diffuse and mild NS meningoencephalitis | - | S | - | [10] |
| 7 | - | - | +++ | ++++ | ++ | +++ | - | + | - | Syncytia | A | - | |
| 8 | - | - | +++ | ++++ | ++ | +++ | - | - | Perivasal oedema | Syncytia | A | - | |
| 9 | + | - | + | - | ++ | ++++ | - | - | - | - | A | - | |
| 10 | - | - | ++ | ++ | ++++ | ++++ | - | + | - | Purkinje cell loss | A | L. ivanovii | [10,12] |
| 11 | - | - | ++ | - | - | ++ | - | - | Mild NS plexus choroiditis | - | A | ˠHV and Photobacterium damselae subsp. damselae | [10,12] |
| 12 | + | ++ | ++ | ++ | ++++ | +++ | - | + | - | Suppurative encephalitis characterized by degenerate neutrophils | S | - | [10,12] |
| 13 | - | - | - | ++ | + | +++ | - | + | - | - | A | - | [10,12] |
| 14 | +++ | +++ | +++ | ++ | ++ | + | - | + | Diffuse and moderate NS meningoencephalitis associated with focal and minimal NS plexus choroiditis | Protozoan tissue cysts and syncytia Granulomatous encephalitis | S | Brucella ceti, T. gondii and Photobacterium damselae subsp. damselae | [28,29] |
| 15 | - | - | + | + | - | ++ | - | - | - | - | A | - | [10] |
| 16 | ++ | +++ | +++ | ++ | ++ | ++ | - | + | Diffuse and moderate NS meningoencephalitis and oedema | Protozoan tissue cysts and syncytia Granulomatous necrotizing encephalitis | S | T. gondii and Salmonella 1,4,[5],12:i:-; . | [10,27] |
| 17 | +++ | ++ | - | - | - | ++ | - | + | Diffuse and moderate NS meningoencephalitis and myelitis | - | C | - | |
| 18 | ++ | - | + | - | ++ | +++ | - | + | - | - | S | - | |
| 19 | ++++ | ++++ | ++++ | - | +++ | +++ | + | + | Multifocal and mild NS plexus choroiditis | Vasculitis, pyogranulomatous encephalitis and syncytia | S | - | |
| 20 | ++ | + | ++ | ++ | - | + | - | - | - | Perivasal oedema | S | ˠHV+ and T. gondii | |
| 21 | ++ | ++ | ++ | +++ | +++ | - | - | + | Diffuse and mild NS meningoencephalitis | Vasculitis, syncytia | S | Photobacterium damselae subsp. damselae, Listeria innocua | |
| 22 | ++ | ++ | +++ | - | +++ | ++ | - | - | Oedema | - | S | - | |
| 23 | ++ | +++ | +++ | +++ | ++++ | +++ | - | - | Diffuse and moderate NS meningoencephalitis | Syncytia | S | - | |
| 24 | ++ | ++ | +++ | - | +++ | ++ | - | + | Focal minimal NS plexus choroiditis | Protozoan tissue cysts, syncytia, and Purkenje cell loss; granulomatous encephalitis | S | T. gondii | |
| 25 | - | - | - | - | - | +++ | - | - | - | - | A | - | |
| 26 | +++ | ++ | - | - | - | ++ | - | - | - | Protozoan tissue cysts; granulomatous encephalitis | S | T. gondii | |
| 27 | - | - | +++ | - | ++ | - | - | - | - | Syncytia | A | - | |
| 28 | ++ | +++ | +++ | - | +++ | ++++ | - | - | Diffuse and moderate NS meningoencephalitis | - | S | - | |
| 29 | +++ | +++ | +++ | ++ | ++ | - | - | - | - | - | S | T. gondii | |
| 30 | - | - | ++ | - | + | +++ | - | - | - | - | A | T. gondii and Photobacterium damselae subsp. damselae | |
| 31 | ++ | +++ | +++ | + | +++ | - | - | + | - | S | Photobacterium damselae subsp. damselae | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorda, F.; Crociara, P.; Iulini, B.; Gazzuola, P.; Favole, A.; Goria, M.; Serracca, L.; Dondo, A.; Crescio, M.I.; Audino, T.; et al. Neuropathological Characterization of Dolphin Morbillivirus Infection in Cetaceans Stranded in Italy. Animals 2022, 12, 452. https://doi.org/10.3390/ani12040452
Giorda F, Crociara P, Iulini B, Gazzuola P, Favole A, Goria M, Serracca L, Dondo A, Crescio MI, Audino T, et al. Neuropathological Characterization of Dolphin Morbillivirus Infection in Cetaceans Stranded in Italy. Animals. 2022; 12(4):452. https://doi.org/10.3390/ani12040452
Chicago/Turabian StyleGiorda, Federica, Paola Crociara, Barbara Iulini, Paola Gazzuola, Alessandra Favole, Maria Goria, Laura Serracca, Alessandro Dondo, Maria Ines Crescio, Tania Audino, and et al. 2022. "Neuropathological Characterization of Dolphin Morbillivirus Infection in Cetaceans Stranded in Italy" Animals 12, no. 4: 452. https://doi.org/10.3390/ani12040452








