Distribution of CRISPR in Escherichia coli Isolated from Bulk Tank Milk and Its Potential Relationship with Virulence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Detection of Virulence Genes
2.3. Phylogenetic Groups
2.4. CRISPR Locus Sequence Typing and Spacer Analysis
2.5. Statistical Analysis
3. Results
3.1. Distribution of Virulence Genes
3.2. Distribution of Phylogenetic Groups and CRISPR Loci
3.3. Distribution of CRISPR 1 and CRISPR 2 by Phylogenetic Group
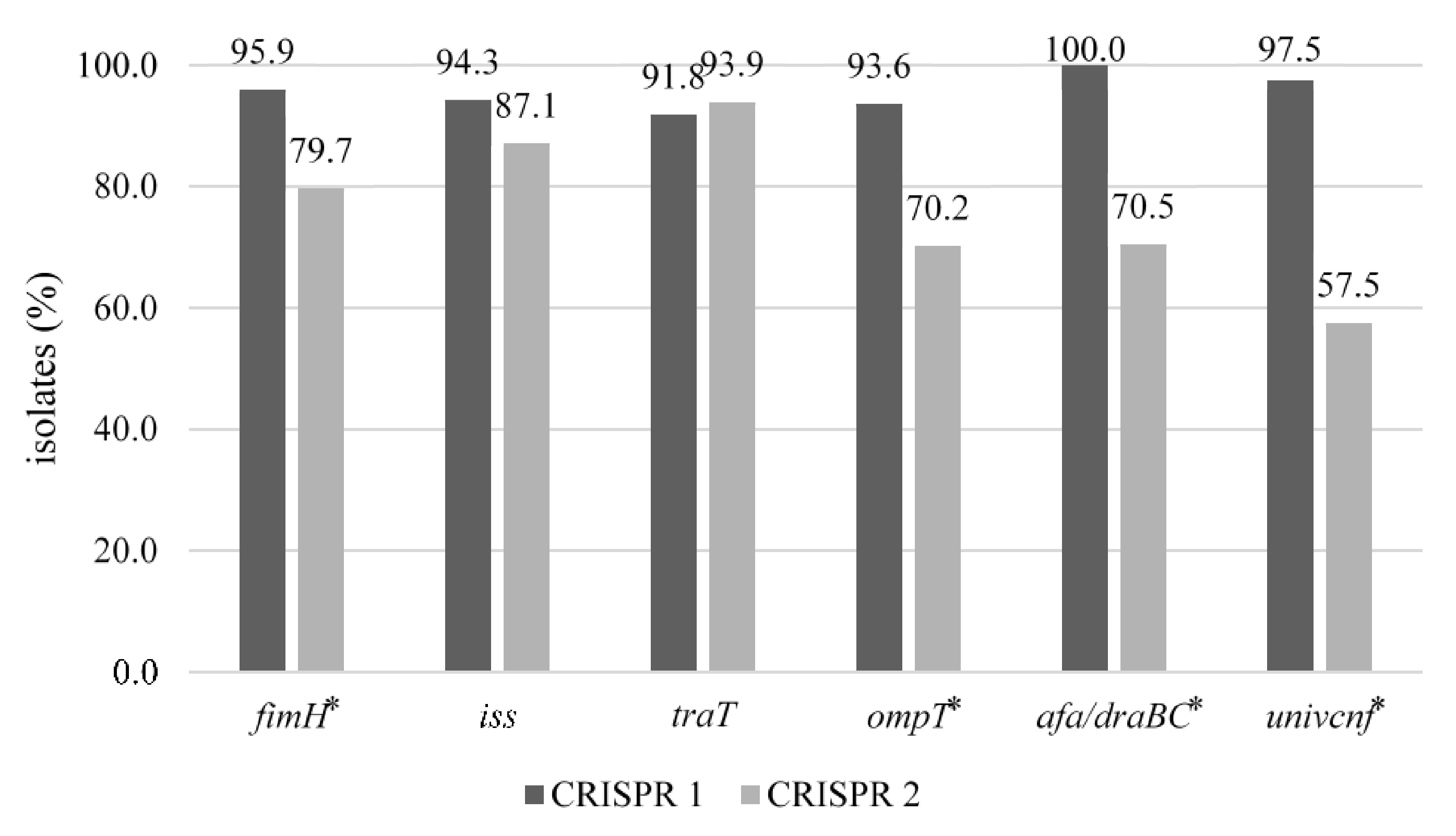
3.4. Distribution of CRISPR 1 and CRISPR 2 by Virulence Genes
3.5. CRISPR-Based Typing of Virulence Gene-Carrying Isolates
3.6. Protospacer Match from Spacer Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Petzl, W.; Zerbe, H.; Günther, J.; Seyfert, H.-M.; Hussen, J.; Schuberth, H.-J. Pathogen-Specific Responses in the Bovine Udder. Models and Immunoprophylactic Concepts. Res. Vet. Sci. 2018, 116, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.T.; Dalanezi, F.M.; de Paula, C.L.; Hernandes, R.T.; Pantoja, J.C.F.; Listoni, F.J.P.; Langoni, H.; Ribeiro, M.G. Putative Virulence Factors of Extra-Intestinal Escherichia coli Isolated from Bovine Mastitis with Different Clinical Scores. Lett. Appl. Microbiol. 2019, 68, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.A.; Grimsrud, A.; Richards, A.C.; Mulvey, M.A.; Wilson, E.; Erickson, D.L. Bile Salts Regulate Zinc Uptake and Capsule Synthesis in a Mastitis-Associated Extraintestinal Pathogenic Escherichia coli Strain. Infect. Immun. 2021, 89, e00357-21. [Google Scholar] [CrossRef]
- Aslam, N.; Khan, S.-U.-H.; Usman, T.; Ali, T. Phylogenetic Genotyping, Virulence Genes and Antimicrobial Susceptibility of Escherichia coli Isolates from Cases of Bovine Mastitis. J. Dairy Res. 2021, 88, 78–79. [Google Scholar] [CrossRef]
- Fernandes, J.B.C.; Zanardo, L.G.; Galvão, N.N.; Carvalho, I.A.; Nero, L.A.; Moreira, M.A.S. Escherichia coli from Clinical Mastitis: Serotypes and Virulence Factors. J. Vet. Diagn. Investig. 2011, 23, 1146–1152. [Google Scholar] [CrossRef] [Green Version]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The Population Genetics of Commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef]
- Alfinete, N.W.; Bolukaoto, J.Y.; Heine, L.; Potgieter, N.; Barnard, T.G. Virulence and Phylogenetic Analysis of Enteric Pathogenic Escherichia coli Isolated from Children with Diarrhoea in South Africa. Int. J. Infect. Dis. 2022, 114, 226–232. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli Phylo-Typing Method Revisited: Improvement of Specificity and Detection of New Phylo-Groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Mora, A.; López, C.; Dabhi, G.; Blanco, M.; Blanco, J.E.; Alonso, M.P.; Herrera, A.; Mamani, R.; Bonacorsi, S.; Moulin-Schouleur, M.; et al. Extraintestinal Pathogenic Escherichia coli O1:K1:H7/NM from Human and Avian Origin: Detection of Clonal Groups B2 ST95 and D ST59 with Different Host Distribution. BMC Microbiol. 2009, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Logue, C.M.; Wannemuehler, Y.; Nicholson, B.A.; Doetkott, C.; Barbieri, N.L.; Nolan, L.K. Comparative Analysis of Phylogenetic Assignment of Human and Avian ExPEC and Fecal Commensal Escherichia coli Using the (Previous and Revised) Clermont Phylogenetic Typing Methods and Its Impact on Avian Pathogenic Escherichia coli (APEC) Classification. Front. Microbiol. 2017, 8, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leimbach, A.; Poehlein, A.; Vollmers, J.; Görlich, D.; Daniel, R.; Dobrindt, U. No Evidence for a Bovine Mastitis Escherichia coli Pathotype. BMC Genom. 2017, 18, 359. [Google Scholar] [CrossRef] [PubMed]
- Bag, M.A.S.; Khan, M.S.R.; Sami, M.D.H.; Begum, F.; Islam, M.S.; Rahman, M.M.; Rahman, M.T.; Hassan, J. Virulence Determinants and Antimicrobial Resistance of E. coli Isolated from Bovine Clinical Mastitis in Some Selected Dairy Farms of Bangladesh. Saudi J. Biol. Sci. 2021, 28, 6317–6323. [Google Scholar] [CrossRef]
- Jung, D.; Park, S.; Ruffini, J.; Dussault, F.; Dufour, S.; Ronholm, J. Comparative Genomic Analysis of Escherichia coli Isolates from Cases of Bovine Clinical Mastitis Identifies Nine Specific Pathotype Marker Genes. Microb. Genom. 2021, 7, 8. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening Sequences of Regularly Spaced Prokaryotic Repeats Derive from Foreign Genetic Elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Hatoum-Aslan, A.; Marraffini, L.A. Impact of CRISPR Immunity on the Emergence and Virulence of Bacterial Pathogens. Curr. Opin. Microbiol. 2014, 17, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Bonomo, M.E.; Deem, M.W. The Physicist’s Guide to One of Biotechnology’s Hottest New Topics: CRISPR-Cas. Phys. Biol. 2018, 15, 041002. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, S.; Kim, Y.B.; Lee, Y.J. Virulence Variation of Salmonella gallinarum Isolates through SpvB by CRISPR Sequence Subtyping, 2014 to 2018. Animals 2020, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Díez-Villaseñor, C.; Almendros, C.; García-Martínez, J.; Mojica, F.J.M. Diversity of CRISPR Loci in Escherichia coli. Microbiology 2010, 156, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Touchon, M.; Rocha, E.P.C. The Small, Slow and Specialized CRISPR and Anti-CRISPR of Escherichia and Salmonella. PLoS ONE 2010, 5, e11126. [Google Scholar] [CrossRef] [Green Version]
- Touchon, M.; Charpentier, S.; Clermont, O.; Rocha, E.P.C.; Denamur, E.; Branger, C. CRISPR Distribution within the Escherichia coli Species Is Not Suggestive of Immunity-Associated Diversifying Selection. J. Bacteriol. 2011, 193, 2460–2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, S.; Jensen, M.A.; Bai, J.; DebRoy, C.; Barrangou, R.; Dudley, E.G. The Evolutionary Divergence of Shiga Toxin-Producing Escherichia coli Is Reflected in Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) Spacer Composition. Appl. Environ. Microbiol. 2013, 79, 5710–5720. [Google Scholar] [CrossRef] [Green Version]
- Toro, M.; Cao, G.; Ju, W.; Allard, M.; Barrangou, R.; Zhao, S.; Brown, E.; Meng, J. Association of Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) Elements with Specific Serotypes and Virulence Potential of Shiga Toxin-Producing Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 1411–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cady, K.C.; White, A.S.; Hammond, J.H.; Abendroth, M.D.; Karthikeyan, R.S.G.; Lalitha, P.; Zegans, M.E.; O’Toole, G.A. Prevalence, Conservation and Functional Analysis of Yersinia and Escherichia CRISPR Regions in Clinical Pseudomonas aeruginosa Isolates. Microbiology 2011, 157, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Food and Drug Safety (MFDS). Processing Standards and Ingredient Specifications for Livestock Products; MFDS: Chongju, Korea, 2018.
- Candrian, U.; Furrer, B.; Höfelein, C.; Meyer, R.; Jermini, M.; Lüthy, J. Detection of Escherichia coli and Identification of Enterotoxigenic Strains by Primer-Directed Enzymatic Amplification of Specific DNA Sequences. Int. J. Food Microbiol. 1991, 12, 339–351. [Google Scholar] [CrossRef]
- Chapman, T.A.; Wu, X.-Y.; Barchia, I.; Bettelheim, K.A.; Driesen, S.; Trott, D.; Wilson, M.; Chin, J.J.-C. Comparison of Virulence Gene Profiles of Escherichia coli Strains Isolated from Healthy and Diarrheic Swine. Appl. Environ. Microbiol. 2006, 72, 4782–4795. [Google Scholar] [CrossRef] [Green Version]
- Grissa, I.; Vergnaud, G.; Pourcel, C. The CRISPRdb Database and Tools to Display CRISPRs and to Generate Dictionaries of Spacers and Repeats. BMC Bioinform. 2007, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Gagnon, J.N.; Brouns, S.J.J.; Fineran, P.C.; Brown, C.M. CRISPRTarget. RNA Biol. 2013, 10, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Kaipainen, T.; Pohjanvirta, T.; Shpigel, N.Y.; Shwimmer, A.; Pyörälä, S.; Pelkonen, S. Virulence Factors of Escherichia coli Isolated from Bovine Clinical Mastitis. Vet. Microbiol. 2002, 85, 37–46. [Google Scholar] [CrossRef]
- Guerra, S.T.; Orsi, H.; Joaquim, S.F.; Guimarães, F.F.; Lopes, B.C.; Dalanezi, F.M.; Leite, D.S.; Langoni, H.; Pantoja, J.C.F.; Rall, V.L.M.; et al. Short Communication: Investigation of Extra-Intestinal Pathogenic Escherichia coli Virulence Genes, Bacterial Motility, and Multidrug Resistance Pattern of Strains Isolated from Dairy Cows with Different Severity Scores of Clinical Mastitis. J. Dairy Sci. 2020, 103, 3606–3614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Z.; Huang, C.; Gao, X.; Wang, Z.; Liu, Y.; Tian, C.; Hong, W.; Niu, S.; Liu, M. The Phylogenetic Group, Antimicrobial Susceptibility, and Virulence Genes of Escherichia coli from Clinical Bovine Mastitis. J. Dairy Sci. 2018, 101, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Connell, I.; Agace, W.; Klemm, P.; Schembri, M.; Marild, S.; Svanborg, C. Type 1 Fimbrial Expression Enhances Escherichia coli Virulence for the Urinary Tract. Proc. Natl. Acad. Sci. USA 1996, 93, 9827–9832. [Google Scholar] [CrossRef] [Green Version]
- Langermann, S.; Palaszynski, S.; Barnhart, M.; Auguste, G.; Pinkner, J.S.; Burlein, J.; Barren, P.; Koenig, S.; Leath, S.; Jones, C.H.; et al. Prevention of Mucosal Escherichia coli Infection by FimH-Adhesin-Based Systemic Vaccination. Science 1997, 276, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Lehtolainen, T.; Pohjanvirta, T.; Pyörälä, S.; Pelkonen, S. Association between Virulence Factors and Clinical Course of Escherichia coli Mastitis. Acta Vet. Scand. 2003, 44, 203–205. [Google Scholar] [CrossRef]
- Wenz, J.R.; Barrington, G.M.; Garry, F.B.; Ellis, R.P.; Magnuson, R.J. Escherichia coli Isolates’ Serotypes, Genotypes, and Virulence Genes and Clinical Coliform Mastitis Severity. J. Dairy Sci. 2006, 89, 3408–3412. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, J.; Muckle, C.A.; Lo, R.Y. Serum Resistance and the TraT Gene in Bovine Mastitis-Causing Escherichia coli. Vet. Microbiol. 1991, 28, 343–351. [Google Scholar] [CrossRef]
- Blum, S.E.; Leitner, G. Genotyping and Virulence Factors Assessment of Bovine Mastitis Escherichia coli. Vet. Microbiol. 2013, 163, 305–312. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular Mechanisms of Escherichia coli Pathogenicity. Nat. Reviews. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, G.; Liu, W.; Liu, Y.; Ali, T.; Chen, W.; Yin, J.; Han, B. Phylogenetic Group, Virulence Factors and Antimicrobial Resistance of Escherichia coli Associated with Bovine Mastitis. Res. Microbiol. 2014, 165, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Müştak, H.K.; Günaydin, E.; Kaya, İ.B.; Salar, M.Ö.; Babacan, O.; Önat, K.; Ata, Z.; Diker, K.S. Phylo-Typing of Clinical Escherichia coli Isolates originating from Bovine Mastitis and Caninepyometra and Urinary Tract Infection by Means Ofquadruplex PCR. Vet. Q. 2015, 35, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ombarak, R.A.; Hinenoya, A.; Awasthi, S.P.; Iguchi, A.; Shima, A.; Elbagory, A.-R.M.; Yamasaki, S. Prevalence and Pathogenic Potential of Escherichia coli Isolates from Raw Milk and Raw Milk Cheese in Egypt. Int. J. Food Microbiol. 2016, 221, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Sashital, D.G. Mechanisms of Type I-E and I-F CRISPR-Cas Systems in Enterobacteriaceae. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, E.; Lopatina, A.; Medvedeva, S.; Kapustin, M.; Shmakov, S.; Tikhonov, A.; Artamonova, I.I.; Logacheva, M.; Severinov, K. Dynamics of Escherichia coli Type I-E CRISPR Spacers over 42,000 Years. Mol. Ecol. 2017, 26, 2019–2026. [Google Scholar] [CrossRef] [Green Version]
- Delannoy, S.; Beutin, L.; Fach, P. Use of Clustered Regularly Interspaced Short Palindromic Repeat Sequence Polymorphisms for Specific Detection of Enterohemorrhagic Escherichia coli Strains of Serotypes O26:H11, O45:H2, O103:H2, O111:H8, O121:H19, O145:H28, and O157:H7 by Real-Time PCR. J. Clin. Microbiol. 2012, 50, 4035–4040. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Nolan, L.K. Pathogenomics of the Virulence Plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. MMBR 2009, 73, 750–774. [Google Scholar] [CrossRef] [Green Version]
- Gyles, C.; Boerlin, P. Horizontally Transferred Genetic Elements and Their Role in Pathogenesis of Bacterial Disease. Vet. Pathol. 2014, 51, 328–340. [Google Scholar] [CrossRef]
- García-Gutiérrez, E.; Almendros, C.; Mojica, F.J.M.; Guzmán, N.M.; García-Martínez, J. CRISPR Content Correlates with the Pathogenic Potential of Escherichia coli. PLoS ONE 2015, 10, e0131935. [Google Scholar] [CrossRef]
- Stern, A.; Keren, L.; Wurtzel, O.; Amitai, G.; Sorek, R. Self-Targeting by CRISPR: Gene Regulation or Autoimmunity? Trends Genet. 2010, 26, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Babu, M.; Beloglazova, N.; Flick, R.; Graham, C.; Skarina, T.; Nocek, B.; Gagarinova, A.; Pogoutse, O.; Brown, G.; Binkowski, A.; et al. A Dual Function of the CRISPR-Cas System in Bacterial Antivirus Immunity and DNA Repair. Mol. Microbiol. 2011, 79, 484–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louwen, R.; Staals, R.H.J.; Endtz, H.P.; van Baarlen, P.; van der Oost, J. The Role of CRISPR-Cas Systems in Virulence of Pathogenic Bacteria. Microbiol. Mol. Biol. Rev. MMBR 2014, 78, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, S.; Personne, Y.; Newire, E.; Laverick, R.; Russell, O.; Roberts, A.P.; Enne, V.I. Presence of Type I-F CRISPR/Cas Systems Is Associated with Antimicrobial Susceptibility in Escherichia coli. J. Antimicrob. Chemother. 2017, 72, 2213–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozic, B.; Repac, J.; Djordjevic, M. Endogenous Gene Regulation as a Predicted Main Function of Type I-E CRISPR/Cas System in E. coli. Molecules 2019, 24, 784. [Google Scholar] [CrossRef] [Green Version]
- Leiman, P.G.; Basler, M.; Ramagopal, U.A.; Bonanno, J.B.; Sauder, J.M.; Pukatzki, S.; Burley, S.K.; Almo, S.C.; Mekalanos, J.J. Type VI Secretion Apparatus and Phage Tail-Associated Protein Complexes Share a Common Evolutionary Origin. Proc. Natl. Acad. Sci. USA 2009, 106, 4154–4159. [Google Scholar] [CrossRef] [Green Version]
- Bingle, L.E.; Bailey, C.M.; Pallen, M.J. Type VI Secretion: A Beginner’s Guide. Curr. Opin. Microbiol. 2008, 11, 3–8. [Google Scholar] [CrossRef] [Green Version]

| Virulence Genes 1 | No (%) of Isolates Included by Company | |||
|---|---|---|---|---|
| A (n 2 = 38) | B (n = 42) | C (n = 103) | Total (n = 183) | |
| fimH | 36 (94.7) a | 41 (100.0) a | 71 (69.0) b | 148 (80.9) A |
| iss | 27 (71.1) a | 14 (34.1) b | 29 (28.2) b | 70 (38.3) B |
| traT | 21 (55.3) a | 12 (29.3) b | 16 (15.5) b | 49 (26.8) B,C |
| ompT | 10 (26.3) | 14 (34.1) | 23 (22.3) | 47 (25.7) B,C |
| afa/draBC | 6 (15.8) | 11 (26.8) | 27 (26.2) | 44 (24.0) B,C |
| univcnf | 6 (15.8) | 15 (36.6) | 19 (18.4) | 40 (21.9) B,C |
| iroN E. coli | 5 (13.2) | 8 (19.5) | 12 (11.7) | 25 (13.7) C,D |
| kpsMT K5 | 2 (5.3) a,b | 7 (17.1) a | 4 (3.9) b | 13 (7.1) D,E |
| fyuA | 5 (13.2) | 3 (7.3) | 4 (3.9) | 12 (6.6) D,E |
| sfaS | 3 (7.9) | 1 (2.4) | 8 (7.8) | 12 (6.6) D,E |
| bmaE | 0 (0.0) | 0 (0.0) | 9 (8.7) | 9 (4.9) D,E |
| cnf1 | 2 (5.3) | 0 (0.0) | 3 (2.9) | 5 (2.7) E |
| cdt | 0 (0.0) | 1 (2.4) | 3 (2.9) | 4 (2.2) E |
| hlyA | 1 (2.6) | 1 (2.4) | 1 (1.0) | 3 (1.6) E |
| cdtB | 0 (0.0) | 0 (0.0) | 2 (1.9) | 2 (1.1) E |
| iutA | 1 (2.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) E |
| papG allele 3 | 1 (2.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) E |
| kpsMT II | 1 (2.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) E |
| Groups | No (%) of Isolates Included by Company | |||
|---|---|---|---|---|
| A (n 1 = 38) | B (n = 41) | C (n = 85) | Total (n = 164) | |
| Phylogenetic groups | ||||
| A | 3 (7.9) b | 13 (31.7) a | 17 (20.0) a,b | 33 (20.1) B |
| B1 | 26 (68.4) | 20 (48.8) | 59 (69.4) | 105 (64.0) A |
| B2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) D |
| C | 1 (2.6) | 5 (12.2) | 6 (7.1) | 12 (7.3) C |
| D | 8 (21.1) a | 3 (7.3) a,b | 3 (3.5) b | 14 (8.5) C |
| E | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) D |
| F | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) D |
| CRISPR loci | ||||
| CRISPR 1 | 37 (97.4) | 38 (92.7) | 82 (96.5) | 157 (95.7) A |
| CRISPR 2 | 33 (86.8) | 31 (75.6) | 58 (68.2) | 122 (74.4) B |
| CRISPR 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) C |
| CRISPR 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) C |
| E. coli Sequence Types | No (%) of Isolates with Virulence Genes | ||||||
|---|---|---|---|---|---|---|---|
| fimH (n 1 = 148) | iss (n = 70) | traT (n = 49) | ompT (n = 47) | afa/darBC (n = 44) | univcnf (n = 40) | Total (n = 164) | |
| EST 1 | 0 (0.0) bB | 2 (2.9) a,b | 0 (0.0) b | 5 (10.6) a,bA | 3 (6.8) a,bB | 9 (22.5) aA | 19 (11.6) C,D |
| EST 2 | 2 (1.4) B | 1 (1.4) | 0 (0.0) | 1 (2.1) B | 0 (0.0) B | 1 (2.5) B | 5 (3.0) E,F |
| EST 3 | 3 (2.0) B | 2 (2.9) | 2 (4.1) | 1 (2.1) B | 0 (0.0) B | 0 (0.0) B | 8 (4.9) E,F |
| EST 4 | 16 (10.8) aA | 2 (2.9) b,c | 0 (0.0) c | 6 (12.8) a,bA | 9 (20.5) a,bA | 5 (12.5) a,bA | 38 (23.2) B |
| EST 5 | 3 (2.0) B | 1 (1.4) | 0 (0.0) | 1 (2.1) B | 0 (0.0) B | 1 (2.5) B | 6 (3.7) E,F |
| EST 6 | 2 (1.4) B | 0 (0.0) | 0 (0.0) | 0 (0.0) B | 0 (0.0) B | 0 (0.0) B | 2 (1.2) F |
| EST 7 | 2 (1.4) B | 0 (0.0) | 1 (2.0) | 0 (0.0) B | 1 (2.3) B | 0 (0.0) B | 4 (2.4) F |
| EST 8 | 3 (2.0) B | 2 (2.9) | 3 (6.1) | 2 (4.3) B | 2 (4.5) B | 0 (0.0) B | 12 (7.3) D,E |
| EST 9 | 3 (2.0) B | 2 (2.9) | 1 (2.0) | 1 (2.1) B | 1 (2.3) B | 0 (0.0) B | 8 (4.9) E,F |
| EST 10 | 7 (4.7) aB | 4 (5.7) a,b | 2 (4.1) a,b | 3 (6.4) a,bB | 0 (0.0) bB | 2 (5.0) a,bB | 18 (11.0) C,D |
| EST 11 | 3 (2.0) B | 3 (4.3) | 2 (4.1) | 0 (0.0) B | 0 (0.0) B | 0 (0.0) B | 8 (4.9) E,F |
| EST 12 | 1 (0.7) B | 0 (0.0) | 1 (2.0) | 0 (0.0) B | 1 (2.3) B | 0 (0.0) B | 3 (1.8) F |
| EST 13 | 7 (4.7) aB | 5 (7.1) a,b | 1 (2.0) a,b | 1 (2.1) a,b B | 1 (2.3) a,b B | 0 (0.0) bB | 15 (9.1) D,E |
| EST 14 | 8 (5.4) aB | 6 (8.6) a,b | 8 (16.3) a | 0 (0.0) bB | 2 (4.5) a,bB | 1 (2.5) a,bB | 25 (15.2) C |
| EST 15 | 2 (1.4) B | 2 (2.9) | 1 (2.0) | 0 (0.0) B | 2 (4.5) B | 0 (0.0) B | 7 (4.3) E,F |
| EST 16 | 8 (5.4) B | 7 (10.0) | 8 (16.3) | 5 (10.6) A | 4 (9.1) B | 1 (2.5) B | 33 (20.1) B |
| EST 17 | 3 (2.0) B | 1 (1.4) | 2 (4.1) | 0 (0.0) B | 0 (0.0) B | 0 (0.0) B | 6 (3.7) E,F |
| EST 18 | 3 (2.0) B | 0 (0.0) | 0 (0.0) | 0 (0.0) B | 0 (0.0) B | 0 (0.0) B | 3 (1.8) F |
| EST 19 | 3 (2.0) B | 3 (4.3) | 0 (0.0) | 0 (0.0) B | 0 (0.0) B | 0 (0.0) B | 6 (3.7) E,F |
| EST 20 | 3 (2.0) B | 0 (0.0) | 3 (6.1) | 0 (0.0) B | 0 (0.0) B | 0 (0.0) B | 6 (3.7) E,F |
| EST 21 | 2 (1.4) B | 2 (2.9) | 0 (0.0) | 1 (2.1) B | 0 (0.0) B | 2 (5.0) B | 7 (4.3) E,F |
| EST 22 | 33 (22.3) aA | 9 (12.9) b | 0 (0.0) c | 10 (21.3) bA | 13 (29.5) bA | 9 (22.5) bA | 74 (45.1) A |
| EST 23 | 7 (4.7) aB | 2 (2.9) a,b | 4 (8.2) a,b | 1 (2.1) a,bB | 1 (2.3) a,bB | 0 (0.0) bB | 15 (9.1) D,E |
| EST 24 | 10 (6.8) aB | 7 (10.0) a,b | 1 (2.0) b | 5 (10.6) a,bA | 0 (0.0) bB | 7 (17.5) a,bA | 30 (18.3) B,C |
| EST 25 | 11 (7.4) aA | 5 (7.1) a,b | 8 (16.3) a,b | 3 (6.4) a,bB | 4 (9.1) a,bB | 1 (2.5) bB | 32 (19.5) B,C |
| EST 26 | 3 (2.0) B | 2 (2.9) | 1 (2.0) | 1 (2.1) B | 0 (0.0) B | 1 (2.5) B | 8 (4.9) E,F |
| CRISPR Array | Name of Protospacer | Sequences (5′ to 3′) | No. (%) of Isolates | Protospacer Match |
|---|---|---|---|---|
| CRISPR 1 | 1 | ACATGAATGTCGGTTCAGACCGTGTTTTTACC | 29 (18.5) | DNA-binding protein |
| TGTACTTACAGCCAAGTCTGGCACAAAAATGG | ||||
| 78 | CCCTCACACCGATTCGCCAAACGGTGGAGAAG | 1 (0.6) | toll/interleukin-1 receptor domain-containing protein | |
| GGGAGTGTGGCTAAGCGGTTTGCCACCTCTTC | ||||
| 81 | TTTTGCTGACACCGGCAATACTGAACGGCTGG | 11 (7.0) | DNA-cytosine methyltransferase | |
| AAAACGACTGTGGCCGTTATGACTTGCCGACC | ||||
| 107 | GCTGGTGGCGCGGGCAAACGGAACAATCCCGC | 1 (0.6) | darB, helicase | |
| CGACCACCGCGCCCGTTTGCCTTGTTAGGGCG | ||||
| 117 | AAACAGATTGTTCGTTTTCCCCATATTCATGA | 12 (7.6) | DUF1380 domain-containing protein | |
| TTTGTCTAACAAGCAAAAGGGGTATAAGTACT | ||||
| 162 | AGTATTAACTGCGGTGGCAGTGAGGCCAATAG | 1 (0.6) | Head decoration protein, Viral protein | |
| TCATAATTGACGCCACCGTCACTCCGGTTATC | ||||
| 163 | GTTGCCCCCCAAAATCATTAAATCCCCGGCGG | 32 (20.4) | tail associated lysozyme | |
| CAACGGGGGGTTTTAGTAATTTAGGGGCCGCC | ||||
| CRISPR 2 | 47 | GAAAAATGCATACGATTCGAGCACCAGTTTGGC | 1 (0.8) | DUF1281 domain-containing protein |
| CTTTTTACGTATGCTAAGCTCGTGGTCAAACCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.-J.; Lee, Y.-J. Distribution of CRISPR in Escherichia coli Isolated from Bulk Tank Milk and Its Potential Relationship with Virulence. Animals 2022, 12, 503. https://doi.org/10.3390/ani12040503
Kang H-J, Lee Y-J. Distribution of CRISPR in Escherichia coli Isolated from Bulk Tank Milk and Its Potential Relationship with Virulence. Animals. 2022; 12(4):503. https://doi.org/10.3390/ani12040503
Chicago/Turabian StyleKang, Hyo-Jung, and Young-Ju Lee. 2022. "Distribution of CRISPR in Escherichia coli Isolated from Bulk Tank Milk and Its Potential Relationship with Virulence" Animals 12, no. 4: 503. https://doi.org/10.3390/ani12040503






