“Move or Not to Move”—Red Deer Stags Movement Activity during the Rut
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Capturing
2.3. GPS Data Processing
2.4. Establishing the Time of Conception
2.5. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates, and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Burbaité, L.; Csányi, S. Red deer population and harvest changes in Europe. Acta Zool. Litu. 2010, 20, 179–199. [Google Scholar] [CrossRef] [Green Version]
- Borowik, T.; Wawrzyniak, P.; Jedrzejewska, B. Red deer (Cervus elaphus) fertility and survival of young in a low-density population subject to predation and hunting. J. Mammal. 2016, 97, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Clutton-Brock, T.H. Review Lecture: Mammalian mating system. Proc. R. Soc. Lond. B 1989, 236, 339–372. [Google Scholar] [CrossRef] [PubMed]
- Bonenfant, C.; Gaillard, J.M.; Klein, F.; Maillard, D.M. Variation in harem size of red deer (Cervus elaphus L.): The effects of adult sex ratio and age-structure. J. Zool. 2004, 264, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Carranza, J.; Valencia, J. Red deer females collect on male clumps at mating areas. Behav. Ecol. 1999, 10, 525–532. [Google Scholar] [CrossRef]
- Allen, A.M.; Singh, N.J. Linking Movement Ecology with Wildlife Management and Conservation. Front. Ecol. Evol. 2016, 3, 155. [Google Scholar] [CrossRef] [Green Version]
- Vervaecke, H.; Roden, C.; De Vries, H. Dominance, fatness and fitness in female American bison. Bison Bison. Anim. Behav. 2005, 70, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.A.; Thomas, L.; Wilcox, C.; Ovaskainen, O.; Matthiopoulos, J. State-space models of individual animal movement. Trends Ecol. Evol. 2008, 23, 87–94. [Google Scholar] [CrossRef]
- Reinecke, H.; Leinen, L.; Thihen, I.; Meihner, M.; Herzog, S.; Schütz, S.; Kiffner, C. Home range size estimates of red deer in Germany: Environmental, individual, and methodological correlates. Eur. J. Wildl. Res. 2014, 60, 237–247. [Google Scholar] [CrossRef]
- Apollonio, M.; Merli, E.; Chirichella, R.; Pokorny, B.; Alagić, A.; Flajšman, K.; Stephens, P.A. Capital-income breeding in male ungulates: Causes and consequences of strategy differences among species. Front. Ecol. Evol. 2020, 8, 521–767. [Google Scholar] [CrossRef]
- Mysterud, A.; Bonenfant, C.; Loe, L.E.; Langvatn, R.; Yoccoz, N.G.; Stenseth, N.C. Age-specific feeding cessation in male red deer during rut. J. Zool. 2008, 275, 407–412. [Google Scholar] [CrossRef]
- Perez-Espona, S.; Pérez-Barbería, F.J.; Goodall-Copestake, W.; Gordon, I.J.; Pemberton, J.M. Genetic diversity and population structure of Scottish Highland red deer (Cervus elaphus) populations: A mitochondrial survey. Heredity 2008, 102, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Wolff, J.O. Breeding strategies, mate choice and reproductive success in American bison. Oikos 1998, 83, 529–544. [Google Scholar] [CrossRef]
- Coté, S.D.; Festa-Bianchet, M. Reproductive success in female mountain goats: The influence of age and social rank. Anim. Behav. 2001, 62, 173–181. [Google Scholar] [CrossRef]
- Gibson, R.M.; Guinness, F.E. Behavioural factors affecting male reproductive success in red deer (Cervus elaphus). Anim. Behav. 1980, 28, 1163–1174. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Rose, K.E.; Guinness, F.E. Density related changes in sexual selection in red deer. Proc. R. Soc. Lond. B 1997, 264, 1509–1516. [Google Scholar] [CrossRef] [Green Version]
- Davies, N.B. Mating systems. In Behavioural Ecology: An Evolutionary Approach 3; Blackwell Scientific Publications: Oxford, UK, 1991; pp. 263–294. [Google Scholar]
- Lincoln, G.A.; Guinness, F.E. The sexual significance of the rut in red deer. J. Reprod. Fertil. Suppl. 1973, 19, 475–489. [Google Scholar]
- Brennan, P.L.R. Sexual Selection. Nat. Educ. Knowl. 2010, 3, 79. [Google Scholar]
- Guinness, F.E.; Lincoln, G.A.; Short, R.V. The reproductive cycle of the female red deer (Cervus elaphus). J. Reprod. Fert. 1971, 27, 427–438. [Google Scholar] [CrossRef]
- Scott, I.C.; Asher, G.W.; Archer, J.A.; Littlejohn, R.P. The effect of conception date on gestation length of red deer (Cervus elaphus). Anim. Reprod. Sci. 2008, 109, 206–217. [Google Scholar] [CrossRef]
- Gaillard, J.M.; Festa-Bianchet, M.; Yoccoz, N.G.; Loison, A.; Toígo, C. Temporal Variation in Fitness Components and Population Dynamics of Large Herbivores. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 367–393. [Google Scholar] [CrossRef]
- Morano, S.; Stewart, K.M.; Sedinger, J.S.; Nicolai, C.A.; Vavra, M. Life-history strategies of North American elk: Trade-offs associated with reproduction and survival. J. Mammal. 2013, 94, 162–172. [Google Scholar] [CrossRef]
- Asher, G.W. Reproductive cycles in female cervids. In Current Therapy in Large Animal Theriogenology, 2nd ed.; Saunders: Philadelphia, PA, USA, 2007; pp. 921–931. [Google Scholar]
- Iason, G.R.; Guinness, F.E. Synchrony of oestrus and conception in red deer (Cervus elaphus L.). Anim. Behav. 1985, 33, 1169–1174. [Google Scholar] [CrossRef]
- Langvatn, R.; Mysterud, A.; Stenseth, N.C.; Yoccoz, N.G. Timing and synchrony of ovulation in red deer constrained by short northern summers. Am. Nat. 2004, 163, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clutton-Brock, T.H.; Guinness, F.E.; Albon, S.D. Red Deer: Behaviour and Ecology of Two Sexes; University of Chicago Press: Chicago, IL, USA, 1982. [Google Scholar]
- Náhlik, A.; Sándor, G.; Tari, T.; Király, G. Space use and activity patterns of red deer in a highly forested and in a patchy forest-agricultural habitat. Acta Silv. Lignaria Hung. 2009, 5, 109–118. [Google Scholar]
- Pépin, D.; Morellet, N.; Goulard, M. Seasonal and daily walking activity patterns of free ranging adult red deer (Cervus elaphus) at the individual level. Eur. J. Wildl. Res. 2009, 55, 479–486. [Google Scholar] [CrossRef]
- Georgii, B. Activity patterns of female red deer (Cervus elaphus L.) in the Alps. Oecologia 1981, 49, 127–136. [Google Scholar] [CrossRef]
- Ruckstuhl, K.E.; Neuhaus, P. Sexual Segregation in Ungulates: A New Approach. Behaviour 2000, 137, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Jarnemo, A. Seasonal migration of male red deer (Cervus elaphus) in southern Sweden and consequences for management. Eur. J. Wildl. Res. 2008, 54, 327–333. [Google Scholar] [CrossRef]
- Georgii, B.; Schröder, W. Home Range and Activity Patterns of Male Red Deer (Cervus elaphus L.) in the Alps. Oecologia 1983, 58, 238–248. [Google Scholar] [CrossRef]
- Carranza, J.; Alvarez, F.; Redondo, T. Territoriality as a mating strategy in red deer. Anim. Behav. 1990, 40, 79–88. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Albon, S.D.; Gibson, R.M.; Guinness, F.E. The logical stag: Adaptive aspects of fighting behaviour in red (Cervus elaphus). Anim. Behav. 1979, 27, 211–225. [Google Scholar] [CrossRef]
- Haugen, A.O. Breeding records of captive white-tailed deer in Alabama. J. Mammal. 1959, 40, 108–113. [Google Scholar] [CrossRef]
- Szegvári, Z.; Nagy, G., VI. Dél-Dunántúl Zöld Szigetei. In Proceedings of the Konferencia a Boronka-melléki kirándulások, Pécs, Hungary, 10 October 2013. [Google Scholar]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: www.r-project.org (accessed on 30 December 2021).
- Worton, B.J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Calenge, C. The package "adehabitat" for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Modell. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Kie, J.G. A rule-based ad hoc method for selecting a bandwidth in kernel home-range analyses. Anim. Biotelemetry. 2013, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Gitzen, R.A.; Millspaugh, J.J.; Kernohan, B.J. Bandwidth Selection for Fixed-Kernel Analysis of Animal Utilization Distributions. J. Wildl. Manag. 2006, 70, 1334–1344. [Google Scholar] [CrossRef]
- Jacques, C.N.; Jenks, J.A.; Klaver, R.W. Seasonal Movements and Home-Range Use by Female Pronghorns in Sagebrush-Steppe Communities of Western South Dakota. J. Mammal. 2009, 90, 433–441. [Google Scholar] [CrossRef]
- Bertrand, M.R.; DeNicola, A.J.; Beissinger, S.R.; Swihart, R.K. Effects of parturition on home ranges and social affiliations of female white-tailed deer. J. Wildl. Manag. 1996, 60, 899–909. [Google Scholar] [CrossRef]
- Ciuti, S.; Muhly, T.B.; Paton, D.G.; McDevitt, A.D.; Musiani, M.; Boyce, M.S. Human selection of elk behavioural traits in a landscape of fear. Proc. R. Soc. Lond. B 2012, 279, 4407–4416. [Google Scholar] [CrossRef] [Green Version]
- Hooge, P.N.; Eichenlaub, B. Animal Movement Extension to ArcView; Version 2.04; Geological Survey; Alaska Science Center—Biological Science Office: Reston, VA, USA, 2000. [Google Scholar]
- Huggett, A.S.G.; Widdas, W.F. The relationship between mammalian foetal weight and conception age. J. Physiol. 1951, 114, 306–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, J.A.; Trainer, C.E.; Wright, P.L. Breeding season in elk as determined from known-age embryos. J. Wildl. Manag. 1959, 23, 27–34. [Google Scholar] [CrossRef]
- Mitchell, B.; Lincoln, G.A. Conception dates in relation to age and condition in two populations of red deer in Scotland. J. Zool. 1973, 171, 141–152. [Google Scholar] [CrossRef]
- Sándor, G.; László, R.; Náhlik, A. Determination of time of conception of fallow deer in a Hungarian free range habitat. Folia Zool. 2014, 63, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, Y.; Matsuura, Y.; Suzuki, M.; Saga, S.; Okuyama, H.; Fukui, D.; Bandou, G.; Katagiri, S.; Takahashi, Y.; Tsubota, T. Fetal Age Estimation of Hokkaido Sika Deer (Cervus nippon yesoensis) Using Ultrasonography During Early Pregnancy. J. Reprod. Dev. 2008, 55, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomé, H. Vergleichend-anatomische Untersuchungen der prae- und postnatalen Entwicklung und der funktionellen Veranderungen des Uterus von Rotwild, (Cervus elaphus Linné, 1758) sowie Altersberechnungen an Feten dieser Art; Enke: Stuttgart, Germany, 1980. [Google Scholar]
- Sugár, L.; Horn, A. The fertility (pregnancy) rate and the time of conception in red deer populations in Hungary. In Proceedings of the CIC Rotwild-Symposium, Graz, Austria, 19–22 June 1986. [Google Scholar]
- Prell, H. Tragzeiten von Cerviden. Zoolog. Gart. 1939, 11, 182–186. [Google Scholar]
- Heck, L. Die Rothirsche. In Grzimeks Tierleben, Bd. XIII, Saugetiere 4; Verlag Kindler: Zürich, Germany, 1968. [Google Scholar]
- Clements, M.N.; Clutton-Brock, T.H.; Guinness, F.E.; Pemberton, J.M.; Kruuk, L.E.B. Variances and Covariances of Phenological Traits in A Wild Mammal Population. Evolution 2010, 65, 788–801. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.T.; Ryan, P.D. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Loe, L.E.; Bonenfant, C.; Mysterud, A.; Gaillard, J.M.; Langvatn, R.; Klein, F.; Calenge, C.; Ergon, T.; Pettorelli, N.; Stenseth, N.C. Climate predictability and breeding phenology in red deer: Timing and synchrony of rutting and calving in Norway and France. J. Anim. Ecol. 2005, 74, 579–588. [Google Scholar] [CrossRef]
- Mysterud, A.; Coulson, T.; Stenseth, N.C. The role of males in the dynamics of ungulate populations. J. Anim. Ecol. 2002, 71, 907–915. [Google Scholar] [CrossRef]
- Coulson, T.; Kruuk, L.E.B.; Tavecchia, G.; Pemberton, J.M.; Clutton-Brock, T.H. Estimating selection on neonatal traits in red deer using elasticity path analysis. Evolution. 2003, 57, 2879–2892. [Google Scholar] [CrossRef] [PubMed]
- Pélabon, C.; Komers, P.E.; Hoglund, J. Do leks limit the frequency of aggressive encounters in fallow deer? Linking local male density and lek occurrence. Can. J. Zool. 1999, 77, 667–670. [Google Scholar] [CrossRef]
- Mysterud, A.; Holand, Ø.; Røed, K.H.; Gjøstein, H.; Kumpula, J.; Nieminen, M. Effects of age, density, and sex ratio on reproductive effort in male reindeer (Rangifer tarandus). J. Zool. 2003, 261, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Stopher, K.V.; Nussey, D.H.; Clutton-Brock, T.H.; Guinness, F.; Morris, A.; Pemberton, J.M. The red deer rut revisited: Female excursions but no evidence females move to mate with preferred males. Behav. Ecol. 2011, 22, 808–818. [Google Scholar] [CrossRef]
- Bowyer, R.; McCullough, D.; Rachlow, J.; Ciuti, S.; Whiting, J. Evolution of ungulate mating systems: Integrating social and environmental factors. Ecol. Evol. 2020, 10, 5160–5178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarnemo, A. Male red deer (Cervus elaphus) dispersal during the breeding season. J. Ethol. 2011, 29, 329–336. [Google Scholar] [CrossRef]
- Jarnemo, A.; Jansson, G.; Mansson, J. Temporal variations in activity patterns during rut-implications for survey techniques of red deer (Cervus elaphus). Wildl. Res. 2017, 44, 106–113. [Google Scholar] [CrossRef]
- Evans, R.M. The relationship between parental input and investment. Anim. Behav. 1990, 39, 797–798. [Google Scholar] [CrossRef]
- Jönsson, K.I. Capital and Income Breeding as Alternative Tactics of Resource Use in Reproduction. Oikos 1997, 78, 57–66. [Google Scholar] [CrossRef]
- Singh, N.J.; Milner-Gulland, E.J. Conserving a moving target: Planning protection for a migratory species as its distribution changes. J. Appl. Ecol. 2011, 48, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Meisingset, E.L.; Loe, L.E.; Brekkum, Ø.; Bischof, R.; Rivrud, I.M.; Lande, U.S.; Zimmermann, B.; Veiberg, V.; Mysterud, A. Spatial mismatch between management units and movement ecology of a partially migratory ungulate. J. Appl. Ecol. 2018, 55, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Hagen, R.; Haydn, A.; Suchant, R. Estimating red deer (Cervus elaphus) population size in the Southern Black Forest: The role of hunting in population control. Eur. J. Wildl. Res. 2018, 64, 42. [Google Scholar] [CrossRef]

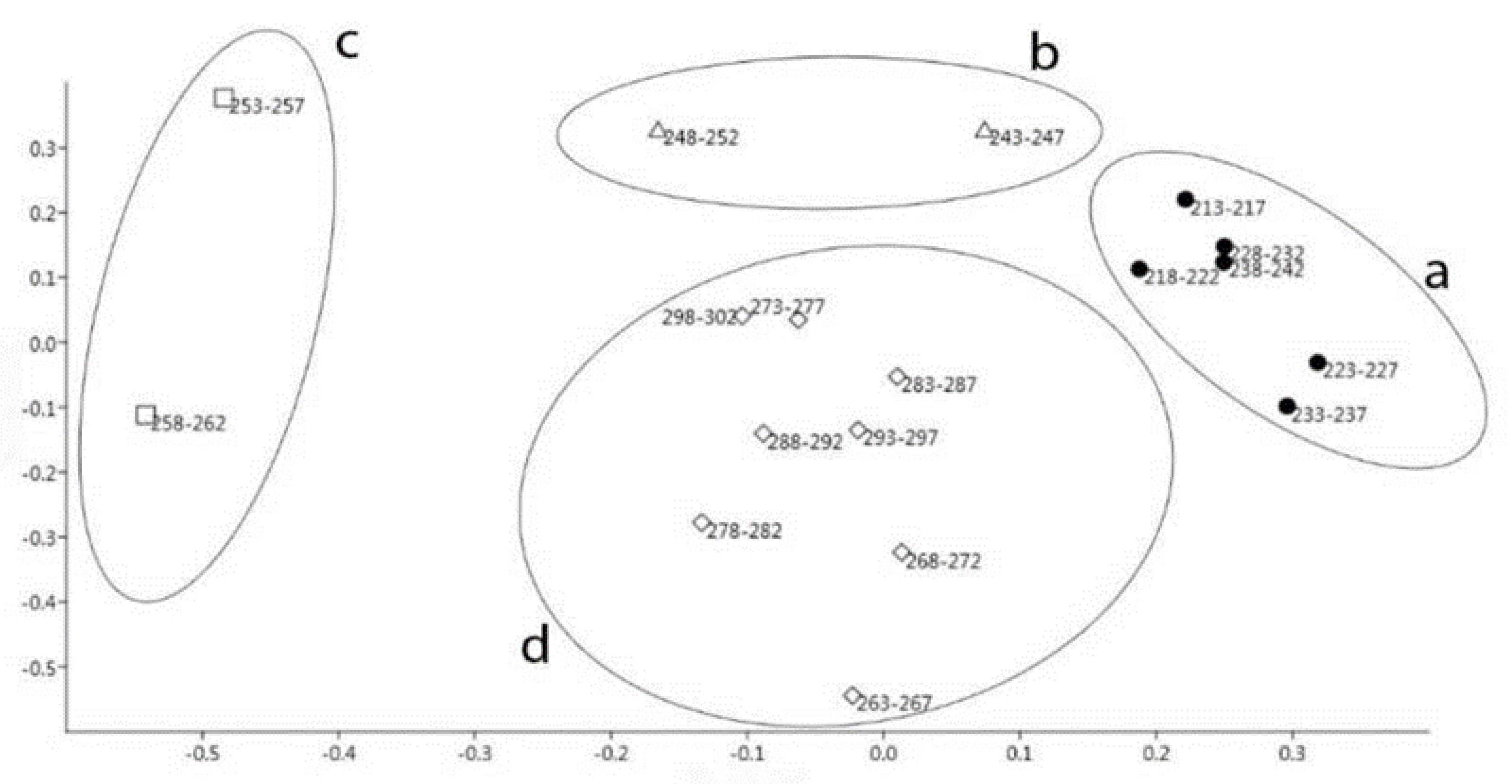

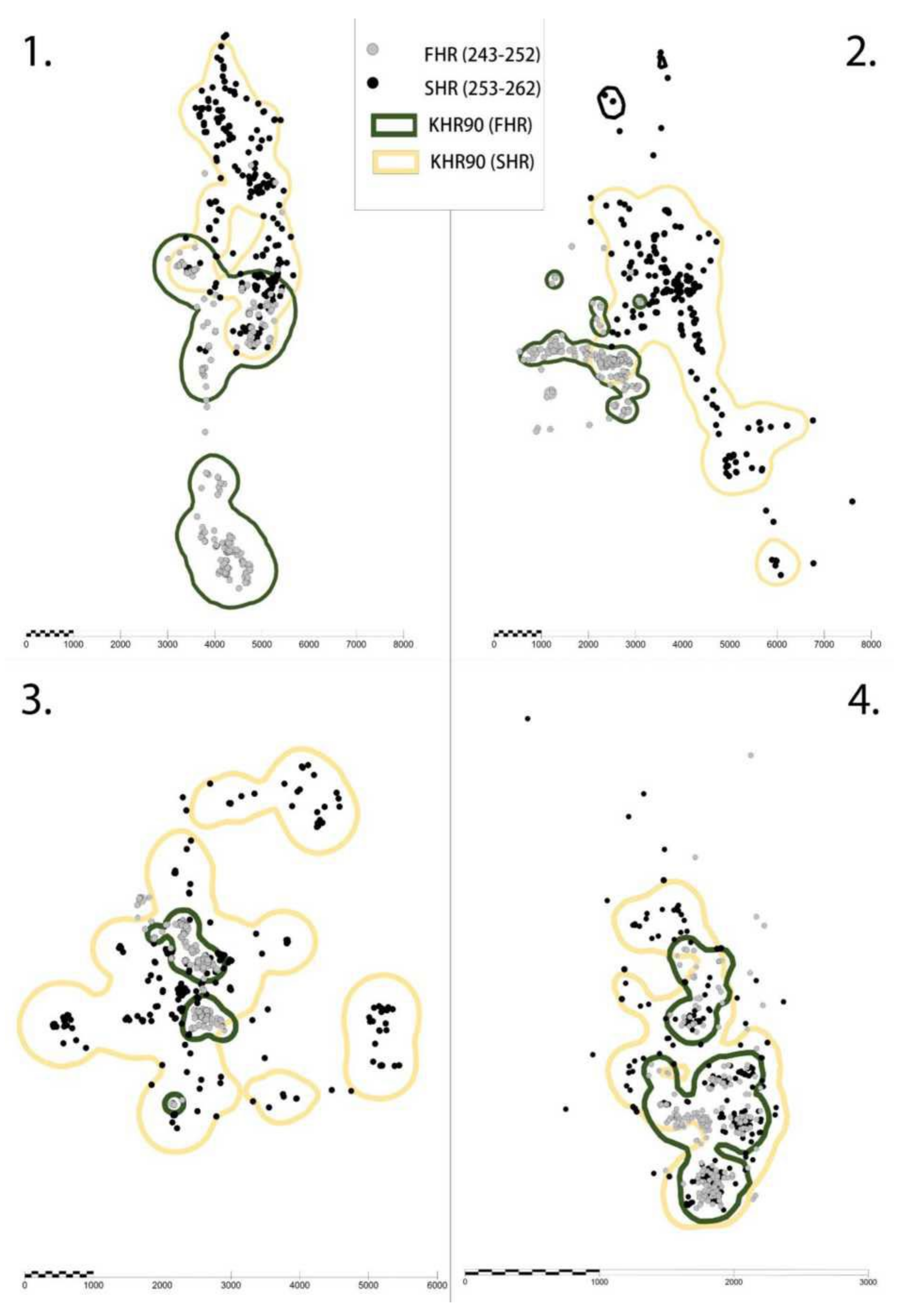
| Average Daily Activity (Meter) | Tukey’s HSD Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BR | FHR | SHR | AR | ||||||||
| Before Rut (BR) | 2403 | BR | * p ≤ 0.001 | * p ≤ 0.001 | * p ≤ 0.001 | ||||||
| First Half of Rut (FHR) | 4870 | FHR | 7.379 | * p ≤ 0.001 | p = 0.983 | ||||||
| Second Half of Rut (SHR) | 6638 | SHR | 18.55 | 9.123 | * p ≤ 0.001 | ||||||
| After the Rut (AR) | 3972 | AR | 10.4 | 0.5199 | 12.06 | ||||||
| Conception Index | χ2 Test Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BR | FHR | SHR | AR | ||||||||
| Before Rut (BR) | 0.47 | BR | *p ≤ 0.001 | p = 0.692 | p = 0.664 | ||||||
| First Half of Rut (FHR) | 4.1 | FHR | 19.822 | *p ≤ 0.001 | p = 0.307 | ||||||
| Second Half of Rut (SHR) | 1.7 | SHR | 0.356 | 15.264 | *p ≤ 0.001 | ||||||
| After the Rut (AR) | 0.28 | AR | 0.424 | 1.546 | 25.2 | ||||||
| Average Movement Range Kernel Home Range 90 (ha) | Tukey’s HSD Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BR | FHR | SHR | AR | ||||||||
| Before Rut (BR) | 67.7 | BR | p = 0.708 | * p ≤ 0.001 | * p = 0.022 | ||||||
| First Half of Rut (FHR) | 176.7 | FHR | 1.516 | * p ≤ 0.001 | p = 0.802 | ||||||
| Second Half of Rut (SHR) | 757.4 | SHR | 9.597 | 6.598 | * p ≤ 0.001 | ||||||
| After the Rut (AR) | 265.8 | AR | 4.167 | 1.281 | 7.065 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csányi, E.; Tari, T.; Németh, S.; Sándor, G. “Move or Not to Move”—Red Deer Stags Movement Activity during the Rut. Animals 2022, 12, 591. https://doi.org/10.3390/ani12050591
Csányi E, Tari T, Németh S, Sándor G. “Move or Not to Move”—Red Deer Stags Movement Activity during the Rut. Animals. 2022; 12(5):591. https://doi.org/10.3390/ani12050591
Chicago/Turabian StyleCsányi, Erika, Tamás Tari, Sándor Németh, and Gyula Sándor. 2022. "“Move or Not to Move”—Red Deer Stags Movement Activity during the Rut" Animals 12, no. 5: 591. https://doi.org/10.3390/ani12050591
APA StyleCsányi, E., Tari, T., Németh, S., & Sándor, G. (2022). “Move or Not to Move”—Red Deer Stags Movement Activity during the Rut. Animals, 12(5), 591. https://doi.org/10.3390/ani12050591






