The Interaction of NO and H2S in Boar Spermatozoa under Oxidative Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Experimental Design
2.2. Statistical Analysis
3. Results
3.1. Sperm Motility
3.2. Plasma Membrane Integrity and Lipid Peroxidation
3.3. Acrosome Integrity
3.4. Total Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buzadzic, B.; Vucetic, M.; Jankovic, A.; Stancic, A.; Korac, A.; Korac, B.; Otasevic, V. New insights into male (in)fertility: The importance of NO. Br. J. Pharmacol. 2015, 172, 1455–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toor, J.S.; Sikka, S.C. Human Spermatozoa and Interactions with Oxidative Stress; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 2, ISBN 9780128125014. [Google Scholar]
- Di Villa Bianca, R.D.E.; Sorrentino, R.; Maffia, P.; Mirone, V.; Imbimbo, C.; Fusco, F.; De Palma, R.; Ignarro, L.J.; Cirino, G. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc. Natl. Acad. Sci. USA. 2009, 106, 4513–4518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintus, E.; Jovičić, M.; Kadlec, M.; Ros-Santaella, J.L. Divergent effect of fast- and slow-releasing H2S donors on boar spermatozoa under oxidative stress. Sci. Rep. 2020, 10, 6508. [Google Scholar] [CrossRef]
- Kadlec, M.; Ros-Santaella, J.L.; Pintus, E. The roles of no and h2s in sperm biology: Recent advances and new perspectives. Int. J. Mol. Sci. 2020, 21, 2174. [Google Scholar] [CrossRef] [Green Version]
- Otasevic, V.; Stancic, A.; Korac, A.; Jankovic, A.; Korac, B. Reactive oxygen, nitrogen, and sulfur species in human male fertility. A crossroad of cellular signaling and pathology. BioFactors 2020, 46, 206–219. [Google Scholar] [CrossRef]
- Lee, N.P.Y.; Cheng, C.Y. Nitric oxide and cyclic nucleotides: Their roles in junction dynamics and spermatogenesis. Oxid. Med. Cell. Longev. 2008, 1, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R. Gasotransmitters: Growing pains and joys. Trends Biochem. Sci. 2014, 39, 227–232. [Google Scholar] [CrossRef]
- Bahmanzadeh, M.; Abolhassani, F.; Amidi, F.; Ejtemaiemehr, S.H.; Salehi, M.; Abbasi, M. The effects of nitric oxide synthase inhibitor (L-NAME) on epididymal sperm count, motility, and morphology in varicocelized rat. Mol. Cell. Neurosci. 2008, 16, 23–38. [Google Scholar]
- Türkyilmaz, Z.; Gülen, Ş.; Sönmez, K.; Karabulut, R.; Dinçer, S.; Başaklar, A.C.; Kale, N. Increased nitric oxide is accompanied by lipid oxidation in adolescent varicocele. Int. J. Androl. 2004, 27, 183–187. [Google Scholar] [CrossRef]
- Hellstrom, W.J.G.; Bell, M.; Wang, R.; Sikka, S.C. Effect of sodium nitroprusside on sperm motility, viability, and lipid peroxidation. Fertil. Steril. 1994, 61, 1117–1122. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Li, S.; Han, Y.; Zhang, P.; Meng, G.; Xiao, Y.; Xie, L.; Wang, X.; Sha, J.; et al. Hydrogen Sulfide As a Potential Target in Preventing Spermatogenic Failure and Testicular Dysfunction. Antioxid. Redox Signal. 2018, 28, 1447–1462. [Google Scholar] [CrossRef]
- Xia, Y.; Ning, J.; Cheng, F.; Yu, W.; Rao, T.; Ruan, Y.; Yuan, R.; Du, Y. GYY4137 a H2S donor, attenuates ipsilateral epididymis injury in experimentally varicocele-induced rats via activation of the PI3K/Akt pathway. Iran. J. Basic Med. Sci. 2019, 22, 729–735. [Google Scholar] [CrossRef]
- Li, G.; Xie, Z.Z.; Chua, J.M.W.; Wong, P.C.; Bian, J. Hydrogen sulfide protects testicular germ cells against heat-induced injury. Nitric Oxide-Biol. Chem. 2015, 46, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Whiteman, M. Hydrogen sulfide signaling: Interactions with nitric oxide and reactive oxygen species. Ann. N. Y. Acad. Sci. 2016, 1365, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function-in sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in Spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, P.; Hao, Y.; Yu, S.; Min, L.; Li, L.; Ma, D.; Chen, L.; Yi, B.; et al. Decrease in male mouse fertility by hydrogen sulfide and/or ammonia can Be inheritable. Chemosphere 2018, 194, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribe, P.; Boguen, R.; Treulen, F.; Sänchez, R.; Villegas, J.V. Peroxynitrite-mediated nitrosative stress decreases motility and mitochondrial membrane potential in human spermatozoa. Mol. Hum. Reprod. 2014, 21, 237–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, R.; Garrido, N.; Céspedes, J.A.; González-Fernández, L.; García-Marín, L.J.; Bragado, M.J. Molecular Mechanisms Involved in the Impairment of Boar Sperm Motility by Peroxynitrite-Induced Nitrosative Stress. Int. J. Mol. Sci. 2020, 21, 1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirino, G.; Vellecco, V.; Bucci, M. Nitric oxide and hydrogen sulfide: The gasotransmitter paradigm of the vascular system. Br. J. Pharmacol. 2017, 174, 4021–4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panthi, S.; Manandhar, S.; Gautam, K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl. Neurodegener. 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Ianaro, A.; de Nucci, G. Gaseous Mediators in Gastrointestinal Mucosal Defense and Injury. Dig. Dis. Sci. 2017, 62, 2223–2230. [Google Scholar] [CrossRef] [Green Version]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.X.; et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef] [Green Version]
- Staicu, F.D.; Lopez-Úbeda, R.; Romero-Aguirregomezcorta, J.; Martínez-Soto, J.C.; Matás Parra, C. Regulation of boar sperm functionality by the nitric oxide synthase/nitric oxide system. J. Assist. Reprod. Genet. 2019, 36, 1721–1736. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.P.Y.; Cheng, C.Y. Nitric Oxide/Nitric Oxide Synthase, Spermatogenesis, and Tight Junction Dynamics. Biol. Reprod. 2004, 70, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangwar, D.K.; Atreja, S.K. Signalling Events and Associated Pathways Related to the Mammalian Sperm Capacitation. Reprod. Domest. Anim. 2015, 50, 705–711. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Yuan, S.; Kevil, C.G. Gasotransmitter heterocellular signaling. Antioxid. Redox Signal. 2017, 26, 936–960. [Google Scholar] [CrossRef]
- Ivanovic-Burmazovic, I.; Filipovic, M.R. Saying NO to H2S: A Story of HNO, HSNO, and SSNO. Inorg. Chem. 2019, 58, 4039–4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; De La Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.L.; Lumsden, N.G.; Farry, J.; Jefferis, A.M.; Kemp-Harper, B.K.; Chin-Dusting, J.P.F. Nitroxyl: A vasodilator of human vessels that is not susceptible to tolerance. Clin. Sci. 2015, 129, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.E.; Shinyashiki, M.; Han, T.H.; Fukuto, J.M. Antioxidant actions of nitroxyl (HNO). Free Radic. Biol. Med. 2007, 42, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Nagpure, B.V.; Bian, J.S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell. Longev. 2016, 2016, 6904327. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, M.; Fukuda, R.; Bateman, R.M.; Yamamoto, T.; Suematsu, M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S Gas Biology. Antioxid. Redox Signal. 2010, 13, 157–192. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R.A.P.; Vickers, S.E. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil. 1990, 88, 343–352. [Google Scholar] [CrossRef]
- García-Vázquez, F.A.; Hernández-Caravaca, I.; Yánez-Quintana, W.; Matás, C.; Soriano-Úbeda, C.; Izquierdo-Rico, M.J. Morphometry of boar sperm head and flagellum in semen backflow after insemination. Theriogenology 2015, 84, 566–574. [Google Scholar] [CrossRef]
- Brzezinska-Slebodzinska, E.; Slebodzinska, A.B.; B, P.; Wieczorek, G. Antioxidant effect of vitamin E and glutathione on lipid peroxidation in boar semen plasma. Biol. Trace Elem. Res. 1995, 47, 69–74. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Yong, Q.C.; Hu, L.F.; Wang, S.; Huang, D.; Bian, J.S. Hydrogen sulfide interacts with nitric oxide in the heart: Possible involvement of nitroxyl. Cardiovasc. Res. 2010, 88, 482–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, Q.C.; Cheong, J.L.; Hua, F.; Deng, L.W.; Khoo, Y.M.; Lee, H.S.; Perry, A.; Wood, M.; Whiteman, M.; Bian, J.S. Regulation of heart function by endogenous gaseous mediators-crosstalk between nitric oxide and hydrogen sulfide. Antioxid. Redox Signal. 2011, 14, 2081–2091. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, M.; Pintus, E.; Fenclová, T.; Imoník, O.S.; Chmelíková, E.; Ros-Santaella, J.L.; Sedmíková, M. Effect of nitric oxide on boar sperm motility, membrane integrity, and acrosomal status during semen storage. Pol. J. Vet. Sci. 2018, 21, 73–82. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Awda, B.J.; Mackenzie-bell, M.; Buhr, M.M. Reactive Oxygen Species and Boar Sperm Function. Biol. Reprod. 2009, 81, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide-Biol. Chem. 2015, 46, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Schulze, M.; Ruediger, K.; Mueller, K.; Jung, M.; Well, C.; Reissmann, M. Development of an in vitro index to characterize fertilizing capacity of boar ejaculates. Anim. Reprod. Sci. 2013, 140, 70–76. [Google Scholar] [CrossRef]
- Sutkeviciene, N.; Riskeviciene, V.; Januskauskas, A.; Zilinskas, H.; Andersson, M. Assessment of sperm quality traits in relation to fertility in boar semen. Acta Vet. Scand. 2009, 51, 53. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.A.; Almiñana, C.; Roca, J.; Vázquez, J.M.; Martínez, E.A. Boar semen variability and its effects on IVF efficiency. Theriogenology 2008, 70, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Anderson, D.L.; Penedo, M.C.T. Porcine sperm fertilizing potential in relationship to sperm functional capacities. Anim. Reprod. Sci. 1996, 44, 231–239. [Google Scholar] [CrossRef]
- Brito, L.F.C.; Barth, A.D.; Bilodeau-Goeseels, S.; Panich, P.L.; Kastelic, J.P. Comparison of methods to evaluate the plasmalemma of bovine sperm and their relationship with in vitro fertilization rate. Theriogenology 2003, 60, 1539–1551. [Google Scholar] [CrossRef]
- Ramu, S.; Jeyendran, R.S. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Methods Mol. Biol. 2013, 927, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, C.; Treviño, C.L.; Mata-Martínez, E.; Chávez, J.C.; Sánchez-Cárdenas, C.; Baker, M.; Darszon, A. Sperm Acrosome Biogenesis and Function during Fertilization; Advances in Anatomy, Embryology and Cell Biology; Buffone, M.G., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 220, ISBN 978-3-319-30565-3. [Google Scholar]
- Aquila, S.; Giordano, F.; Guido, C.; Rago, V.; Carpino, A. Nitric oxide involvement in the acrosome reaction triggered by leptin in pig sperm. Reprod. Biol. Endocrinol. 2011, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Staicu, F.-D.; Parra, C.M. Nitric Oxide: Key Features in Spermatozoa. In Nitric Oxide Synthase-Simple Enzyme-Complex Roles; InTech: London, UK, 2017; Volume i, p. 13. [Google Scholar]
- Revelli, A.; Costamagna, C.; Moffa, F.; Aldieri, E.; Ochetti, S.; Bosia, A.; Massobrio, M.; Lindblom, B.; Ghigo, D. Signaling pathway of nitric oxide-induced acrosome reaction in human spermatozoa. Biol. Reprod. 2001, 64, 1708–1712. [Google Scholar] [CrossRef] [Green Version]
- Sengoku, K.; Tamate, K.; Yoshida, T.; Takaoka, Y.; Miyamoto, T.; Ishikawa, M. Effects of low concentrations of nitric oxide on the zona pellucida binding ability of human spermatozoa. Fertil. Steril. 1998, 69, 522–527. [Google Scholar] [CrossRef]
- Hou, M.L.; Huang, S.Y.; Lai, Y.K.; Lee, W.C. Geldanamycin augments nitric oxide production and promotes capacitation in boar spermatozoa. Anim. Reprod. Sci. 2008, 104, 56–68. [Google Scholar] [CrossRef]
- Funahashi, H. Induction of capacitation and the acrosome reaction of boar spermatozoa by L-arginine and nitric oxide synthesis associated with the anion transport system. Reproduction 2002, 124, 857–864. [Google Scholar] [CrossRef] [PubMed]
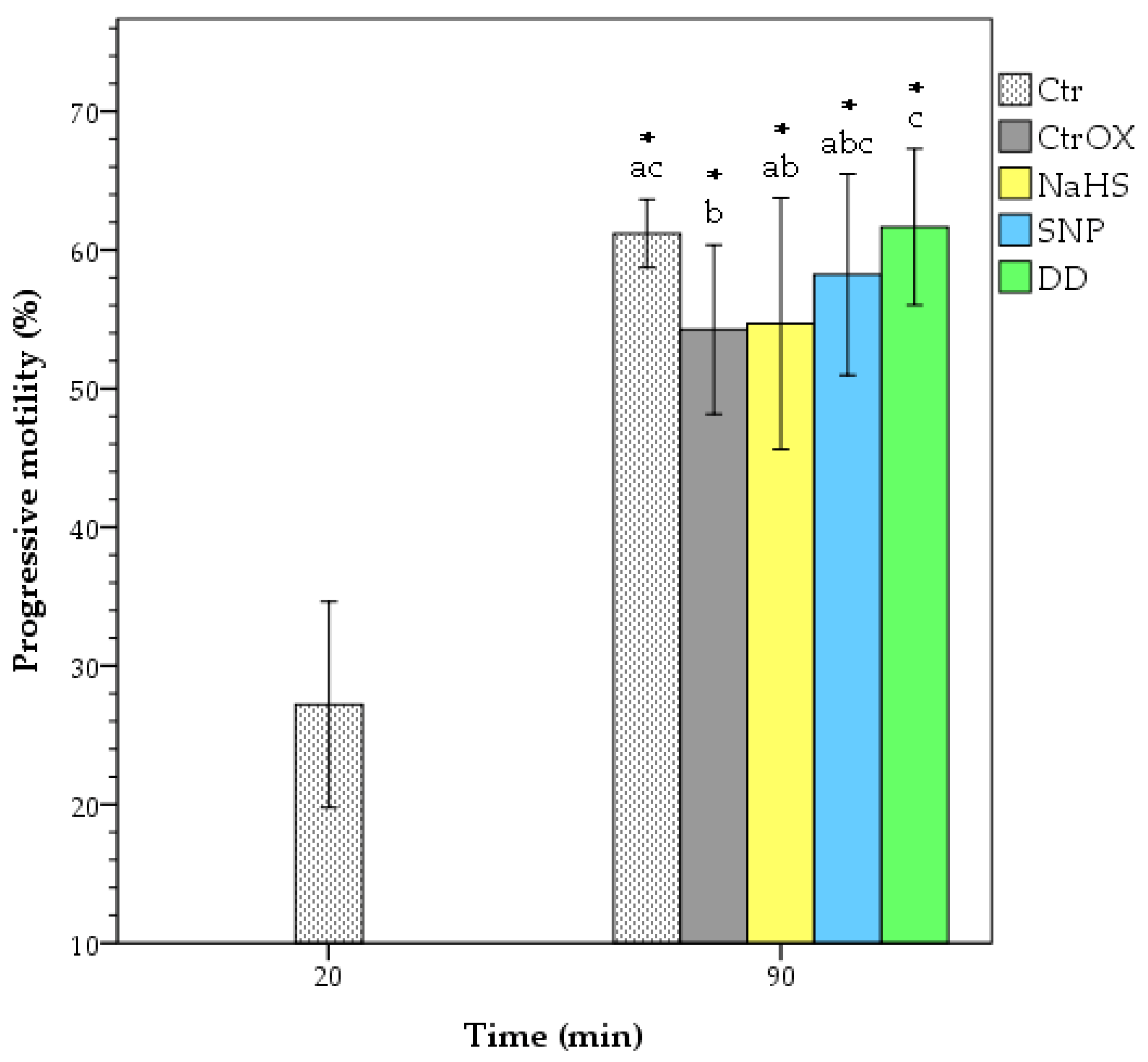
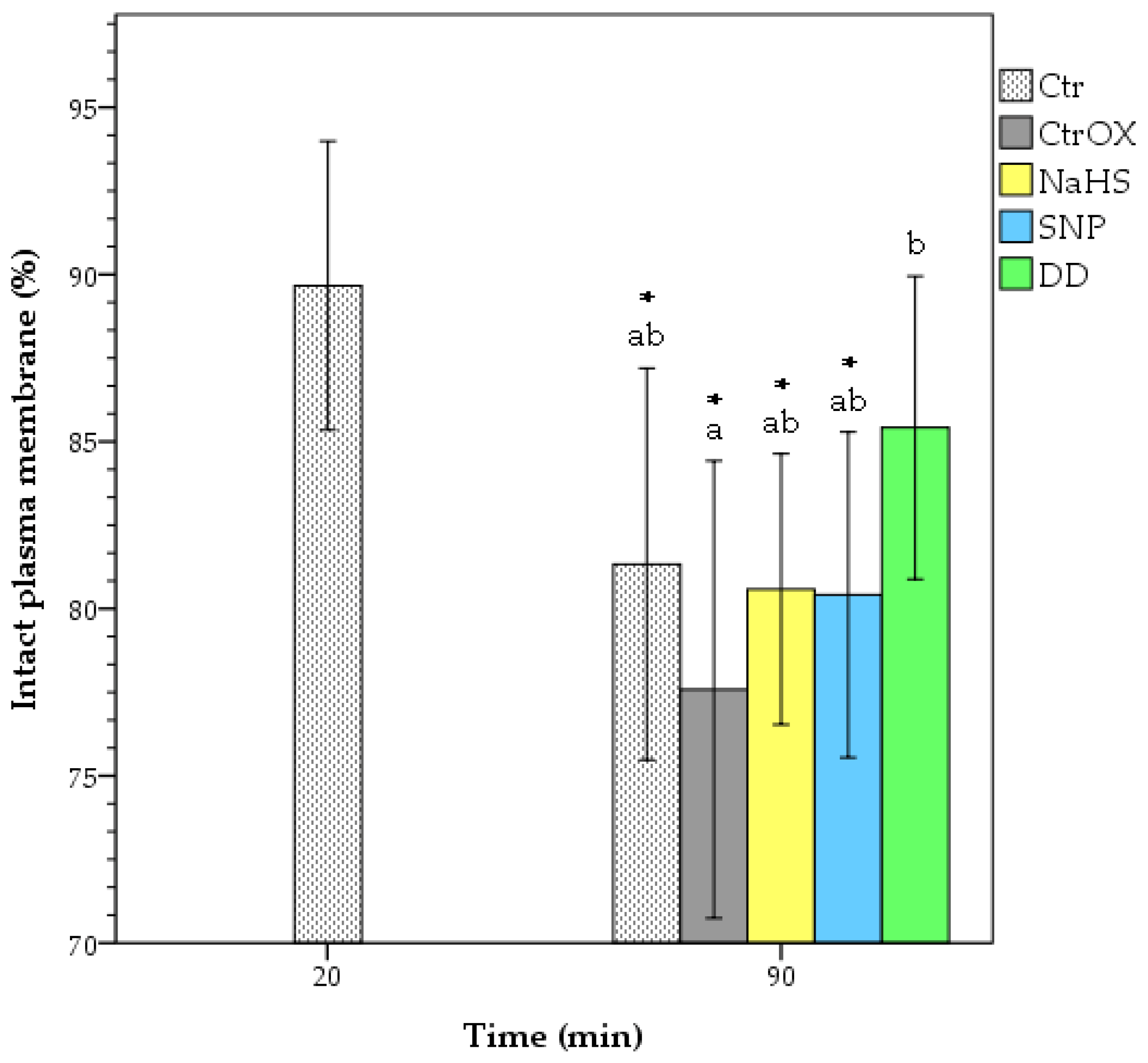

| Treatment | Time (min) | TMot (%) | VAP (µm/s) | VCL (µm/s) | VSL (µm/s) | ALH (µm) | BCF (Hz) | LIN (%) | STR (%) | WOB (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | 20 | 65.9 ± 8.2 | 40.1 ± 6.1 | 95.2 ± 8.2 | 24.9 ± 4.7 | 3.0 ± 0.3 | 11.4 ± 0.7 | 28.4 ± 4.7 | 66.6 ± 6.0 | 40.8 ± 3.6 |
| Ctr | 90 | 66.8 ± 2.9 a | 40.5 ± 4.3 | 71.7 ± 6.9 * | 36.7 ± 4.1 * | 2.8 ± 0.3 | 14.0 ± 0.5 ab,* | 50.0 ± 2.8 * | 88.4 ± 1.6 * | 54.9 ± 2.4 * |
| CtrOX | 90 | 55.7 ± 11.2 b | 41.0 ± 8.4 | 77.5 ± 17.5 * | 34.4 ± 7.1 * | 2.7 ± 0.6 | 14.2 ± 0.8 ab,* | 48.8 ± 7.6 * | 85.4 ± 6.3 * | 55.3 ± 5.1 * |
| NaHS | 90 | 56.7 ± 13.1 ab | 38.8 ± 4.8 | 71.0 ± 13.7 * | 33.8 ± 3.7 * | 2.6 ± 0.4 | 13.8 ± 0.5 a,* | 49.5 ± 8.2 * | 86.7 ± 7.9 * | 55.2 ± 5.0 * |
| SNP | 90 | 59.4 ± 12.2 ab | 40.9 ± 5.9 | 72.2 ± 11.3 * | 36.4 ± 4.8 * | 2.7 ± 0.4 | 14.3 ± 1.0 ab,* | 51.9 ± 5.5 * | 88.7 ± 4.4 * | 57.1 ± 4.3 * |
| DD | 90 | 60.5 ± 9.8 ab | 40.2 ± 6.1 | 70.0 ± 15.5 * | 36.2 ± 4.8 * | 2.7 ± 0.5 | 14.7 ± 1.1 b,* | 53.4 ± 7.2 * | 89.7 ± 5.6 * | 58.2 ± 5.0 * |
| Treatment | Time (min) | Intact Acrosome (%) |
|---|---|---|
| Ctr | 20 | 98.0 ± 1.4 |
| Ctr | 90 | 97.8 ± 2.0 |
| CtrOX | 90 | 96.2 ± 1.2 |
| NaHS | 90 | 97.1 ± 1.6 |
| SNP | 90 | 95.5 ± 5.3 |
| DD | 90 | 97.9 ± 1.4 |
| Treatment | Time (min) | Total Antioxidant Capacity (mM) |
|---|---|---|
| Ctr | 20 | 0.60 ± 0.06 |
| Ctr | 90 | * 0.54 ± 0.06 a |
| CtrOX | 90 | * 0.43 ± 0.04 b |
| NaHS | 90 | * 0.42 ± 0.04 b |
| SNP | 90 | * 0.41 ± 0.04 b |
| DD | 90 | * 0.42 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadlec, M.; Pintus, E.; Ros-Santaella, J.L. The Interaction of NO and H2S in Boar Spermatozoa under Oxidative Stress. Animals 2022, 12, 602. https://doi.org/10.3390/ani12050602
Kadlec M, Pintus E, Ros-Santaella JL. The Interaction of NO and H2S in Boar Spermatozoa under Oxidative Stress. Animals. 2022; 12(5):602. https://doi.org/10.3390/ani12050602
Chicago/Turabian StyleKadlec, Martin, Eliana Pintus, and José Luis Ros-Santaella. 2022. "The Interaction of NO and H2S in Boar Spermatozoa under Oxidative Stress" Animals 12, no. 5: 602. https://doi.org/10.3390/ani12050602
APA StyleKadlec, M., Pintus, E., & Ros-Santaella, J. L. (2022). The Interaction of NO and H2S in Boar Spermatozoa under Oxidative Stress. Animals, 12(5), 602. https://doi.org/10.3390/ani12050602






