Screening of Colistin-Resistant Bacteria in Domestic Pets from France
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Ethics Statement
2.3. Screening for Colistin-Resistant Bacteria
2.4. Bacterial Identification
2.5. Antibiotic Susceptibility Tests (AST)
2.6. DNA Extraction
2.7. Molecular Characterization of Colistin Resistance Genes
2.8. Statistical Analysis
3. Results and Discussion
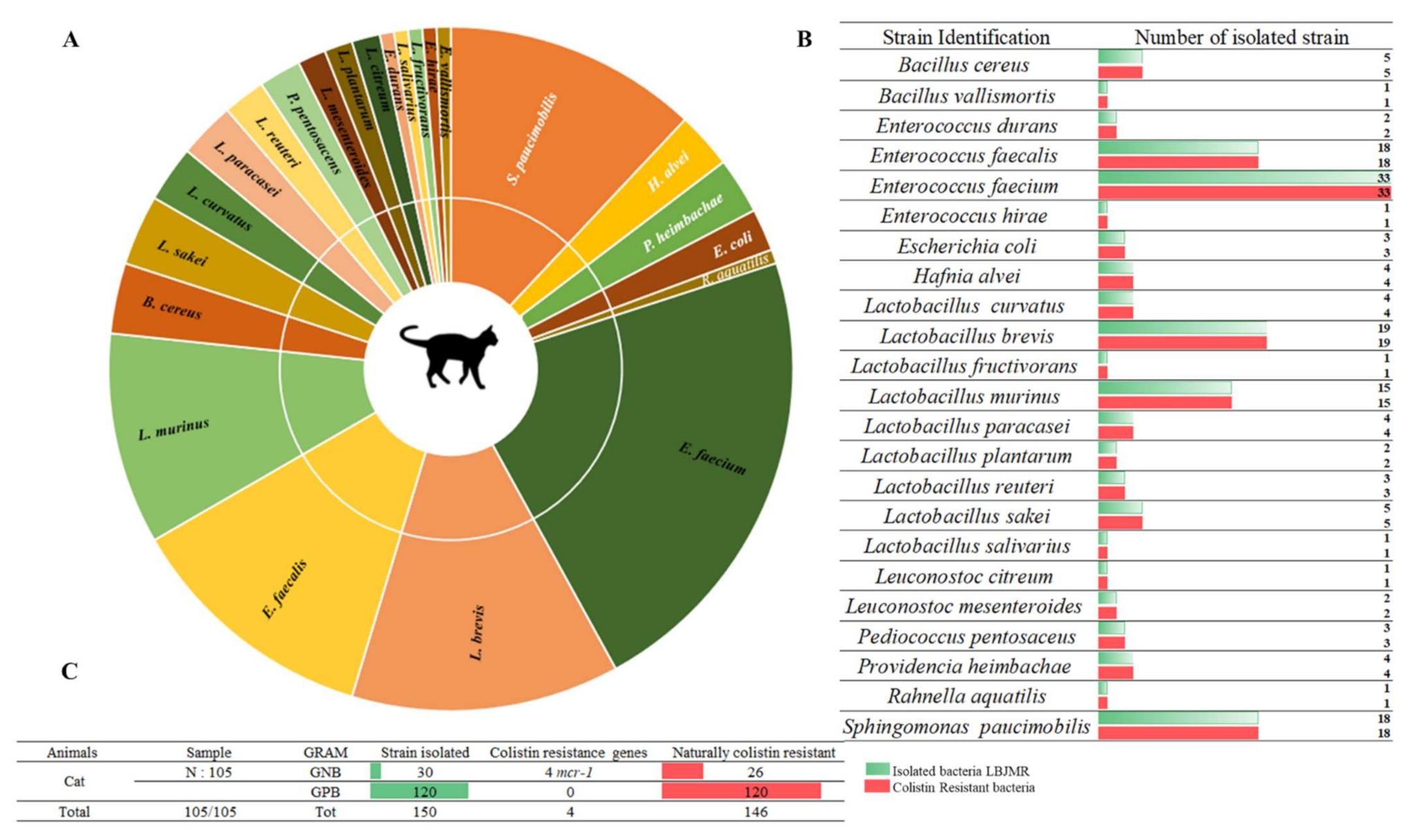
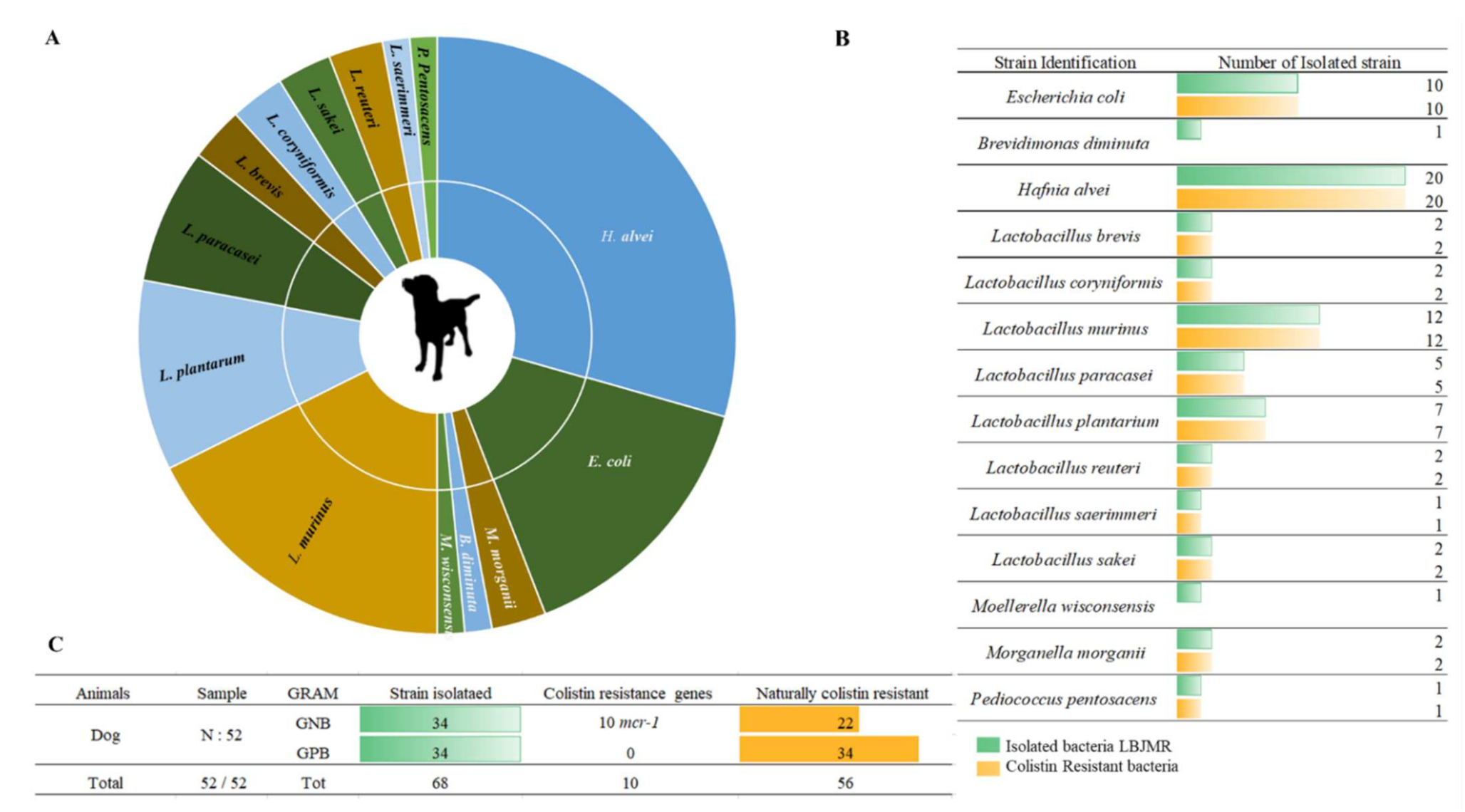
3.1. Screening of Colistin-Resistant Bacteria in Pets
3.2. Phenotype of Antibiotic Resistance
3.3. Screening of Mobile Colistin Resistance (mcr) Genes in Fecal Samples from Pets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kempf, I.; Jouy, E.; Chauvin, C. Colistin use and colistin resistance in bacteria from animals. Int. J. Antimicrob. Agents 2016, 48, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Andrasevic, A.T.; Antunovic, I.A. The world-wide spread of carbapenem-resistant Enterobacterales. One Health Risk Manag. 2020, 2, 4–12. [Google Scholar] [CrossRef]
- Kontopidou, F.; Plachouras, D.; Papadomichelakis, E.; Koukos, G.; Galani, I.; Poulakou, G.; Dimopoulos, G.; Antoniadou, A.; Armaganidis, A.; Giamarellou, H. Colonization and infection by colistin-resistant Gram-negative bacteria in a cohort of critically ill patients. Clin. Microbiol. Infect. 2011, 17, E9–E11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. Bull. Eur. Mal. Transm. Eurosurveillance 2016, 21, 30280. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli. mBio 2017, 8, e00543–e00617. [Google Scholar] [CrossRef] [Green Version]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance 2017, 22, 30589. [Google Scholar] [CrossRef] [Green Version]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef] [Green Version]
- AbuOun, M.; Stubberfield, E.J.; Duggett, N.A.; Kirchner, M.; Dormer, L.; Nunez-Garcia, J.; Randall, L.P.; Lemma, F.; Crook, D.W.; Teale, C.; et al. mcr-1 and mcr-2 (mcr-6.1) variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J. Antimicrob. Chemother. 2017, 72, 2745–2749. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-Q.; Li, Y.-X.; Lei, C.-W.; Zhang, A.; Wang, H.-N. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2018, 73, 1791–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Y.; Zhou, Y.; Wang, Z.; Wang, Y.; Zhang, S.; Shen, Z. Emergence of Colistin Resistance Gene mcr-8 and Its Variant in Raoultella ornithinolytica. Front. Microbiol. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, L.M.; Gaballa, A.; Guldimann, C.; Sullivan, G.; Henderson, L.O.; Wiedmann, M. Identification of Novel Mobilized Colistin Resistance Gene mcr-9 in a Multidrug-Resistant, Colistin-Susceptible Salmonella enterica Serotype Typhimurium Isolate. mBio 2019, 10, e00853–e00919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, S.; Johnson, A.P. Transferable resistance to colistin: A new but old threat. J. Antimicrob. Chemother. 2016, 71, 2066–2070. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Doi, Y.; Huang, X.; Li, H.-Y.; Zhong, L.-L.; Zeng, K.-J.; Zhang, Y.-F.; Patil, S.; Tian, G.-B. Possible Transmission ofmcr-1–HarboringEscherichia colibetween Companion Animals and Human. Emerg. Infect. Dis. 2016, 22, 1679–1681. [Google Scholar] [CrossRef] [Green Version]
- Dierikx, C.M.; Van Duijkeren, E.; Schoormans, A.H.W.; Van Essen-Zandbergen, A.; Veldman, K.; Kant, A.; Huijsdens, X.W.; Van Der Zwaluw, K.; Wagenaar, J.A.; Mevius, D.J. Occurrence and characteristics of extended-spectrum-β-lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 2012, 67, 1368–1374. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Y.; Schwarz, S.; Walsh, T.R.; Ou, Y.; Wu, Y.; Li, M.; Shen, Z. mcr-1 in Enterobacteriaceae from Companion Animals, Beijing, China, 2012–2016. Emerg. Infect. Dis. 2017, 23, 710–711. [Google Scholar] [CrossRef]
- Ortega-Paredes, D.; Haro, M.; Leoro-Garzón, P.; Barba, P.; Loaiza, K.; Mora, F.; Fors, M.; Vinueza-Burgos, C.; Fernández-Moreira, E. Multidrug-resistant Escherichia coli isolated from canine faeces in a public park in Quito, Ecuador. J. Glob. Antimicrob. Resist. 2019, 18, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lei, L.; Zhang, H.; Dai, H.; Song, Y.; Li, L.; Wang, Y.; Xia, Z. Molecular Investigation of Klebsiella pneumoniae from Clinical Companion Animals in Beijing, China, 2017–2019. Pathogens 2021, 10, 271. [Google Scholar] [CrossRef]
- De Jong, A.; Thomas, V.; Simjee, S.; Godinho, K.; Schiessl, B.; Klein, U.; Butty, P.; Vallé, M.; Marion, H.; Shryock, T.R. Pan-European monitoring of susceptibility to human-use antimicrobial agents in enteric bacteria isolated from healthy food-producing animals. J. Antimicrob. Chemother. 2011, 67, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Delannoy, S.; Le Devendec, L.; Jouy, E.; Fach, P.; Drider, D.; Kempf, I. Characterization of Colistin-Resistant Escherichia coli Isolated from Diseased Pigs in France. Front. Microbiol. 2017, 8, 2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolakos, I.; Piccirillo, A. A review on the current situation and challenges of colistin resistance in poultry production. Avian Pathol. 2018, 47, 546–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, D.C.; Mechesso, A.F.; Kang, H.Y.; Kim, S.-J.; Choi, J.-H.; Kim, M.H.; Song, H.-J.; Yoon, S.-S.; Lim, S.-K. First Report of an Escherichia coli Strain Carrying the Colistin Resistance Determinant mcr-1 from a Dog in South Korea. Antibiotics 2020, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-H.; Chen, G.-J.; Lo, D.-Y. Chromosomal Locations of mcr-1 IN Klebsiella pneumoniae AND Enterobacter cloacae from dogs. Taiwan Veter. J. 2019, 45, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Hussein, N.H.; Al-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized colistin resistance (mcr) genes from 1 to 10: A comprehensive review. Mol. Biol. Rep. 2021, 48, 2897–2907. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H. Bacterial zoonoses transmitted by household pets and as reservoirs of antimicrobial resistant bacteria. Microb. Pathog. 2021, 155, 104891. [Google Scholar] [CrossRef] [PubMed]
- Bardet, L.; Le Page, S.; Leangapichart, T.; Rolain, J.-M. LBJMR medium: A new polyvalent culture medium for isolating and selecting vancomycin and colistin-resistant bacteria. BMC Microbiol. 2017, 17, 220. [Google Scholar] [CrossRef] [Green Version]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 17 August 2021).
- Sulaiman, A.A.A.; Kassem, I.I. First report of the plasmid-borne colistin resistance gene (mcr-1) in Proteus mirabilis isolated from domestic and sewer waters in Syrian refugee camps. Travel Med. Infect. Dis. 2019, 33, 101482. [Google Scholar] [CrossRef]
- Chabou, S.; Leangapichart, T.; Okdah, L.; Le Page, S.; Hadjadj, L.; Rolain, J.-M. Real-time quantitative PCR assay with Taqman® probe for rapid detection of MCR-1 plasmid-mediated colistin resistance. New Microbes New Infect. 2016, 13, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Lee, S.; Sung, J.; Ko, G. Analysis of human and animal fecal microbiota for microbial source tracking. ISME J. 2010, 5, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Deng, P.; Swanson, K. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2014, 113, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Intestinal Microbiota of Dogs and Cats: A Bigger World than We Thought. Veter. Clin. N. Am. Small Anim. Pract. 2011, 41, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Dowd, S.E.; Poulsen, J.; Steiner, J.M.; Suchodolski, J.S. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. MicrobiologyOpen 2012, 1, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Viel, A. Usages de la Colistine en Médecine Humaine et Vétérinaire: Exploration Pharmacocinétique et Problématique D’Antibiorésistance. Ph.D. Thesis, Université de Poitiers, Poitiers, France, 2017. [Google Scholar]
- European Medicines Agency. Updated advice on the use of colistin products in animals within the European Union: Development of resistance and possible impact on human and animal health. Eur. Med. Agency 2016, 44, 56. [Google Scholar]
- Walsh, T.R.; Wu, Y. China bans colistin as a feed additive for animals. Lancet Infect. Dis. 2016, 16, 1102. [Google Scholar] [CrossRef]
- Catry, B.; Cavaleri, M.; Baptiste, K.; Grave, K.; Grein, K.; Holm, A.; Jukes, H.; Liebana, E.; Navas, A.L.; Mackay, D.; et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents 2015, 46, 297–306. [Google Scholar] [CrossRef]
- Furet, J.-P.; Firmesse, O.; Gourmelon, M.; Bridonneau, C.; Tap, J.; Mondot, S.; Dorã, J.; Corthier, G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol. Ecol. 2009, 68, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Matuschek, E.; Åhman, J.; Webster, C.; Kahlmeter, G. Antimicrobial susceptibility testing of colistin—Evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin. Microbiol. Infect. 2018, 24, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K.; Jensen, H.; Finster, K.; Jensen, V.F.; Heuer, O.E. Occurrence of antimicrobial resistance in bacteria from diagnostic samples from dogs. J. Antimicrob. Chemother. 2007, 60, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Wang, Y.; He, J.; Cai, C.; Liu, Q.; Yang, D.; Zou, Z.; Shi, L.; Jia, J.; Wang, Y.; et al. Prevalence and risk analysis of mobile colistin resistance and extended-spectrum β-lactamase genes carriage in pet dogs and their owners: A population based cross-sectional study. Emerg. Microbes Infect. 2021, 10, 242–251. [Google Scholar] [CrossRef]
- Simmen, S.; Zurfluh, K.; Nüesch-Inderbinen, M.; Schmitt, S. Investigation for the Colistin Resistance Genes mcr-1 and mcr-2 in Clinical Enterobacteriaceae Isolates from Cats and Dogs in Switzerland. ARC J. Anim. Veter. Sci. 2016, 2, 26–29. [Google Scholar] [CrossRef]
- Du, C.; Feng, Y.; Wang, G.; Zhang, Z.; Hu, H.; Yu, Y.; Liu, J.; Qiu, L.; Liu, H.; Guo, Z.; et al. Co-Occurrence of the mcr-1.1 and mcr-3.7 Genes in a Multidrug-Resistant Escherichia coli Isolate from China. Infect. Drug Resist. 2020, 13, 3649–3655. [Google Scholar] [CrossRef]
- Kobs, V.C.; Valdez, R.E.; De Medeiros, F.; Fernandes, P.P.; Deglmann, R.C.; Gern, R.M.; França, P.H. mcr-1-carrying Enterobacteriaceae isolated from companion animals in Brazil. Pesquisa Veterinária Brasileira 2020, 40, 690–695. [Google Scholar] [CrossRef]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial Usage and Resistance in Companion Animals: A Cross-Sectional Study in Three European Countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumi, M.V.; Mas, J.; Elena, A.; Cerdeira, L.; Muñoz, M.E.; Lincopan, N.; Gentilini, R.; Di Conza, J.; Gutkind, G. Co-occurrence of clinically relevant β-lactamases and MCR-1 encoding genes in Escherichia coli from companion animals in Argentina. Veter- Microbiol. 2019, 230, 228–234. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Oreiby, A.F.; El-Hafeez, A.A.A.; Okanda, T.; Haque, A.; Anwar, K.S.; Tanaka, M.; Miyako, K.; Tsuji, S.; Kato, Y.; et al. First Report of Multidrug-Resistant Carbapenemase-Producing Bacteria Coharboring mcr-9 Associated with Respiratory Disease Complex in Pets: Potential of Animal-Human Transmission. Antimicrob. Agents Chemother. 2020, 65, e01890–e01920. [Google Scholar] [CrossRef]

| Geographical Location | Animal | Strain | Colistin Resistance Gene | q-PCR (Ct) | St-PCR | MIC UMIC (µg/mL) | Identity (%) | E-Value |
|---|---|---|---|---|---|---|---|---|
| Provence Alpes-Côte D’Azur (Marseille) | Cat | E. coli | mcr-1 | 34 | + | 8 | 99 | 0.0 |
| E. coli | mcr-1 | 32 | + | 4 | 98 | 0.0 | ||
| E. coli | mcr-1 | 31 | + | 2 | 99.1 | 0.0 | ||
| Rahnella aquatilis | mcr-1 | 29 | + | 64 | 97.2 | 0.0 | ||
| Dog | E. coli | mcr-1 | 34 | + | 4 | 99.4 | 0.0 | |
| E. coli | mcr-1 | 35 | + | 4 | 99.1 | 0.0 | ||
| E. coli | mcr-1 | 30 | + | 2 | 96 | 0.0 | ||
| E. coli | mcr-1 | 34 | + | 12 | 99 | 0.0 | ||
| E. coli | mcr-1 | 30 | + | 4 | 99.4 | 0.0 | ||
| E. coli | mcr-1 | 25 | + | 8 | 97.8 | 0.0 | ||
| E. coli | mcr-1 | 21 | + | 6 | 98 | 0.0 | ||
| E. coli | mcr-1 | 29 | + | 2 | 99.4 | 0.0 | ||
| E. coli | mcr-1 | 30 | + | 8 | 99.3 | 0.0 | ||
| E. coli | mcr-1 | 29 | + | 2 | 98.5 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamame, A.; Davoust, B.; Rolain, J.-M.; Diene, S.M. Screening of Colistin-Resistant Bacteria in Domestic Pets from France. Animals 2022, 12, 633. https://doi.org/10.3390/ani12050633
Hamame A, Davoust B, Rolain J-M, Diene SM. Screening of Colistin-Resistant Bacteria in Domestic Pets from France. Animals. 2022; 12(5):633. https://doi.org/10.3390/ani12050633
Chicago/Turabian StyleHamame, Afaf, Bernard Davoust, Jean-Marc Rolain, and Seydina M. Diene. 2022. "Screening of Colistin-Resistant Bacteria in Domestic Pets from France" Animals 12, no. 5: 633. https://doi.org/10.3390/ani12050633






