Effects of Sophorolipid on Growth Performance, Organ Characteristics, Lipid Digestion Markers, and Gut Functionality and Integrity in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Housing
2.2. Experimental Procedures and Sample Collection
2.3. Morphological Assay
2.4. RNA and Microbial DNA Extraction
2.5. qRT-PCR
2.6. Short-Chain Fatty Acid Measurement
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Relative Organ Weights
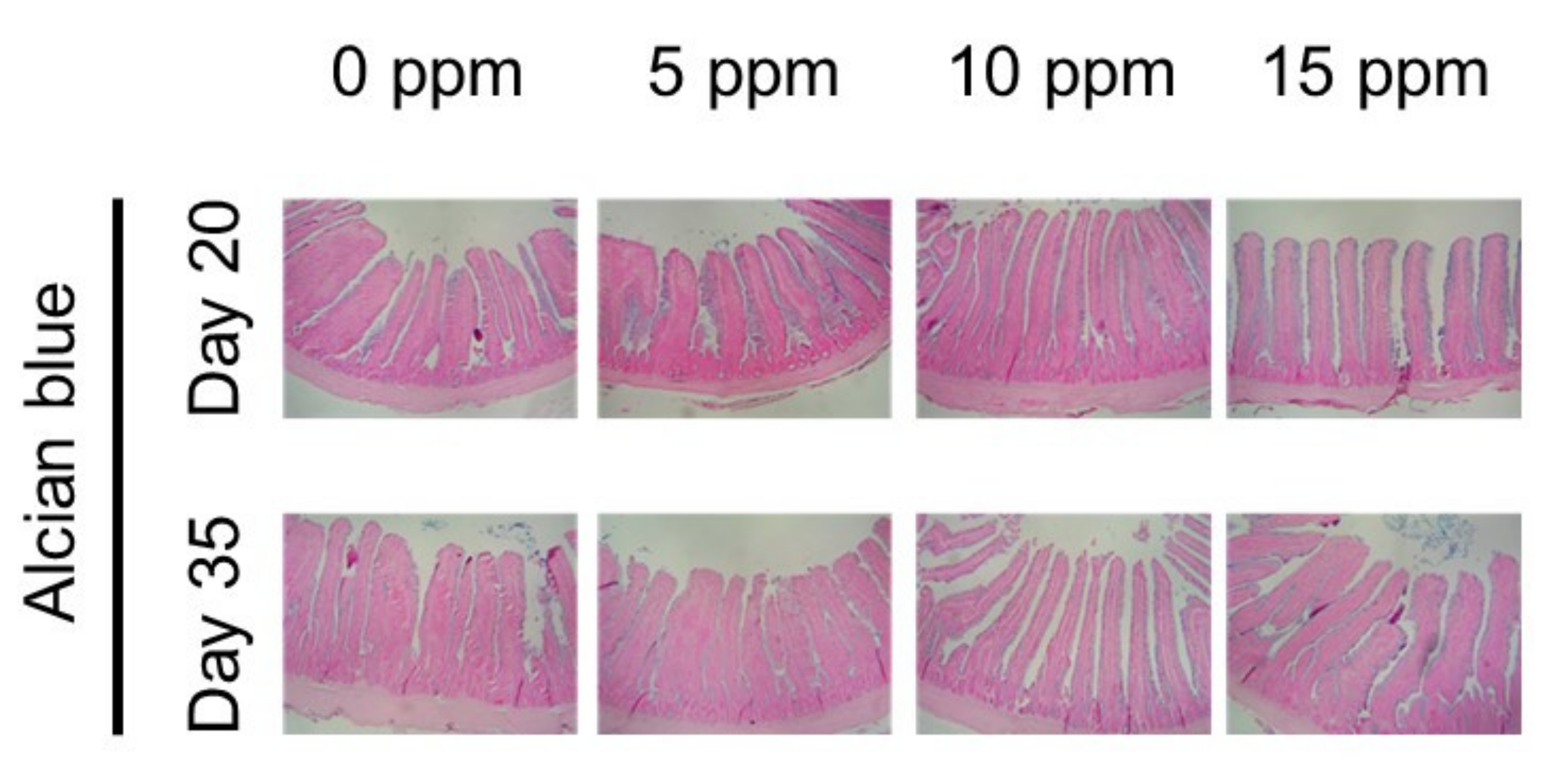
3.3. Morphology of Small Intestine
3.4. Gene Expression Levels of Lipid Digestion Markers in Jejunum
3.5. Microbial Population in Cecal Contents
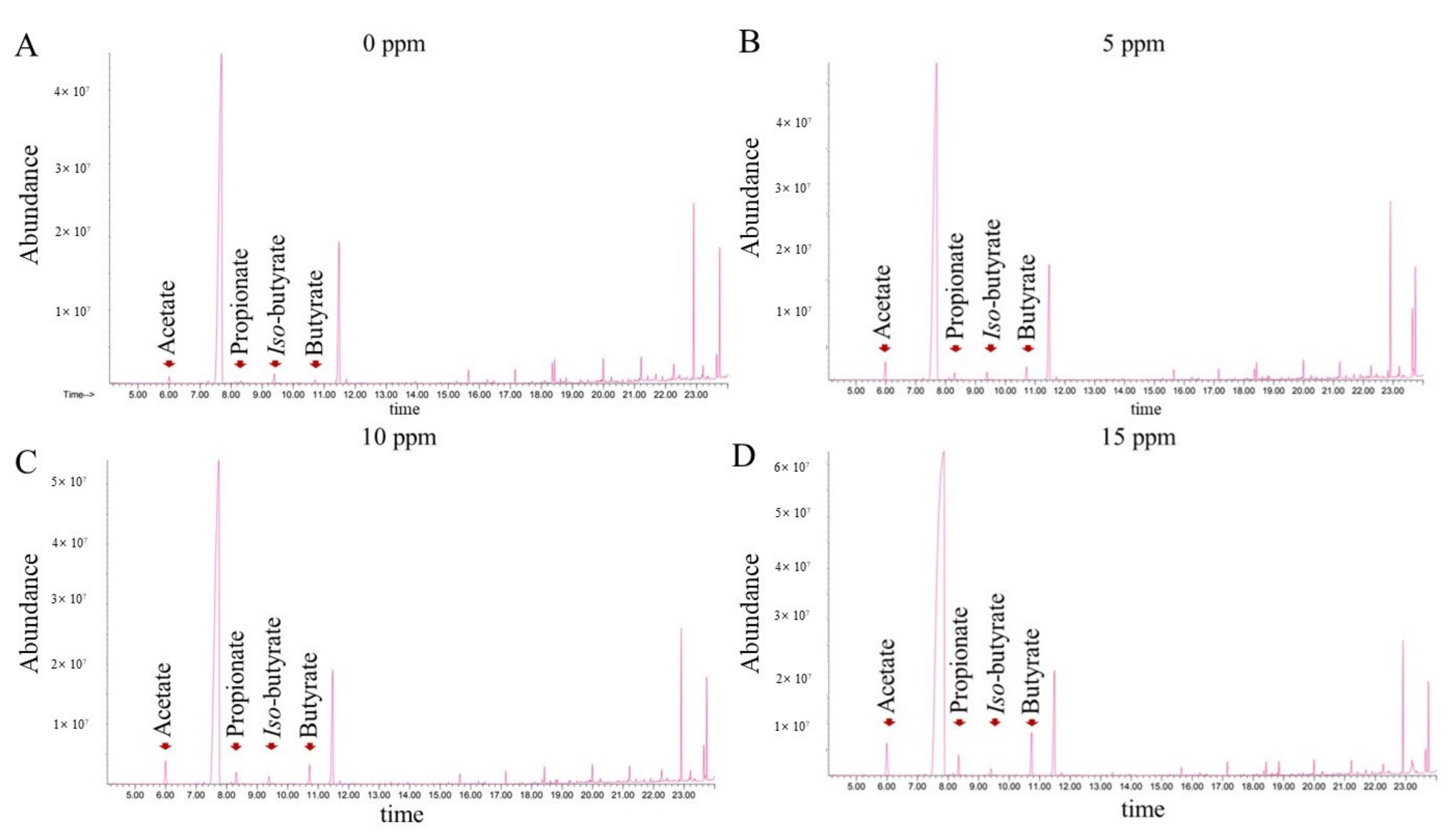
3.6. Short Chain Fatty Acid Concentration in Cecal Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiseman, J.; Lewis, C.E. Influence of dietary energy and nutrient concentration on the growth of body weight and of carcass components of broiler chickens. J. Agric. Sci. 1998, 131, 361–371. [Google Scholar] [CrossRef]
- Firman, J.D.; Kamyab, A.; Leigh, H. Comparison of fat sources in rations of broilers from hatch to market. Int. J. Poult. Sci. 2008, 7, 1152–1155. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, V.; Tancharoenrat, P.; Zaefarian, F.; Ravindran, G. Fats in poultry nutrition: Digestive physiology and factors influencing their utilization. Anim. Feed Sci. Technol. 2016, 213, 1–21. [Google Scholar] [CrossRef]
- Rochell, S.J.; Helmbrecht, A.; Parsons, C.M.; Dilger, R.N. Influence of dietary amino acid reductions and Eimeria acervuline infection on growth performance and intestinal cytokine responses of broilers fed low crude protein diets. Poult. Sci. 2016, 95, 2602–2614. [Google Scholar] [CrossRef]
- Tancharoenrat, P.; Ravindran, V.; Zaefarian, F.; Ravindran, G. Digestion of fat and fatty acids along the gastrointestinal tract of broiler chickens. Poult. Sci. 2014, 93, 371–379. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Attia, A.I.; Reda, F.M.; Ismail, I.E. Impact of dietary supplemental bile salts on growth performance, carcass, immunity and antioxidant parameters and bacteriology of broiler chicks. Ital. J. Anim. Sci. 2020, 19, 1406–1416. [Google Scholar] [CrossRef]
- Arshad, M.A.; Bhatti, S.A.; Rehman, M.S.U.; Yousaf, W.; Younus, G.; Sizmaz, O.; Bilalm, M.Q. Supplementation of bile acids and lipase in broiler diets for better nutrient utilization and performance: Potential effects and future implications–A review. Ann. Anim. Sci. 2021, 21, 757–787. [Google Scholar] [CrossRef]
- Hu, Y.D.; Lan, D.; Zhu, Y.; Pang, H.Z.; Mu, X.P.; Hu, X.F. Effect of diets with different energy and lipase levels on performance, digestibility, and carcass trait in broilers. Asian-Australas. J. Anim. Sci. 2018, 31, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yan, J.; Zhang, X.; Han, B. Tolerance properties and growth performance assessment of Yarrowia lipolytic lipase in broilers. J. Appl. Anim. Res. 2018, 46, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Boontiam, W.; Jung, B.; Kim, Y.Y. Effects of lysophospholipid supplementation to lower nutrient diets on growth performance, intestinal morphology and blood metabolites in broiler chickens. Poult. Sci. 2017, 96, 593–601. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Lee, J.S.; Jung, K.J.; Kim, I.H. Influence of emulsifier blends having different hydrophilic-lipophilic balance value on growth performance, nutrient digestibility, serum lipid profiles and meat quality of broilers. Poult. Sci. 2018, 97, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Kim, Y.B.; Kim, E.K. Production and application of sophorolipid, a microbial surfactant. Korean Soc. Biotechnol. Bioeng. J. 1999, 14, 747–753. [Google Scholar]
- Develter, D.W.; Lauryssen, L.M. Properties and industrial applications of sophorolipids. Eur. J. Lipid Sci. Tech. 2010, 112, 628–638. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Bio. R. 1997, 61, 47–64. [Google Scholar]
- Finnerty, W.R. Biosurfactants in environmental biotechnology. Curr. Opin. Biotech. 1994, 5, 291–295. [Google Scholar] [CrossRef]
- Hardin, R.; Pierre, J.; Schulze, R.; Mueller, C.M.; Fu, S.L.; Wallner, S.R.; Stanek, A.; Shah, V.; Gross, R.A.; Weedon, J.; et al. Sophorolipids improve sepsis survival: Effects of dosing and derivatives. J. Surg. Res. 2008, 142, 314–319. [Google Scholar] [CrossRef]
- Kwak, M.J.; Park, M.Y.; Choi, Y.S.; Cho, J.; Pathiraja, D.; Kim, J.; Lee, H.B.; Choi, I.G.; Whang, K.Y. Dietary sophorolipid accelerates growth by modulation of gut microbiota population and intestinal environments in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Song, Z.; Zhang, X.; Wang, X.; Jiao, H.; Lin, H. Increased de novo lipogenesis in liver contributes to the augmented fat deposition in dexamethasone exposed broiler chickens (Gallus gallus domesticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 164–169. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Saleh, K.M.M.; Jaradat, Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult. Sci. 2019, 98, 4113–4122. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.; Suh, Y.; Cressman, M.; Lee, S.S.; Lee, K. Adipogenic and myogenic potentials of chicken embryonic fibroblasts in vitro: Combination of fatty acids and insulin induces adipogenesis. Lipids 2020, 55, 163–171. [Google Scholar] [CrossRef]
- Castillo, M.; Martín-Orúe, S.M.; Roca, M.; Manzanilla, E.G.; Badiola, I.; Perez, J.F.; Gasa, J. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. J. Anim. Sci. 2006, 84, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Vink, C.; Driessen, C.; London, N.; Thijs, C.; Stobberingh, E.E. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol. Lett. 2005, 243, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Li, A. Isolation and characterization of Streptococcus dysgalactiae from diseased Acipenser schrenckii. Aquaculture 2009, 294, 14–17. [Google Scholar] [CrossRef]
- Cheng, C.M.; Lin, W.; Van, K.T.; Phan, L.; Tran, N.N.; Farmer, D. Rapid detection of Salmonella in foods using real-time PCR. J. Food Prot. 2008, 71, 2436–2441. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Khonyoung, D.; Yamauchi, K.; Suzuki, K. Influence of dietary fat sources and lysolecithin on growth performance, visceral organ size, and histological intestinal alteration in broiler chickens. Livest. Sci. 2015, 76, 111–120. [Google Scholar] [CrossRef]
- Boontiam, W.; Hyun, Y.K.; Jung, B.; Kim., Y.Y. Effects of lysophospholipid supplementation to reduced energy, crude protein, and amino acid diets on growth performance, nutrient digestibility, and blood profiles in broiler chickens. Poult. Sci. 2019, 98, 6693–6701. [Google Scholar] [CrossRef]
- Mueller, A.P.; Wolfe, H.R.; Meyer, R.K.; Aspinall, R.L. Further studies on the role of the bursa of fabricius in antibody production. J. Immunol. 1962, 88, 354–360. [Google Scholar]
- Bontempo, V.; Comi, M.; Jiang, X.R.; Rebucci, R.; Capararulo, V.; Giromini, C.; Gottardo, D.; Fusi, E.; Stella, S.; Tirloni, E.; et al. Evaluation of a synthetic emulsifier product supplementation on broiler chicks. Anim. Feed Sci. Techonol. 2018, 240, 157–164. [Google Scholar] [CrossRef]
- Ockner, R.K.; Manning, J.A. Fatty acid-binding protein in small intestine identification, isolation, and evidence for its role in cellular fatty acid transport. J. Clin. Investig. 1974, 54, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yang, D.; Gao, S.; Wang, T. Effects of soy-lecithin on lipid metabolism and hepatic expression of lipogenic genes in broiler chickens. Livest. Sci. 2008, 118, 53–60. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Y.; Braunstein, E.; Georgeson, K.E.; Harmon, C.M. Gut expression and regulation of FAT/CD36: Possible role in fatty acid transport in rat enterocytes. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E916–E923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhotnik, I.; Gork, A.S.; Chen, M.; Drongowski, R.A.; Coran, A.G.; Harmon, C.M. Effect of low fat diet on lipid absorption and fatty-acid transport following bowel secretion. Pediatr. Surg. Int. 2001, 17, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.X.; Guo, H.W.; Li, C.; Jiang, R.R.; Li, Z.J.; Wang, Y.B.; Kang, X.T.; Han, R.L. Polymorphism of exon 7 of DGAT2 gene and its association with growth traits in chicken (Gallus Gallus). J. Agric. Biotech. 2016, 24, 689–696. [Google Scholar]
- Meimandipour, A.; Shuhaimi, M.; Soleimani, A.F.; Azhar, K.; Hair-Bejo, M.; Kabeir, B.M.; Javanmard, A.; Muhammad, A.; Yazid, A.M. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of Lactobacillus. Poult. Sci. 2010, 89, 470–476. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.; van Knapen, F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef] [Green Version]
- Dalmasso, G.; Nguyen, H.T.T.; Yan, Y.; Charrier-Hisamuddin, L.; Sitaraman, S.V.; Merlin, D. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS ONE 2008, 3, e2476. [Google Scholar] [CrossRef] [Green Version]
- Adil, S.; Banday, T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010, 2010, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.J.; Park, M.Y.; Kim, J.; Lee, H.; Whang, K.Y. Curative effects of sophorolipid on physical wounds: In vitro and in vivo studies. Vet. Med. Sci. 2021, 7, 1400–1408. [Google Scholar] [CrossRef]
- Kwak, M.J.; Ha, D.J.; Choi, Y.S.; Lee, H.; Whang, K.Y. Protective and restorative effects of sophorolipid on intestinal dystrophy in dextran sulfate sodium-induced colitis mouse model. Food Func. 2022, 13, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Choi, S.W.; Choi, Y.S.; Lee, H.; Whang, K.Y. Sophorolipid protects against early-weaning syndrome by improving the gut microenvironment in early-weaned piglets. BMC Vet. Res. 2022, 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Starter (day 0–10) | Grower (day 11–20) | Finisher (day 21–35) | |
|---|---|---|---|
| Ingredients | |||
| Corn | 54.23 | 49.10 | 55.95 |
| Soybean meal | 30.38 | 22.03 | 14.05 |
| Fermented soybean meal | 5.00 | 0.00 | 0.00 |
| Distilled dried grains with solubles | 0.00 | 5.00 | 5.00 |
| Unpolished rice | 0.00 | 4.00 | 3.00 |
| Rice bran polished | 0.00 | 1.00 | 1.50 |
| Rapeseed mineral | 0.00 | 4.00 | 3.00 |
| Sesame seed meal | 0.00 | 0.00 | 0.50 |
| Poultry meal | 2.50 | 5.50 | 8.00 |
| Animal fat | 2.47 | 5.41 | 5.47 |
| Soy oil | 0.90 | 0.00 | 0.00 |
| L-Lysine sulfate (55%) | 0.46 | 0.57 | 0.62 |
| L-Methionine (90%) | 0.45 | 0.32 | 0.29 |
| Threonine (98%) | 0.17 | 0.14 | 0.15 |
| L-Tryptophan (99%) | 0.00 | 0.01 | 0.02 |
| Choline chloride (50%) | 0.10 | 0.10 | 0.12 |
| Monocalcium phosphate | 1.53 | 1.07 | 0.80 |
| Limestone | 1.18 | 1.20 | 1.00 |
| Salt | 0.25 | 0.25 | 0.25 |
| Sodium bicarbonate | 0.05 | 0.05 | 0.05 |
| Vitamin premix 1 | 0.20 | 0.14 | 0.11 |
| Mineral premix 2 | 0.15 | 0.12 | 0.12 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated value | |||
| Metabolizable energy (kcal/kg) | 3002.00 | 3100.00 | 3200.00 |
| Crude protein (%) | 23.00 | 21.50 | 20.00 |
| Crude fat (%) | 5.96 | 8.63 | 9.39 |
| Calcium (%) | 0.90 | 0.90 | 0.85 |
| Phosphate (%) | 0.77 | 0.71 | 0.65 |
| Lysine (%) | 1.50 | 1.33 | 1.20 |
| Methionine (%) | 0.74 | 0.61 | 0.56 |
| Threonine (%) | 1.03 | 0.95 | 0.90 |
| Tryptophan (%) | 0.26 | 0.23 | 0.20 |
| Gene Name | Sequence (Forward, Reverse) | Reference |
|---|---|---|
| Housekeeping gene | ||
| GAPDH | F: 5′-CTACACACGGACACTTCAAG-3′ | [18] |
| R: 5′-GACTACGGGGGTACAAACA-3′ | ||
| Lipid absorption proteins | ||
| FABP1 | F: 5′-ACTGGCTCCAAAGAATGACCAATG-3′ | [19] |
| R: 5′-TGTCTCCGTTGAGTTCGGTCAC-3′ | ||
| CD36 | F: 5′-GCGATTTGGTTAATGGCACT-3′ | Self-made |
| R: 5′-TCTCCAACATCAATCGGTGA-3′ | ||
| DGAT2 | F: 5′-AAAAGGGGATGCTGCCTATCT-3′ | [20] |
| R: 5′-GCTTACGCAGCTCCATCTTCT-3′ | ||
| FATP4 | F: 5′-AGGGATTTGTGAAACTGGCACT-3′ | [20] |
| R: 5′-CTTTGGGATGGTGATGGGTT-3′ | ||
| Intestinal microbial species | ||
| Total bacteria | F: 5′-GCAGGCCTAACACATGCAAGTC-3′ | [21] |
| R: 5′-CTGCTGCCTCCCGTAGGAGT-3′ | ||
| E. coli | F: 5′-CATGCCGCGTGTATGAAGAA-3′ | [22] |
| R: 5′-CGGGTAACGTCAATGAGCAAA-3′ | ||
| Sterptococcus spp. | F: 5′-GTACAGTTGCTTCAGGACGTATC-3′ | [23] |
| R: 5′-ACGTTCGATTTCATCACGTTG-3′ | ||
| Salmonella spp. | F: 5′-AACGTGTTTCCGTGCGTAAT-3′ | [24] |
| R: 5′-TCCATCAAATTAGCGGAGGC-3′ | ||
| Treatment | 0 ppm | 5 ppm | 10 ppm | 15 ppm | SEM | p-Value | Linear | Quadratic | Cubic |
|---|---|---|---|---|---|---|---|---|---|
| Body weight, g | |||||||||
| Day 0 | 40.09 | 40.14 | 40.18 | 40.15 | 0.029 | 0.599 | 0.476 | 0.257 | 0.962 |
| Day 10 | 259.74 | 256.88 | 261.32 | 247.78 | 3.498 | 0.083 | 0.072 | 0.181 | 0.175 |
| Day 20 | 868.27 ab | 866.79 ab | 878.80 a | 841.43 b | 5.164 | 0.043 | 0.084 | 0.057 | 0.141 |
| Day 35 | 2004.80 | 2060.65 | 2047.47 | 2005.17 | 13.184 | 0.338 | 0.915 | 0.080 | 0.747 |
| ADG, g/day | |||||||||
| Starter | 21.97 | 21.67 | 22.11 | 20.76 | 0.216 | 0.087 | 0.073 | 0.188 | 0.180 |
| Grower | 60.85 | 60.99 | 61.75 | 59.37 | 0.345 | 0.077 | 0.173 | 0.055 | 0.204 |
| Finisher | 75.77 | 79.59 | 77.91 | 77.58 | 0.779 | 0.418 | 0.583 | 0.206 | 0.364 |
| Overall | 56.13 | 57.73 | 57.35 | 56.14 | 0.377 | 0.338 | 0.914 | 0.080 | 0.747 |
| ADFI, g/day | |||||||||
| Starter | 27.92 | 28.58 | 27.93 | 27.24 | 0.313 | 0.547 | 0.337 | 0.311 | 0.685 |
| Grower | 86.40 | 87.52 | 90.16 | 84.91 | 0.728 | 0.062 | 0.739 | 0.023 | 0.131 |
| Finisher | 134.37 | 135.98 | 138.26 | 133.92 | 1.474 | 0.769 | 0.943 | 0.362 | 0.624 |
| Overall | 89.33 | 90.15 | 91.58 | 88.39 | 0.682 | 0.441 | 0.823 | 0.167 | 0.431 |
| FCR | |||||||||
| Starter | 1.27 | 1.32 | 1.26 | 1.31 | 0.012 | 0.269 | 0.494 | 0.993 | 0.067 |
| Grower | 1.42 | 1.44 | 1.46 | 1.43 | 0.009 | 0.488 | 0.472 | 0.244 | 0.451 |
| Finisher | 1.77 | 1.71 | 1.78 | 1.73 | 0.014 | 0.255 | 0.529 | 0.795 | 0.070 |
| Overall | 1.59 | 1.56 | 1.60 | 1.58 | 0.008 | 0.546 | 0.839 | 0.856 | 0.170 |
| Treatment | 0 ppm | 5 ppm | 10 ppm | 15 ppm | SEM | p-Value | Linear | Quadratic | Cubic |
|---|---|---|---|---|---|---|---|---|---|
| Day 20 | |||||||||
| Intestine, g/kg | 49.35 | 45.13 | 46.75 | 50.21 | 1.063 | 0.353 | 0.658 | 0.088 | 0.672 |
| Gut weight/length, g/m | 27.52 a | 23.85 b | 24.54 b | 27.34 a | 0.664 | 0.037 | 0.980 | 0.016 | 0.669 |
| Spleen, g/kg | 0.79 | 0.89 | 0.81 | 0.85 | 0.040 | 0.540 | 0.474 | 0.635 | 0.219 |
| Bursa of Fabricius, g/kg | 1.85 | 1.78 | 1.86 | 1.90 | 0.078 | 0.838 | 0.842 | 0.677 | 0.446 |
| Day 35 | |||||||||
| Intestine, g/kg | 31.01 | 30.83 | 28.81 | 30.16 | 0.816 | 0.808 | 0.570 | 0.669 | 0.521 |
| Gut weight/length, g/m | 31.90 | 30.34 | 30.33 | 29.74 | 0.787 | 0.808 | 0.389 | 0.770 | 0.777 |
| Spleen, g/kg | 1.42 a | 0.80 b | 0.75 b | 0.93 a | 0.082 | 0.001 | 0.004 | 0.001 | 0.476 |
| Bursa of Fabricius, g/kg | 1.13 a | 0.92 ab | 0.71 b | 1.21 a | 0.069 | 0.021 | 0.946 | 0.005 | 0.145 |
| Treatment | 0 ppm | 5 ppm | 10 ppm | 15 ppm | SEM | p-Value | Linear | Quadratic | Cubic |
|---|---|---|---|---|---|---|---|---|---|
| Day 20 | |||||||||
| Villus height, μm | 316.53 c | 335.94 b | 403.85 a | 364.62 ab | 11.173 | 0.013 | 0.013 | 0.096 | 0.053 |
| Crypt depth, μm | 107.78 a | 75.99 b | 80.22 b | 81.79 b | 3.734 | 0.001 | 0.001 | 0.002 | 0.062 |
| Villus crypt ratio | 2.95 b | 4.42 a | 5.04 a | 4.48 a | 0.213 | <0.001 | <0.001 | <0.001 | 0.673 |
| Goblet cells/villus, μm | 0.21 b | 0.36 ab | 0.44 a | 0.51 a | 0.032 | <0.001 | <0.001 | 0.205 | 0.756 |
| Day 35 | |||||||||
| Villus height, μm | 346.11 b | 416.61 ab | 435.86 a | 449.26 a | 14.836 | 0.044 | 0.010 | 0.256 | 0.679 |
| Crypt depth, μm | 103.72 | 102.16 | 93.43 | 87.00 | 3.183 | 0.214 | 0.047 | 0.690 | 0.728 |
| Villus crypt ratio | 3.34 b | 4.09 ab | 4.68 a | 5.26 a | 0.224 | 0.003 | <0.001 | 0.776 | 0.907 |
| Goblet cells/villus, μm | 0.27 | 0.32 | 0.35 | 0.30 | 0.016 | 0.426 | 0.423 | 0.175 | 0.641 |
| Treatment | 0 ppm | 5 ppm | 10 ppm | 15 ppm | SEM | p-Value | Linear | Quadratic | Cubic |
|---|---|---|---|---|---|---|---|---|---|
| Day 20 | |||||||||
| FABP1, fold change | 1.00 | 0.99 | 1.30 | 3.00 | 0.383 | 0.284 | 0.110 | 0.370 | 0.841 |
| CD36, fold change | 1.00 b | 1.13 b | 1.22 b | 2.04 a | 0.177 | 0.035 | 0.036 | 0.298 | 0.611 |
| FATP4, fold change | 1.00 a | 0.57 ab | 0.61 ab | 0.47 b | 0.064 | 0.029 | 0.008 | 0.142 | 0.197 |
| DGAT2, fold change | 1.00 | 1.58 | 1.03 | 1.19 | 0.150 | 0.562 | 0.956 | 0.543 | 0.200 |
| Day 35 | |||||||||
| FABP1, fold change | 1.00 | 1.33 | 1.67 | 1.38 | 0.199 | 0.460 | 0.128 | 0.850 | 0.934 |
| CD36, fold change | 1.00 b | 1.04 b | 1.60 ab | 3.07 a | 0.278 | 0.048 | 0.021 | 0.282 | 0.998 |
| FATP4, fold change | 1.00 | 0.74 | 1.52 | 1.41 | 0.175 | 0.140 | 0.064 | 0.474 | 0.255 |
| DGAT2, fold change | 1.00 b | 0.92 b | 2.06 ab | 3.52 a | 0.381 | 0.019 | 0.004 | 0.176 | 0.758 |
| Treatment | 0 ppm | 5 ppm | 10 ppm | 15 ppm | SEM | p-Value | Linear | Quadratic | Cubic |
|---|---|---|---|---|---|---|---|---|---|
| Day 20 | |||||||||
| E. coli, fold change | 1.00 | 0.46 | 0.89 | 0.51 | 0.164 | 0.636 | 0.245 | 0.930 | 0.701 |
| Streptococcus spp., fold change | 1.00 a | 0.17 b | 0.16 b | 0.23 b | 0.132 | 0.035 | 0.017 | 0.092 | 0.327 |
| Salmonella spp., fold change | 1.00 | 1.06 | 1.17 | 1.10 | 0.109 | 0.973 | 0.912 | 0.691 | 0.808 |
| Day 35 | |||||||||
| E. coli, fold change | 1.00 | 1.64 | 0.64 | 1.45 | 0.184 | 0.224 | 0.105 | 0.435 | 0.268 |
| Streptococcus spp., fold change | 1.00 | 0.32 | 0.89 | 0.84 | 0.144 | 0.203 | 0.219 | 0.253 | 0.282 |
| Salmonella spp., fold change | 1.00 | 0.77 | 0.45 | 0.72 | 0.092 | 0.256 | 0.584 | 0.119 | 0.257 |
| Treatment | 0 ppm | 5 ppm | 10 ppm | 15 ppm | SEM | p-Value | Linear | Quadratic | Cubic |
|---|---|---|---|---|---|---|---|---|---|
| Day 20 | |||||||||
| Acetate, mmol/g | 107.99 | 169.39 | 148.70 | 127.02 | 12.007 | 0.328 | 0.732 | 0.108 | 0.453 |
| Propionate, mmol/g | 8.44 | 13.95 | 13.52 | 14.99 | 1.410 | 0.402 | 0.161 | 0.489 | 0.545 |
| Iso-butyrate, mmol/g | 2.67 | 3.16 | 2.55 | 2.77 | 0.223 | 0.839 | 0.905 | 0.790 | 0.412 |
| Butyrate, mmol/g | 8.97 b | 19.03 a | 21.02 a | 11.25 b | 2.301 | 0.029 | 0.637 | 0.039 | 0.844 |
| Total, mmol/g | 128.06 | 205.53 | 185.79 | 156.04 | 15.007 | 0.304 | 0.627 | 0.096 | 0.512 |
| Day 35 | |||||||||
| Acetate, mmol/g | 149.20 | 165.49 | 187.14 | 206.04 | 12.292 | 0.430 | 0.117 | 0.959 | 0.943 |
| Propionate, mmol/g | 12.97 b | 16.17 ab | 22.01 a | 22.91 a | 1.758 | 0.016 | 0.026 | 0.705 | 0.578 |
| Iso-butyrate, mmol/g | 3.39 | 3.96 | 3.96 | 4.24 | 0.330 | 0.872 | 0.466 | 0.844 | 0.805 |
| Butyrate, mmol/g | 15.47 | 19.68 | 23.45 | 25.32 | 2.494 | 0.578 | 0.190 | 0.829 | 0.951 |
| Total, mmol/g | 181.04 | 205.30 | 236.56 | 258.51 | 16.284 | 0.390 | 0.102 | 0.972 | 0.912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, M.-J.; Choi, S.-W.; Choi, Y.-S.; Lee, H.; Park, M.-Y.; Whang, K.-Y. Effects of Sophorolipid on Growth Performance, Organ Characteristics, Lipid Digestion Markers, and Gut Functionality and Integrity in Broiler Chickens. Animals 2022, 12, 635. https://doi.org/10.3390/ani12050635
Kwak M-J, Choi S-W, Choi Y-S, Lee H, Park M-Y, Whang K-Y. Effects of Sophorolipid on Growth Performance, Organ Characteristics, Lipid Digestion Markers, and Gut Functionality and Integrity in Broiler Chickens. Animals. 2022; 12(5):635. https://doi.org/10.3390/ani12050635
Chicago/Turabian StyleKwak, Min-Jin, Sun-Woo Choi, Yong-Soon Choi, Hanbae Lee, Min-Young Park, and Kwang-Youn Whang. 2022. "Effects of Sophorolipid on Growth Performance, Organ Characteristics, Lipid Digestion Markers, and Gut Functionality and Integrity in Broiler Chickens" Animals 12, no. 5: 635. https://doi.org/10.3390/ani12050635





