Effects of Competitive ELISA-Positive Results of Piroplasmosis on the Performance of Endurance Horses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. Parasitological Methods
2.4. Hematological and Biochemical Analysis
2.5. Statistical Analysis
3. Results
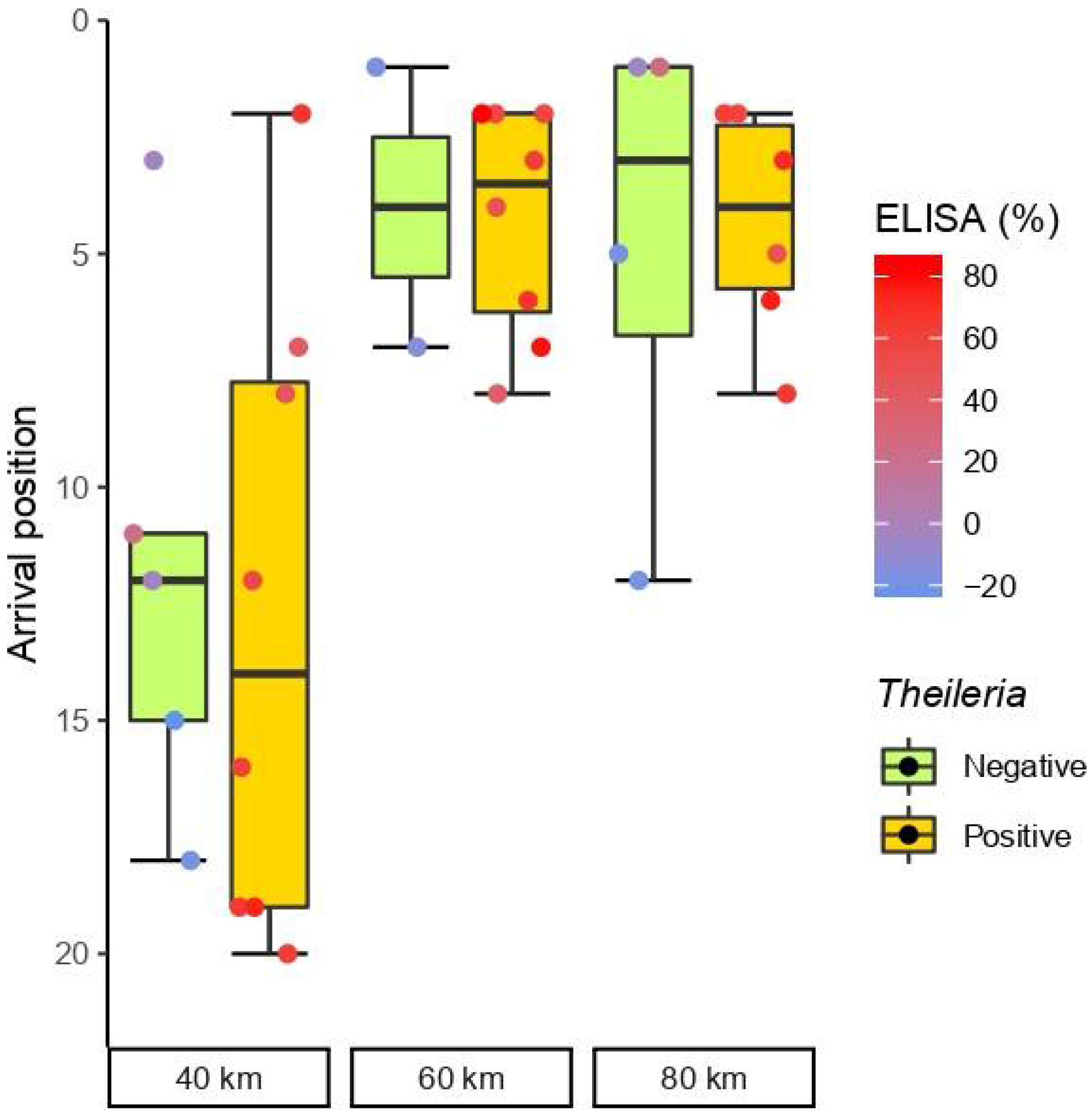
3.1. Equine Piroplasmosis Test
3.2. Horse Performance and Endurance
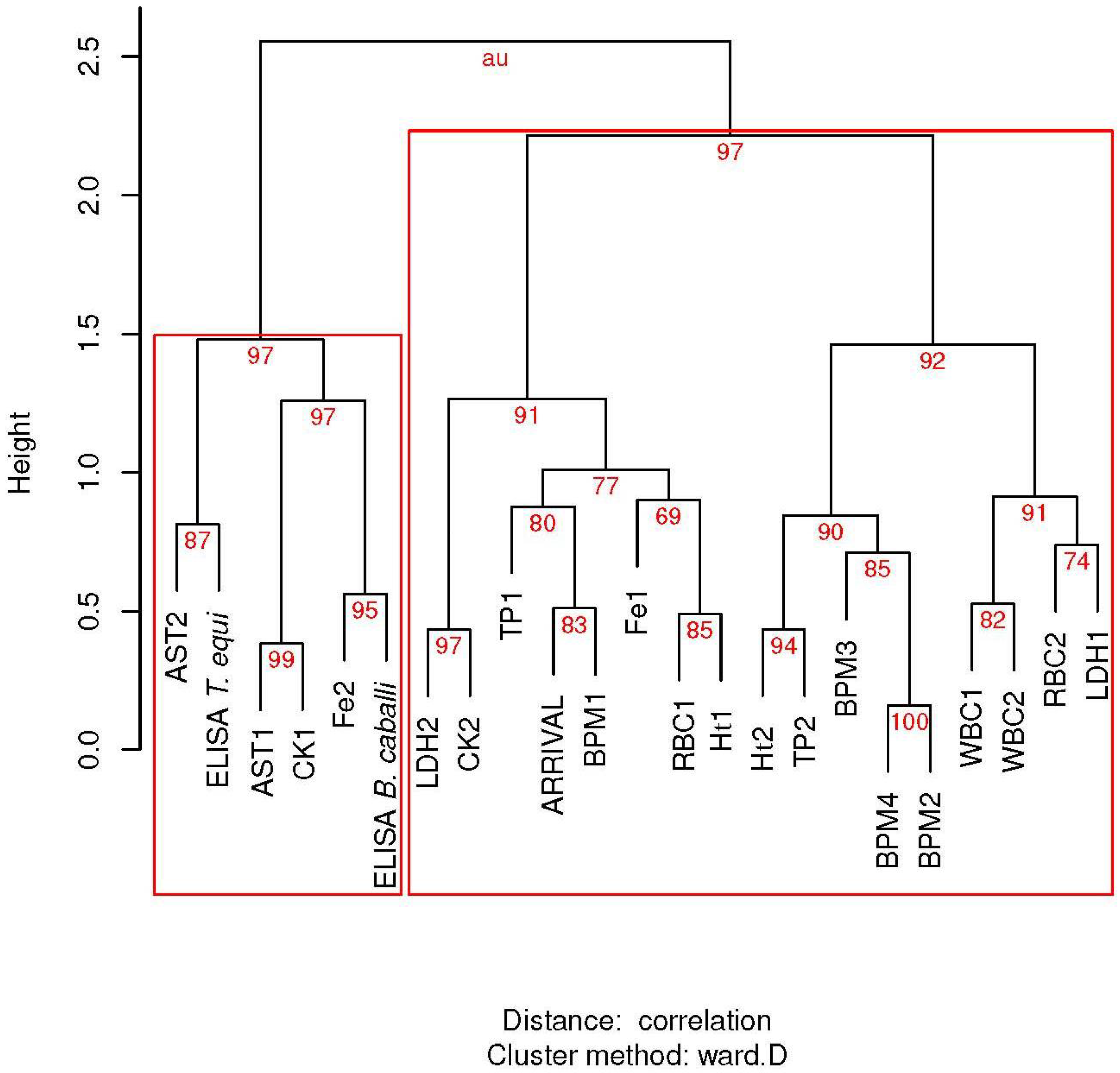
3.3. Influence of Piroplasmosis Status in Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FEI Endurance Rules. Available online: https://inside.fei.org/fei/disc/endurance/rules (accessed on 11 December 2021).
- Barnes, A.; Kingston, J.; Beetson, S.; Kuiper, C. Endurance veterinarians detect physiologically compromised horses in a 160 km ride. Equine Vet. J. 2010, 42, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Riber, C.; Trigo, P.; Castejón-Riber, C.; Castejon, F.M. Dehydration, electrolyte imbalances and renin-angiotensin-aldosterone-vasopressin axis in successful and unsuccessful endurance horses. Equine Vet. J. 2010, 42, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Schott, H.C.; Marlin, D.J.; Geor, R.J.; Holbrook, T.C.; Deaton, C.M.; Vincent, T.; Dacre, K.; Schroter, R.C.; Jose-Cunilleras, E.; Cornelisse, C.J. Changes in selected physiological and laboratory measurements in elite horses competing in a 160 km endurance ride. Equine Vet. J. 2006, 38, 37–42. [Google Scholar] [CrossRef]
- Burger, D.; Dollinger, S. Raisons d’élimination, état de santé et carrière sportive des chevaux dans les raids d’endurance en Europe et dans les pays Arabes: Approche statistique. Prat. Vet. Equine 1998, 30, 91–97. [Google Scholar]
- Langlois, C.; Robert, C. Épidémiologie des troubles métaboliques chez les chevaux d’endurance—Pratique Vétérinaire Equine n° 157 du 01/01/2008. Prat. Vet. Equine 2008, 40, 51–60. [Google Scholar]
- Fielding, C.L.; Meier, C.A.; Balch, O.K.; Kass, P.H. Risk factors for the elimination of endurance horses from competition. J. Am. Vet. Med. Assoc. 2011, 239, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Nagy, A.; Murray, J.K.; Dyson, S.J. Horse-, rider-, venue- and environment-related risk factors for elimination from Fédération Equestre Internationale endurance rides due to lameness and metabolic reasons. Equine Vet. J. 2014, 46, 294–299. [Google Scholar] [CrossRef]
- Wilberger, M.S.; Mckenzie, E.C.; Payton, M.E.; Rigas, J.D.; Valberg, S.J. Prevalence of exertional rhabdomyolysis in endurance horses in the Pacific Northwestern United States. Equine Vet. J. 2015, 47, 165–170. [Google Scholar] [CrossRef]
- Flethøj, M.; Schwarzwald, C.C.; Haugaard, M.M.; Carstensen, H.; Kanters, J.K.; Olsen, L.H.; Buhl, R. Left ventricular function after prolonged exercise in equine endurance athletes. J. Vet. Intern. Med. 2016, 30, 1260–1269. [Google Scholar] [CrossRef] [Green Version]
- van der Kolk, J.H.; Thomas, S.; Mach, N.; Ramseyer, A.; Burger, D.; Gerber, V.; Nuoffer, J.M. Serum acylcarnitine profile in endurance horses with and without metabolic dysfunction. Vet. J. 2020, 255, 105419. [Google Scholar] [CrossRef]
- Wise, L.N.; Kappmeyer, L.S.; Mealey, R.H.; Knowles, D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013, 27, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; Arenas-Montes, A.; Hernández, E.; Adaszek, Ł.; Carbonero, A.; Almería, S.; Jaén-Téllez, J.A.; Gutiérrez-Palomino, P.; Arenas, A. Seroprevalence and risk factors associated with Babesia caballi and Theileria equi infection in equids. Vet. J. 2013, 195, 172–178. [Google Scholar] [CrossRef]
- Cortés, M.G.M.; Fernández-García, J.L.; Martínez-Estéllez, M.Á.H. Seroprevalence of Theileria equi and Babesia caballi in horses in Spain. Parasite 2017, 24, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, S.; Bayly, W.; Sellon, D. Equine Internal Medicine, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Rothschild, C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013, 33, 497–508. [Google Scholar] [CrossRef]
- Sumbria, D.; Moudgil, A.D.; Singla, L. Equine Piroplasmosis: Current status. Veterinaria 2014, 1, 9–14. [Google Scholar]
- Laus, F.; Spaterna, A.; Faillace, V.; Veronesi, F.; Ravagnan, S.; Beribé, F.; Cerquetella, M.; Meligrana, M.; Tesei, B. Clinical investigation on Theileria equi and Babesia caballi infections in Italian donkeys. BMC Vet. Res. 2015, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Camino, E.; Pozo, P.; Dorrego, A.; Carvajal, K.A.; Buendia, A.; Gonzalez, S.; de Juan, L.; Dominguez, L.; Cruz-Lopez, F. Importance of equine piroplasmosis antibody presence in Spanish horses prior to export. Ticks Tick Borne Dis. 2020, 11, 101329. [Google Scholar] [CrossRef]
- Anonymous. Equine Piroplasmosis. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 3.5.8; OIE: Paris, France, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 10 January 2022).
- Xie, Y. Knitr: A General-Purpose Package for Dynamic Report Generation in R. 2019. Available online: https://rdrr.io/cran/knitr/ (accessed on 10 January 2022).
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef]
- Suzuki, R.; Shimodaira, H.; Suzuki, M.R. Suggests MASS. Package ‘Pvclust’. 2019. Available online: https://cran.r-project.org/web/packages/pvclust/pvclust.pdf (accessed on 10 January 2022).
- Amrhein, V.; Korner-Nievergelt, F.; Roth, T. The earth is flat (p > 0.05): Significance thresholds and the crisis of unreplicable research. PeerJ 2017, 5, e3544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, E.P.; Vidotto, O.; Almeida, J.C.; Ribeiro, L.P.; Borges, M.V.; Pequeno, W.H.; Stipp, D.T.; de Oliveira, C.J.; Biondo, A.W.; Vieira, T.S.; et al. Serological and molecular detection of Theileria equi in sport horses of northeastern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2016, 47, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Carapeto, M.V.; Habela, M.A.; Zaragoza, C. Electrophoretic pattern of serum proteins in horses with babesiosis. Arch. Med. Vet. 2010, 42, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Camacho, A.T.; Guitian, F.J.; Pallas, E.; Gestal, J.J.; Olmeda, A.S.; Habela, M.A.; Telford, S.R.; Spielman, A. Theileria (Babesia) equi and Babesia caballi infections in horses in Galicia, Spain. Trop. Anim. Health Prod. 2005, 37, 293–302. [Google Scholar] [CrossRef]
- Camino, E.; Buendia, A.; Dorrego, A.; Pozo, P.; de Juan, L.; Dominguez, L.; Cruz-Lopez, F. Sero-molecular survey and risk factors of equine piroplasmosis in horses in Spain. Equine Vet. J. 2021, 53, 771–779. [Google Scholar] [CrossRef]
- Habela, M.; Reina, D.; Nieto, C.; Verdugo, S.G.; Navarrete, I. Epidemiología de la babesiosis equina en Extremadura: Estudio preliminar. Med. Vet. 1989, 6, 31–39. [Google Scholar]
- Kouam, M.K.; Kantzoura, V.; Gajadhar, A.A.; Theis, J.H.; Papadopoulos, E.; Theodoropoulos, G. Seroprevalence of equine piroplasms and host-related factors associated with infection in Greece. Vet. Parasitol. 2010, 169, 273–278. [Google Scholar] [CrossRef]
- Grandi, G.; Molinari, G.; Tittarelli, M.; Sassera, D.; Kramer, L.H. Prevalence of Theileria equi and Babesia caballi infection in horses from Northern Italy. Vector-Borne Zoonotic Dis. 2011, 11, 955–956. [Google Scholar] [CrossRef]
- Guidi, E.; Pradier, S.; Lebert, I.; Leblond, A. Piroplasmosis in an endemic area: Analysis of the risk factors and their implications in the control of theileriosis and babesiosis in horses. Parasitol. Res. 2015, 114, 71–83. [Google Scholar] [CrossRef]
- Bartolomé Del Pino, L.E.; Nardini, R.; Veneziano, V.; Iacoponi, F.; Cersini, A.; Autorino, G.L.; Buono, F.; Scicluna, M. Babesia caballi and Theileria equi infections in horses in Central-Southern Italy: Sero-molecular survey and associated risk factors. Ticks Tick Borne Dis. 2016, 7, 462–469. [Google Scholar] [CrossRef]
- Sigg, L.; Gerber, V.; Gottstein, B.; Doherr, M.G.; Frey, C.F. Seroprevalence of Babesia caballi and Theileria equi in the Swiss horse population. Parasitol. Int. 2010, 59, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Zobba, R.; Ardu, M.; Niccolini, S.; Chessa, B.; Manna, L.; Cocco, R.; Parpaglia, M.L.P. Clinical and laboratory findings in equine piroplasmosis. J. Equine Vet. Sci. 2008, 28, 301–308. [Google Scholar] [CrossRef]
- Rousseau, N.; Pige, R.; Cohen, R.; Pecquet, M. What is the acceptable hemolysis index for the measurements of plasma potassium, LDH and AST? Ann. Biol. Clin. 2016, 74, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Hailat, N.Q.; Lafi, S.Q.; Al-Darraji, A.M.; Al-Ani, F.K. Equine babesiosis associated with strenuous exercise: Clinical and pathological studies in Jordan. Vet. Parasitol. 1997, 69, 1–8. [Google Scholar] [CrossRef]
- Pasolini, M.P.; Pagano, T.B.; Costagliola, A.; Biase, D.D.; Lamagna, B.; Auletta, L.; Fatone, G.; Greco, M.; Coluccia, P.; Vincenzo, V.; et al. Inflammatory myopathy in horses with chronic piroplasmosis. Vet. Pathol. 2018, 55, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Maśko, M.; Domino, M.; Jasiński, T.; Witkowska-Piłaszewicz, O. The physical activity-dependent hematological and biochemical changes in school horses in comparison to blood profiles in endurance and race horses. Animals 2021, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Jagrič-Munih, S.; Nemec-Svete, A.; Zrimšek, P.; Kramarič, P.; Kos-Kadunc, V.; Vovk, T.; Kobal, S. Plasma malondialdehyde, biochemical and haematological parameters in standardbred horses during a selected field exercise test. Acta Vet. 2012, 62, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Jović, S.; Stevanović, J.; Borozan, S.; Dimitrijević, B.; Milosavljević, P. Influence of physical activity of racehorses on lactate dehydrogenase and creatine kinase activities, and protein synthesis. Acta Vet. 2013, 63, 549–568. [Google Scholar] [CrossRef]
- Larsson, J.; Pilborg, P.H.; Johansen, M.; Christophersen, M.T.; Holte, A.; Roepstorff, L.; Olsen, L.H.; Harrison, A.P. Physiological parameters of Endurance horses pre-compared to post-race, correlated with performance: A two race study from Scandinavia. ISRN Vet. Sci. 2013, 18, 684353. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Kaneps, A.J.; Geor, R.J. Equine Exercise Physiology: The Science of Exercise in the Athletic Horse; Elsevier Ltd.: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Goachet, A.G.; Fraipont, A.; Votion, D.M.; Van Erck, E.; Leclerc, J.L. Hydration and electrolyte balance in horses during an endurance season. Equine Vet. J. 2010, 42, 98–104. [Google Scholar] [CrossRef]
- Allen, P.C.; Frerichs, W.M.; Holbrook, A.A. Experimental acute Babesia caballi infections. I. Red blood cell dynamics. Exp. Parasitol. 1975, 37, 67–77. [Google Scholar] [CrossRef]
- Knowles, D.P. Control of Babesia equi parasitemia. Parasitol. Today 1996, 12, 195–198. [Google Scholar] [CrossRef]
- Kerber, C.E.; Ferreira, F.; Pereira, M.C. Control of equine piroplasmosis in Brazil. Onderstepoort J. Vet. Res. 1999, 66, 123–127. [Google Scholar] [PubMed]
- Garba, U.M.; Sackey, A.K.B.; Agbede, R.I.S.; Tekdek, L.B.; Bisalla, M. Hemogram changes in Nigerian royal horses with natural piroplasmosis. J. Indian Vet. Assoc. 2011, 9, 5–9. [Google Scholar]
- Scoppetta, F.; Tartaglia, M.; Renzone, G.; Avellini, L.; Gaiti, A.; Scaloni, A.; Chiaradia, E. Plasma protein changes in horse after prolonged physical exercise: A proteomic study. J. Proteom. 2012, 75, 4494–4504. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Bąska, P.; Czopowicz, M.; Żmigrodzka, M.; Szczepaniak, J.; Szarska, E.; Winnicka, A.; Cywińska, A. Changes in serum Amyloid A (SAA) Concentration in arabian endurance horses during first training season. Animals 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Trigo, P.; Castejon, F.; Riber, C.; Muñoz, A. Use of biochemical parameters to predict metabolic elimination in endurance rides. Equine Vet. J. 2010, 42, 142–146. [Google Scholar] [CrossRef]
- Azevedo, J.F.; Miranda, A.C.T.; Ramos, M.T.; Oliveira, C.A.A.; Padilha, F.G.F.; Silva, V.P.; Baldani, C.D.; Almeida, F.Q. Evaluation of physiological responses of cardiac and blood parameters after incremental exercise on field and laboratory tests in eventing horses. Comp. Exerc. Physiol. 2014, 10, 33–37. [Google Scholar] [CrossRef]
- Hodgson, D.; McKeever, K.; McGowan, C. The Athletic Horse: Principles and Practice of Equine Sports Medicine, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Dias, D.C.R.; da Silva Rocha, J.; Mello, F.M.; dos Santos El-Bachá, R.; Ayres, M.C. Influência do exercício sobre o hemograma, enzimas marcadoras de lesão muscular e índice de peroxidação de biomoléculas em eqüinos submetidos à atividade de salto. Rev. Bras. Cien. Vet. 2011, 18, 32–35. [Google Scholar]
- Tirosh-Levy, S.; Gottlieb, Y.; Steinman, A. Stress conditions do not affect Theileria equi parasitemia levels in sub-clinically infected horses. Ticks Tick Borne Dis. 2020, 11, 101384. [Google Scholar] [CrossRef]

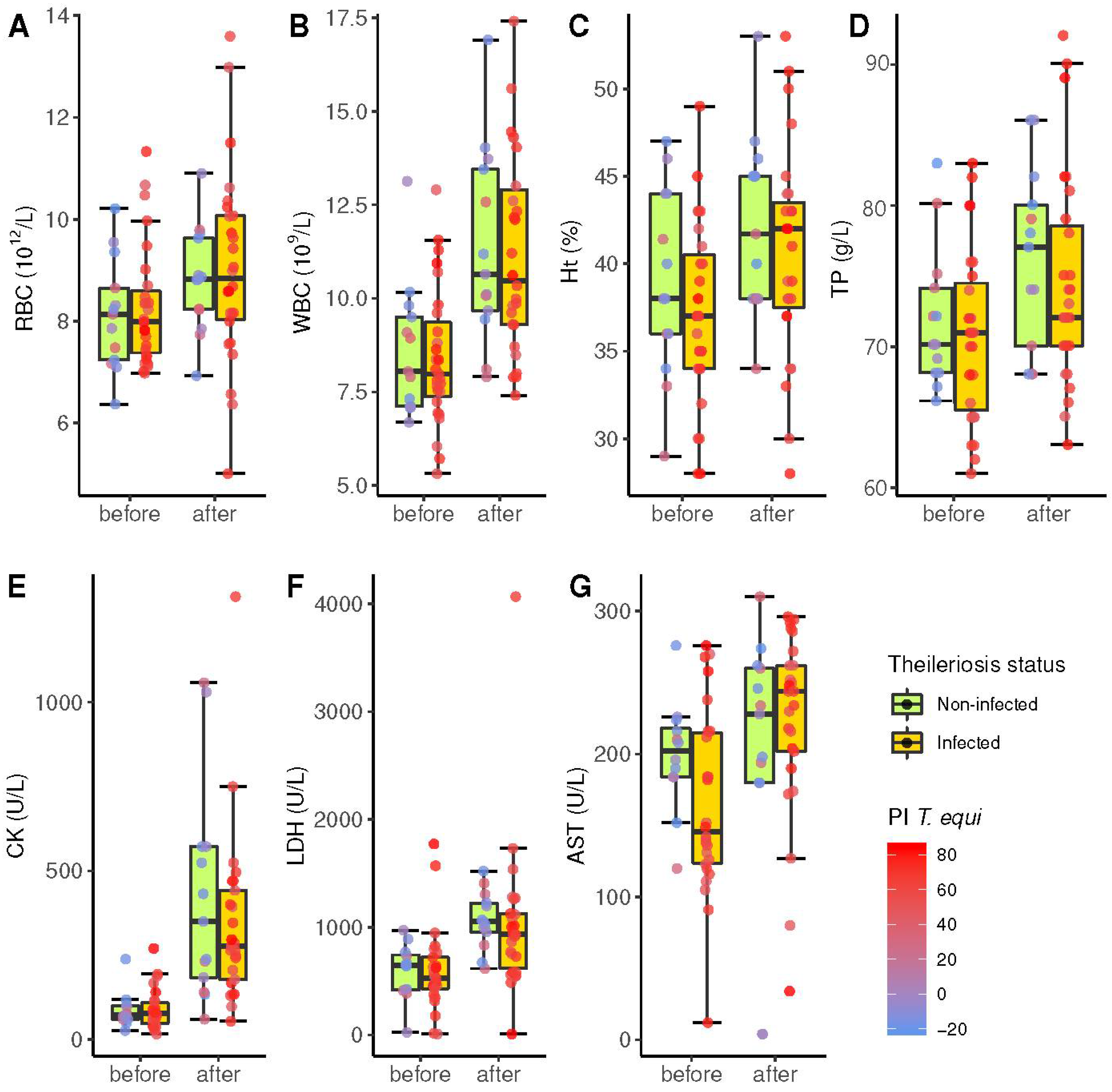
| Global Group (n = 40) | Seropositive EP (n = 28) | Seronegative EP (n = 12) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter (SI Units) | Reference Values | n1 | n2 | Before Exercise | After Exercise | p-Value | Before Exercise | After Exercise | Before Exercise | After Exercise | p-Value |
| RBC (1012/L) | 6.40−10.40 | 40 | 39 | 8.2 ± 1.1 | 9.0 ± 1.7 | 0.0046 | 8.2 ± 1.2 | 9.0 ± 1.9 | 8.3 ± 1.2 | 9.0 ± 1.9 | 0.41 |
| Ht (%) | 30−47 | 40 | 40 | 37.7 ± 5.3 | 41.2 ± 5.7 | 4.8 × 10−4 | 37.1 ± 5.2 | 40.6 ± 5.9 | 38.9 ± 5.2 | 42.6 ± 5.9 | 0.12 |
| WBC (109/L) | 4.90−11.10 | 40 | 39 | 8.5 ± 1.8 | 10.8 ± 2.6 | 9.2 × 10−8 | 8.4 ± 1.8 | 10.8 ± 2.6 | 8.7 ± 1.8 | 11.0 ± 2.6 | 0.34 |
| TP (g/L) | 56−79 | 40 | 40 | 71 ± 6 | 75 ± 7 | 37 × 10−4 | 71 ± 6 | 74 ± 8 | 70 ± 6 | 77 ± 8 | 0.08 |
| Iron (µmol/L) | 18.8−49.6 | 38 | 37 | 28.5 ± 4.5 | 29.4 ± 10.2 | 3.7 × 10−4 | 28.1 ± 4.1 | 30.1 ± 11.8 | 29.5 ± 4.1 | 27.6 ± 11.8 | 0.68 |
| CK (U/L) | 10−350 | 38 | 38 | 88 ± 57 | 371 ± 281 | 1.8 × 10−10 | 90 ± 59 | 350 ± 256 | 85 ± 59 | 417 ± 256 | 0.33 |
| LDH (U/L) | 250−2070 | 37 | 39 | 592 ± 354 | 1039 ± 601 | 3.6 × 10−5 | 575 ± 388 | 1027 ± 704 | 600 ± 388 | 1066 ± 704 | 0.25 |
| AST (U/L) | 100−600 | 38 | 38 | 176 ± 59 | 214 ± 76 | 0.0018 | 167 ± 64 | 224 ± 65 | 197 ± 64 | 193 ± 65 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Barriga, D.; Serrano-Aguilera, F.J.; Barrasa-Rita, R.; Habela, M.Á.; Chacón, R.B.; Ezquerra, L.J.; Martín-Cuervo, M. Effects of Competitive ELISA-Positive Results of Piroplasmosis on the Performance of Endurance Horses. Animals 2022, 12, 637. https://doi.org/10.3390/ani12050637
Bravo-Barriga D, Serrano-Aguilera FJ, Barrasa-Rita R, Habela MÁ, Chacón RB, Ezquerra LJ, Martín-Cuervo M. Effects of Competitive ELISA-Positive Results of Piroplasmosis on the Performance of Endurance Horses. Animals. 2022; 12(5):637. https://doi.org/10.3390/ani12050637
Chicago/Turabian StyleBravo-Barriga, Daniel, Francisco J. Serrano-Aguilera, Rafael Barrasa-Rita, Miguel Ángel Habela, Rafael Barrera Chacón, Luis Javier Ezquerra, and María Martín-Cuervo. 2022. "Effects of Competitive ELISA-Positive Results of Piroplasmosis on the Performance of Endurance Horses" Animals 12, no. 5: 637. https://doi.org/10.3390/ani12050637
APA StyleBravo-Barriga, D., Serrano-Aguilera, F. J., Barrasa-Rita, R., Habela, M. Á., Chacón, R. B., Ezquerra, L. J., & Martín-Cuervo, M. (2022). Effects of Competitive ELISA-Positive Results of Piroplasmosis on the Performance of Endurance Horses. Animals, 12(5), 637. https://doi.org/10.3390/ani12050637







