Molecular Biomarkers of Adult Human and Dog Stress during Canine-Assisted Interventions: A Systematic Scoping Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Which molecular biomarkers have been used to measure the effects of AAIs on human and/or dog stress?
- What stress-related outcomes have been found for humans and canines?
- In studies that measure molecular biomarkers of stress, do any also include physiological measures or subjective or behavioral assessments of the same or related outcomes? If so, what measures were used and what outcomes were found?
2.1. Identifying Relevant Studies
2.2. Study Selection
2.3. Data Extraction and Synthesis
3. Results
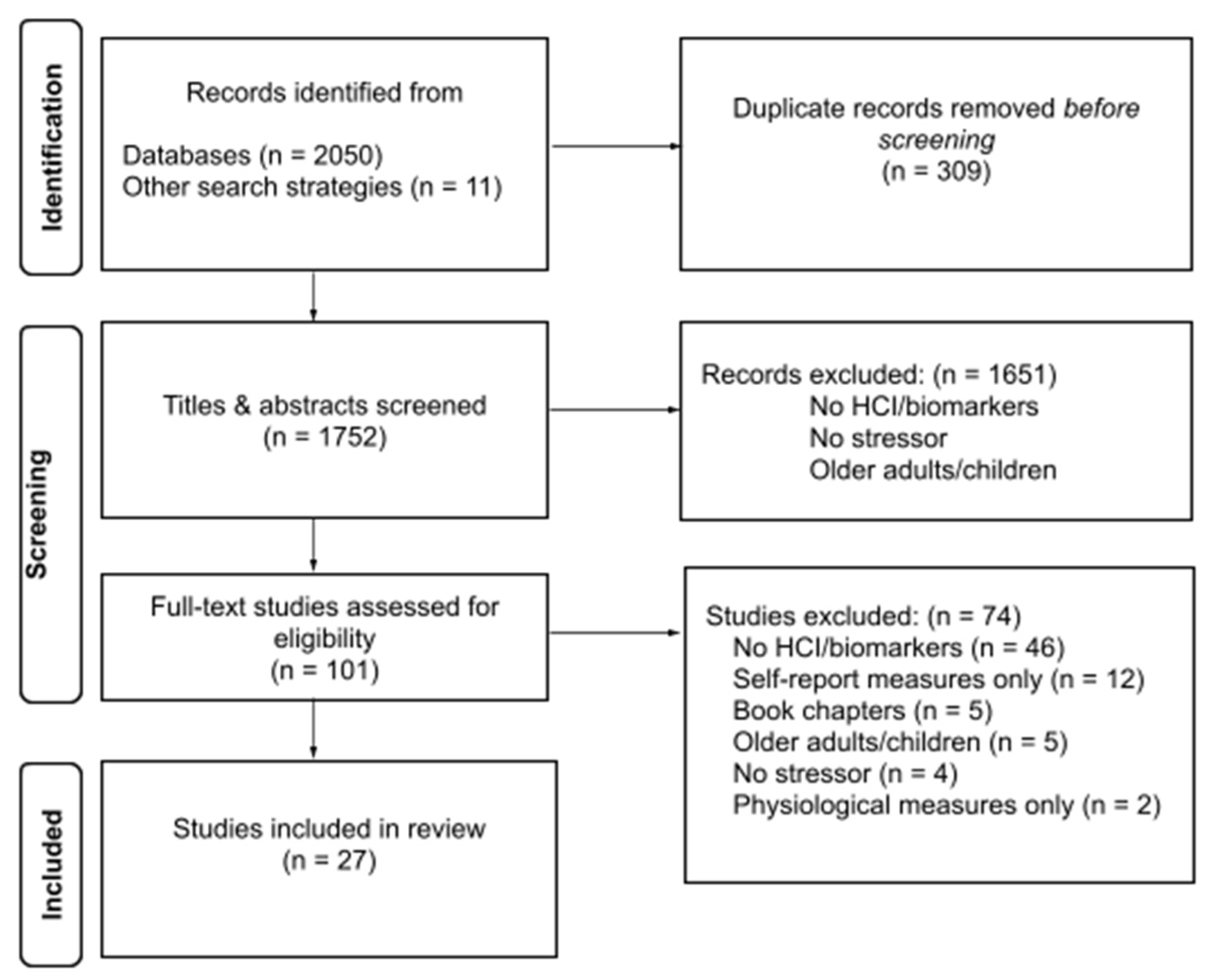
3.1. Overview of Search Results
3.2. Study Characteristics and Key Results: Humans
3.3. Study Characteristics and Key Results: Dogs
4. Discussion
4.1. Overview of Results
4.2. Methodological Issues
4.3. Limitations
4.4. Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stress in America 2020: A National Mental Health Crisis. Available online: https://www.apa.org/news/press/releases/stress/2020/rehttps://www.apa.org/news/press/releases/stress/2020/report-octoberport-october (accessed on 12 February 2021).
- Salleh, M.R. Life event, stress and illness. Malays. J. Med. Sci. 2008, 4, 9–18. [Google Scholar]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef]
- Herbet, T.B. Stress and the immune system. World Health 2004, 2, 4–5. [Google Scholar]
- Guilliams, T.G.; Edwards, L. Chronic stress and the HPA axis: Clinical assessment and therapeutic considerations. Point Inst. Nutraceutical Res. 2018. Available online: https://www.pointinstitute.org/wp-content/uploads/2012/10/standard_v_9.2_hpa_axis.pdf (accessed on 9 September 2021).
- Kudielka, B.M.; Hellhammer, D.H.; Wüst, S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology 2008, 34, 2–18. [Google Scholar] [CrossRef]
- Morales-Jinez, A.; López-Rincón, F.J.; Ugarte-Esquivel, A.; Andrade-Valles, I.; Rodríguez-Mejía, L.E.; Hernández-Torres, J.L. Allostatic load and canine companionship: A comparative study using biomarkers in older adults. Rev. Lat.-Am. 2018, 26, E3071. [Google Scholar] [CrossRef]
- Heady, B.; Na, F.; Zheng, R. Pet dogs benefit owners’ health: A ‘natural experiment’ in China. Soc. Indic. Res. 2008, 87, 481–493. [Google Scholar] [CrossRef]
- Krause-Parello, C.A.; Gulick, E.E. Forensic interviews for child sexual abuse allegations: An investigation into the effects of animal-assisted intervention on stress biomarkers. J. Child Sex. Abuse 2015, 24, 873–886. [Google Scholar] [CrossRef]
- Allen, K.M.; Blascovich, J.; Tomaka, J.; Kelsey, R.M. Presence of human friends and pet dogs as moderators of autonomic responses to stress in women. J. Pers. Soc. Psychol. 1991, 61, 582–589. [Google Scholar] [CrossRef]
- Campo, R.A.; Uchino, B.N. Humans’ bonding with their companion dogs: Cardiovascular benefits during and after stress. J. Soc. Soc. Welf. 2013, 40, 237–260. [Google Scholar]
- Polheber, J.P.; Matchock, R.L. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J. Behav. Med. 2014, 37, 860–867. [Google Scholar] [CrossRef]
- Flynn, E.; Gandenberger, J.; Mueller, M.K.; Morris, K.N. Animal-assisted interventions as an adjunct to therapy for youth: Clinician perspectives. Child Adolexc. Soc. Work J. 2020, 37, 631–642. [Google Scholar] [CrossRef]
- Fine, A.H.; Beck, A.M.; Ng, Z. The state of animal-assisted interventions: Addressing the contemporary issues that will shape the future. Int. J. Environ. Res. Public Health 2019, 16, 3997. [Google Scholar] [CrossRef] [Green Version]
- Glenk, L.M. Current perspectives on therapy dog welfare in animal-assisted interventions. Animals 2017, 7, 7. [Google Scholar] [CrossRef]
- Pendry, P.; Kuzara, S.; Gee, N.R. Evaluation of undergraduate students’ responsiveness to a 4-week university-based animal-assisted stress prevention program. Int. J. Environ. Res. Public Health 2019, 16, 3331. [Google Scholar] [CrossRef] [Green Version]
- Buttner, A.P.; Thompson, B.; Strasser, R.; Santo, J. Evidence for a synchronization of hormonal states between humans and dogs during competition. Physiol. Behav. 2015, 147, 54–62. [Google Scholar] [CrossRef]
- Sundman, A.S.; Van Poucke, E.; Svensson Holm, A.C.; Faresjö, Å.; Theodorsson, E.; Jensen, P.; Roth, L.S. Long-term stress levels are synchronized in dogs and their owners. Sci. Rep. 2019, 9, 7391. [Google Scholar] [CrossRef] [Green Version]
- Wojtaś, J.; Karpiński, M.; Czyżowski, P. Salivary cortisol interactions in search and rescue dogs and their handlers. Animals 2020, 10, 595. [Google Scholar] [CrossRef] [Green Version]
- Cobb, M.L.; Iskandarani, K.; Chinchilli, V.M.; Dreschel, N.A. A systematic review and meta-analysis of salivary cortisol measurement in domestic canines. Deomest. Anim. Endocrinol. 2016, 57, 31–42. [Google Scholar] [CrossRef]
- Coppola, C.L.; Grandin, T.; Enns, R.M. Human interaction and cortisol: Can human contact reduce stress for shelter dogs? Physiol. Behav. 2006, 87, 537–541. [Google Scholar] [CrossRef]
- Haubenhofer, D.K.; Kirchengast, S. Dog handlers’ and dogs’ emotional and cortisol secretion responses associated with animal-assisted therapy sessions. Soc. Anim. 2007, 15, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, M.B.; Williams, M.T.; Miller, D.D.; Douglas, C.W.; Voith, V.L. Influence of male and female petters on plasma cortisol and behaviour: Can human interaction reduce the stress of dogs in a public animal shelter? Appl. Anim. Behav. Sci. 1998, 61, 63–77. [Google Scholar] [CrossRef]
- King, C.; Watter, J.; Mungre, S. Effect of a time-out session with working animal-assisted therapy dogs. J. Vet. Behav. 2011, 6, 232–238. [Google Scholar] [CrossRef]
- Willen, R.M.; Mutwill, A.; MacDonald, L.J.; Schiml, P.A.; Hennessy, M.B. Factors determining the effects of human interaction on the cortisol levels of shelter dogs. Appl. Anim. Behav. Sci. 2017, 186, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.D.; Smidt, J.M.; Bauer, B.A. Therapy dogs’ and handlers’ behavior and salivary cortisol during initial visits in a complex medical institution: A pilot study. Front. Vet. Sci. 2020, 13, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Maclean, E.L.; Gesquiere, L.R.; Gee, N.; Levy, K.; Martin, W.L.; Carter, S.C. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J. Neurosci. Methods 2018, 293, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Cochrane Consumers and Communication Review Group: Data Synthesis and Analysis. Available online: https://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/AnalysisRestyled.pdf (accessed on 8 October 2020).
- Barker, S.B.; Knisely, J.S.; McCain, N.L.; Schubert, C.M.; Pandurangi, A.K. Exploratory study of stress-buffering response patterns from interactions with a therapy dog. Anthrozoös 2010, 23, 79–91. [Google Scholar] [CrossRef]
- Barker, S.B.; Barker, R.T.; McCain, N.L.; Schubert, C.M. A randomized cross-over exploratory study of the effect of visiting therapy dogs on college student stress before final exams. Anthrozoös 2016, 29, 35–46. [Google Scholar] [CrossRef]
- Clark, S.D.; Smidt, J.M.; Bauer, B.A. Welfare considerations: Salivary cortisol concentrations on frequency of therapy dog visits in an outpatient hospital setting: A pilot study. J. Vet. Behav. 2019, 30, 88–91. [Google Scholar] [CrossRef]
- Clark, S.D.; Martin, F.; McGowan, R.T.S.; Smidt, J.M.; Anderson, R.; Wang, L.; Turpin, T.; Langenfeld-McCoy, N.; Bauer, B.A.; Mohabbat, A.B. The impact of a 20-minute animal-assisted activity session on the physiological and emotional states in patients with fibromyalgia. May Clin. Proc. 2020, 95, 2442–2461. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.D.; Martin, F.; McGowan, R.T.S.; Smidt, J.M.; Anderson, R.; Wang, L.; Turpin, T.; Langenfeld-McCoy, N.; Bauer, B.A.; Mohabbat, A.B. Physiological state of therapy dogs during animal-assisted activities in an outpatient setting. Animals 2020, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Coakley, A.B.; Annese, C.D.; Empoliti, J.H.; Flanagan, J.M. The experience of animal assisted therapy on patients in an acute care setting. Clin. Nurs. Res. 2020, 30, 401–405. [Google Scholar] [CrossRef]
- Cole, K.M.; Gawlinski, A.; Steers, N.; Kotlerman, J. Animal-assisted therapy in patients hospitalized with heart failure. Am. J. Crit. Care 2007, 16, 575–585. [Google Scholar] [CrossRef]
- de Carvalho, I.R.; Nunes, T.; de Sousa, L.; Almeida, V. The combined use of salivary cortisol concentrations, heart rate, and respiratory rate for the welfare assessment of dogs involved in AAI programs. J. Vet. Behav. 2019, 36, 26–33. [Google Scholar] [CrossRef]
- Fecteau, S.-M.; Boivin, L.; Trudel, M.; Corbett, B.A.; Harrell, F.E., Jr.; Viau, R.; Picard, F. Parenting stress and salivary cortisol in parents of children with autism spectrum disorder: Longitudinal variations in the context of a service dog’s presence in the family. Biol. Psychol. 2017, 123, 187–195. [Google Scholar] [CrossRef]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Therapy dogs’ salivary cortisol levels vary during animal-assisted interventions. Anim. Welf. 2013, 22, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Salivary cortisol and behavior in therapy dogs during animal-assisted interventions: A pilot study. J. Vet. Behav. 2014, 9, 98–106. [Google Scholar] [CrossRef]
- Kline, J.A.; VanRyzin, K.; Davis, J.C.; Parra, J.A.; Todd, M.L.; Shaw, L.L.; Haggard, B.R.; Fisher, M.A.; Pettit, K.L.; Beck, A.M. Randomized trial of therapy dogs versus deliberative coloring (art therapy) to reduce stress in emergency medicine providers. Acad. Emerg. Med. 2020, 27, 266–275. [Google Scholar] [CrossRef]
- Koda, N.; Watanabe, G.; Miyaji, Y.; Kuniyoshi, M.; Miyaji, C.; Hirata, T. Effects of a dog-assisted intervention assessed by salivary cortisol concentrations in inmates of a Japanese prison. Asian J. Criminol. 2016, 11, 309–319. [Google Scholar] [CrossRef]
- Krause-Parello, C.A.; Levy, C.; Holman, E.; Kolassa, J.E. Effects of VA facility dog on hospitalized veterans seen by a palliative care psychologist: An innovative approach to impacting stress indicators. Am. J. Hosp. Palliat. Care 2018, 35, 5–14. [Google Scholar] [CrossRef]
- Krause-Parello, C.A.; Friedmann, E.; Wilson, C.; Hatzfeld, J.J.; Kolassa, J.; Hackney, A.; Morales, K.A. Relation of post-traumatic stress disorder symptom severity to the efficacy of an animal-assisted intervention for stress reduction after military aeromedical evacuation. Stress Health 2019, 35, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Krause-Parello, C.A.; Friedmann, E.; Blanchard, K.; Payton, M.; Gee, N.R. Veterans and shelter dogs: Examining the impact of a dog-walking intervention on physiological and Post-Traumatic Stress symptoms. Anthrozoös 2020, 33, 225–241. [Google Scholar] [CrossRef]
- Lass-Hennemann, J.; Peyk, P.; Streb, M.; Holz, E.; Michael, T. Presence of a dog reduces subjective but not physiological stress responses to an analog trauma. Front. Psychol. 2014, 5, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lass-Hennemann, J.; Schäfer, S.K.; Römer, S.; Holz, E.; Streb, M.; Michael, T. Therapy dogs as a crisis intervention after traumatic events?—An experimental study. Front. Psychol. 2018, 9, 1627–1637. [Google Scholar] [CrossRef]
- Machová, K.; Součková, M.; Procházková, R.; Vaníčková, Z.; Mezian, K. Canine-assisted therapy improves well-being in nurses. Int. J. Environ. Res. Public Health 2019, 16, 3670. [Google Scholar] [CrossRef] [Green Version]
- Menna, L.F.; Santaniello, A.; Amato, A.; Ceparano, G.; Di Maggio, A.; Sansone, M.; Formisano, P.; Cimmino, I.; Perruolo, G.; Fioretti, A. Changes of oxytocin and serotonin values in dialysis patients after animal assisted activities (AAAs) with a dog—A preliminary study. Animals 2019, 9, 526. [Google Scholar] [CrossRef] [Green Version]
- Nepps, P.; Stewart, C.N.; Bruckno, S.R. Animal-assisted activity: Effects of a complementary intervention program on psychological and physiological variables. J. Evid. Based Complementary Altern. Med. 2014, 19, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Ng, Z.Y.; Pierce, B.J.; Otto, C.M.; Buechner-Maxwell, V.A.; Siracusa, C.; Werre, S.R. The effect of dog–human interaction on cortisol and behavior in registered animal-assisted activity dogs. Appl. Anim. Behav. Sci. 2014, 159, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Pirrone, F.; Ripamonti, A.; Garoni, E.C.; Stradiotti, S.; Albertini, M. Measuring social synchrony and stress in the handler-dog dyad during animal-assisted activities: A pilot study. J. Vet. Behav. 2017, 21, 45–52. [Google Scholar] [CrossRef]
- Rodriguez, K.E.; Bryce, C.I.; Granger, D.A.; O’Haire, M.E. The effect of a service dog on salivary cortisol awakening response in a military population with posttraumatic stress disorder (PTSD). Pyschoneuroendocrinology 2018, 98, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, L.; Sharp, N.C.C.; Doherty, M. Circadian effects on the acute responses of salivary cortisol and IgA in well trained swimmers. Br. J. Sports Med. 2002, 36, 260–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiciuc, C.M.; Cozma-Dima, C.L.; Pasquali, V.; Renzi, P.; Simeoni, S.; Lupusoru, C.E.; Patacchioli, F.R. Awakening responses and diurnal fluctuations of salivary cortisol, DHEA-S and alpha-amylase in healthy male subjects. Neuro Endocrinol. Lett. 2011, 32, 475–480. [Google Scholar]
- Wingenfeld, K.; Schulz, M.; Damkroeger, A.; Philippsen, C.; Rose, M.; Driessen, M. The diurnal course of salivary alpha-amylase in nurses: An investigation of potential confounders and associations with stress. Biol. Psychol. 2010, 85, 179–181. [Google Scholar] [CrossRef]
- Narvaez Linares, N.F.; Charron, V.; Ouimet, A.J.; Labelle, P.R.; Plamondon, H. A systematic review of the Trier Social Stress Test methodology: Issues in promoting study comparison and replicable research. Neurobiol. Stress 2020, 13, 100235. [Google Scholar] [CrossRef]
- Goodman, W.K.; Janson, J.; Wolf, J.M. Meta-analytical assesment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology 2017, 80, 26–35. [Google Scholar] [CrossRef]
- Chong, R.Y.; Uhart, M.; McCaul, M.E.; Johnson, E.; Wand, G.S. Whites have a more robust hypothalamic-pituitary-adrenal axis resopnse to a psychological stressor than blacks. Psychoneuroendocrinology 2008, 33, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Hackler, E.; Lew, J.; Gore, M.O.; Ayers, C.R.; Atzler, D.; Khera, A.; Rohatgi, A.; Lewis, A.; Neeland, I.; Omland, T.; et al. Racial differences in cardiovascular biomarkers in the general population. J. Am. Heart Assoc. 2019, 8, e012729. [Google Scholar] [CrossRef]
- Juster, R.P.; de Torre, M.B.; Kerr, P.; Kheloui, S.; Rossi, M.; Bourdon, O. Sex differences and gender diversity in stress responses and allostatic load among workers and LGBT people. Sex Gend. 2019, 21, 1104. [Google Scholar] [CrossRef]
| Article | Key Demographics | Stressor | Intervention | Stress Measures | Key Outcomes |
|---|---|---|---|---|---|
| Barker et al., 2010 [32] | 10 healthy adults; 8 female, 2 male; 90% White 6 therapy dogs | Stroop Color Word Test | 30-min AAI with own or an unfamiliar (AAA) therapy dog | Biomarkers: Human: sCort, sAA Physiological: Human: BP, HR Subjective: STAI, VAS | sAA, BP, and HR showed little change AAA group showed small increase to sCort with stressor and “negligible” decrease from baseline. Own dog group showed smaller response to stressor and significant decrease post-intervention. Both groups’ subjective stress fell below baseline post-intervention |
| Barker et al., 2016 [33] | 57 adult college students; 44 female, 13 male; 52.6% White 10 therapy dogs | The week before final exams | Control: 15-min attention-control Intervention: 15 min with therapy dog | Biomarkers: Human: sAA, Subjective: PSS, SVAS | No significant pre-post differences to sAA between groups SVAS was lower following intervention: large effect size |
| Clark et al., 2019 [34] | 24 nurses; 23 female, 1 male 4 therapy dogs | AAA stress on dog | AAT visits to outpatient nursing units | Biomarkers: Dogs: sCort | More frequent visits (up to two/week) associated with lower cortisol levels |
| Clark et al., 2020a [35] | 221 adults with fibromyalgia. 93.2% White 19 therapy dogs | Fibromyalgia | Treatment: 20-min AAA with a certified therapy dog and handler Control: 20-min session with handler only | Biomarkers: Humans: sCort, salivary oxytocin Physiological: Humans: Tympanic membrane temperature, HR, HRV Subjective: FIQR, Pain NRS, VAS for “various emotions” | No significant differences between groups in FIQR, NRS VAS, or sCort Treatment group showed significant increase to oxytocin, right tympanic membrane temp, and HRV, and decrease in HR |
| Clark et al., 2020b [36] | 222 adults with fibromyalgia 16 therapy dogs | AAA stress on dog | 5 20-min unstructured AAA visits with patients with fibromyalgia | Biomarkers: Dogs: sCort, salivary oxytocin Physiological: Dogs: HR, HRV, tympanic membrane temperature | Dogs showed “neutral to positive response” to AAA sessions. HR and right tympanic membrane temp lower post-session, all other indicators stable |
| Clark et a., 2020c [26] | 9 therapy dog handlers. 8 female, 1 male 9 therapy dogs | Stress on dogs from their first 3 AAT visits to a hospital | 1st: walking around hospital 2nd: sitting in a waiting room; people interested in the dog could approach 3rd: 47-min inpatient visit | Biomarkers: Humans: sCort (handlers) Dogs: sCort Subjective: Modified PSS (1–4 scale), Handlers’ rating of dogs’ stress | sCort: Nonsignificant decreases post-visit Handlers’ perceptions of dogs’ stress levels aligned with changes in dogs’ cortisol levels |
| Coakley et al., 2020 [37] | 59 patients; 2 female, 27 male; 93.2% White Therapy dogs | Patients hospitalized in an acute care setting | A 15-min AAT session | Biomarkers: Humans: sCort Physiological: HR, RR Subjective: STAI, Wellbeing VAS, Comfort VAS | Significant improvements in anxiety, comfort and well-being; significant reductions in HR and RR. Nonsignificant changes to cortisol |
| Cole et al., 2007 [38] | 76 adults No details on dogs | Patients with advanced heart failure admitted to a cardiac care or cardiac observation unit of a hospital | Group 1: 12-min hospital visit with a therapy dog Group 2: 12-min visit from a volunteer only Group 3: Usual care | Biomarkers: Humans: Epinephrine and norepinephrine Physiological: Humans: BP; HR; right atrial, pulmonary artery, and capillary wedge pressure; cardiac index; systemic vascular resistance Subjective: STAI | Dog group had lower cardiopulmonary pressures, epinephrine and norepinephrine, and anxiety. Other measures not significantly impacted |
| De Carvalho et al., 2019 [39] | 19 therapy dog handlers, all female 19 therapy dogs | AAA stress on dog | AAI sessions (details varied by team but were typically familiar) | Biomarkers: Dogs: sCort Physiological: Dogs: HR, RR | Dogs had higher HR, RR, and sCort after AAIs than at home, but all HR values were “around the normal range” |
| Fecteau et al., 2017 [40] | Parents of 114 autistic children Service dogs | Stress related to parenting an autistic child | Service dog or waitlist control | Biomarkers: Humans: sCort Subjective: PSI-SF | Dog group reported reduced parenting stress after 9 months and lower morning cortisol in first 12 weeks |
| Glenk et al., 2013 [41] | Dog handlers, all female 21 therapy dogs and therapy dogs in training | AAA stress on dog | 8 weekly AAIs on-leash or off-leash at three inpatient mental health facilities | Biomarkers: Dogs: sCort | No significant increases in sCort. Off-leash group had lower working cortisol levels than on-leash |
| Glenk et al., 2014 [42] | Dog handlers 5 therapy dogs | AAA stress on dog | 5 weekly AAAs at an inpatient substance abuse treatment facility | Biomarkers: Dogs: sCort | sCort decreased post-session, with significant decreases in last 2 sessions. No significant difference in sCort between working and nonworking days |
| Haubenhofer and Kirchengast, 2007 [22] | 13 dog handlers; 12 female, 1 male 18 therapy dogs | AAA stress on dogs and handlers | AAT sessions over 3 months (details varied by handler-dog team) | Biomarkers: Humans; sCort (handlers) Dogs: sCort Subjective: Emotion questionnaire | Handlers and dogs had higher sCort on AAT days compared to control days In handlers, sCort increased steadily with session duration; in dogs, with number of sessions/week |
| Kline et al., 2020 [43] | 122 emergency medicine providers; 86.8% White | Occupational stress of emergency medicine providers | Group 1: no intervention Group 2: 5 min coloring Group 3: 5-min AAI | Biomarkers: Humans: sCort Subjective: SVAS, PSS-10, FACES stress scale | SVAS showed reduction in stress in dog group, but PSS-10 did not. sCort decreased significantly in both coloring and dog groups compared to control. |
| Koda et al., 2016 [44] | 78 inmates in a Japanese men’s prison 48 therapy dogs | Stress related to imprisonment. Many also had psychiatric and/or developmental disorders | 12 weekly, 70-min group AAT session | Biomarkers: Humans: sCort Subjective: Mood questionnaire | 35% reported mood improvements after AAT; 6% mood reductions Inmates with psychiatric but not developmental disorders showed decreased sCort post-AAT; inmates with both types of disorders or developmental disorders only did not show significant changes |
| Krause-Parello et al., 2018 [45] | 25 military veterans; 21 male, 4 female; 68% White 1 facility dog | Hospitalized veterans being seen by a palliative care psychologist | Group 1: 20-min AAT visit with a psychologist Group 2: 20-min psychologist visit only | Biomarkers: Humans: sCort, sAA, IgA Physiological: Humans: BP, HR Subjective: Coping Strategy Indicator, Seeking Support subscale; CDC Health-Related Quality of Life; UCLA Loneliness Scale; PSS | Significant decreases in sCort and HR in both groups, dog group showed lower HR than psychologist-only group sAA and IgA not significantly different between conditions |
| Krause-Parello et al., 2019 [46] | 120 patients; 95 male, 25 female; 59.1% White Therapy dogs | Military personnel who had recently been aeromedically evacuated | Group 1: 20-min AAI Group 2: 20-min info session about assistance dogs | Biomarkers: Humans: sCort, sAA, IgA Subjective: PTSSS, PCL-M | sCort decreased significantly in the AAI group compared to control group Patients in experimental condition with higher PTSSS had greater reduction in stress as assessed by IgA No significant difference in sAA between groups |
| Krause-Parello et al., 2020 [47] | 33 military veterans; 26 male, 7 female; 75.8% White Shelter dogs | Military veterans | Group 1: 4 30-min weekly dog walks Group 2: 4 30-min weekly walks with another human | Biomarkers: Humans: sCort, sAA Physiological: Humans: HRV Subjective: PCL-M, PSS | Walking with a dog or another person led to decreases in sCort among those with low PTSD symptom severity, but sAA did not change significantly Individuals with high PTSD symptoms did not show significant change to sAA in dog walk group, but did in human walk group. In this group, average HRV increased in dog walk group but decreased in human walk group |
| Lass-Hennemann et al., 2014 [48] | 80 healthy female university students Therapy dogs | 11 min “trauma film” with fictional scenes of physical and sexual violence | Watched film with: Group 1: therapy dog Group 2: toy dog Group 3: friendly person Group 4: alone | Biomarkers: Humans: sCort Physiological: Humans: BP, HR Subjective: STAI, PANAS | Dog group showed lower STAI and PANAS scores than toy dog or alone groups, and similar to friendly human group No significant differences in physiological or sCort stress between groups |
| Lass-Hennemann et al., 2018 [49] | 60 healthy female university students Therapy dogs | 11 min “trauma film” with fictional scenes of physical and sexual violence | After film: Group 1: Interacted with a friendly dog for 15 min Group 2: Watched a film clip showing a person interacting with a friendly dog Group 3: Told to relax | Biomarkers: Humans: sCort Physiological: Humans: BP, HR Subjective: STAI, PANAS, BDI-II, record of intrusive thoughts and distress | Dog group reported less anxiety, and more positive and less negative affect, but had smaller decrease in physiological arousal after film, compared to other groups. No differences in intrusive thoughts between the groups |
| Machová et al., 2019 [50] | 22 female nurses; 13 worked in rehabilitation and physical medicine (PRM), 9 worked in internal medicine and long-term care 1 therapy dog | Occupational stress of nurses | Condition 1: normal work, no break Condition 2: normal work, break of choice Condition 3: normal work, AAT break | Biomarkers: Humans: sCort | sCort levels of PRM nurses did not decrease after AAT, but did in those working in internal medicine; likely due to low initial cortisol levels from PRM nurses “Break of choice” groups did not show decrease in sCort |
| Menna et al., 2019 [51] | 10 dialysis patients; 7 male, 3 female, with comparable stage of renal damage and “relational difficulties” 1 therapy dog | Dialysis patients affected by end-stage renal disease | 11 weekly hour-long AAA sessions | Biomarkers: Humans: serotonin, oxytocin | No significant changes to serotonin before and after session, but serotonin and oxytocin increased from one session to the next |
| Nepps et al., 2014 [52] | 218 patients, relatively balanced between men and women (exact details not shared to protect privacy) 80% of sessions occurred with the same female border collie; other details not provided | Patients hospitalized in a mental health unit | Group 1: 1-h AAA session Group 2: 1-h stress management program | Biomarkers: Humans: sCort Physiological: Humans: BP, pulse Subjective: Burns Depression Checklist, Burns Anxiety Inventory, 0–10 pain scale | Significant decreases in depression, anxiety, pain, and pulse after AAA, comparable to those in the traditional stress management group. No changes in BP and sCort |
| Ng et al., 2014 [53] | 16 therapy dog handlers; 2 male, 14 female 15 therapy dogs | AAA stress on dog | Setting 1: 60-min AAA with college students Setting 2: 60 min in novel room near a stranger Setting 3: 60 min of normal activity at home | Biomarkers: Dogs: sCort | sCort levels significantly higher in novel setting compared to AAA or home settings. sCort not statistically different between AAA and home settings |
| Pirrone et al., 2017 [54] | 4 female therapy dog handlers 4 therapy dogs | Familiar AAA stress on dogs and handlers | 5 weekly, 55-min AAAs with 2–5 adults Control: HR and saliva collected at similar times of day from home | Biomarkers: Humans: sCort Dogs: sCort Physiological: Humans; HR Dogs: HR | Handlers’ sCort levels decreased over time during both activity and control days. Dogs showed similar pattern, but it was not statically significant. No difference in handlers’ sCort levels on AAA compared to control days. Dogs’ HR was higher during AAA days than in control days |
| Polheber and Matchock, 2014 [12] | 48 university students; 26 males; 64% White 1 therapy dog, female Golden Retriever | TSST | TSST alone, with a human friend, or with a novel dog | Biomarkers: Humans: sCort Physiological: Humans: HR Subjective: STAI | Participants’ sCort levels were lower with dogs’, as compared to with a friend or alone. STAI responses not associated with sCort, but HR was |
| Rodriguez et al., 2018 [55] | 73 post-9/11 military veterans with PTSD; 59 male 45 service dogs | Veterans with PTSD | Service dog. Both groups continued to receive usual care. | Biomarkers: Humans: sCort Subjective: PCL, PROMIS, PSQI | Participants with a service dog showed higher cortisol awakening response and reported lower anxiety, anger, and sleep disturbance, and less alcohol abuse, compared to waitlist controls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandenberger, J.; Flynn, E.; Moratto, E.; Wendt, A.; Morris, K.N. Molecular Biomarkers of Adult Human and Dog Stress during Canine-Assisted Interventions: A Systematic Scoping Review. Animals 2022, 12, 651. https://doi.org/10.3390/ani12050651
Gandenberger J, Flynn E, Moratto E, Wendt A, Morris KN. Molecular Biomarkers of Adult Human and Dog Stress during Canine-Assisted Interventions: A Systematic Scoping Review. Animals. 2022; 12(5):651. https://doi.org/10.3390/ani12050651
Chicago/Turabian StyleGandenberger, Jaci, Erin Flynn, Em Moratto, Ashley Wendt, and Kevin N. Morris. 2022. "Molecular Biomarkers of Adult Human and Dog Stress during Canine-Assisted Interventions: A Systematic Scoping Review" Animals 12, no. 5: 651. https://doi.org/10.3390/ani12050651






