In Silico Predictions on the Productive Life Span and Theory of Its Developmental Origin in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study I. Analysis of Age Dynamics of Disposal Rate and In Silico PLS Forecasting
2.2. Study II. Age Dynamics of 305 d Milk Yield in Cows with Various Values of PLS
3. Results
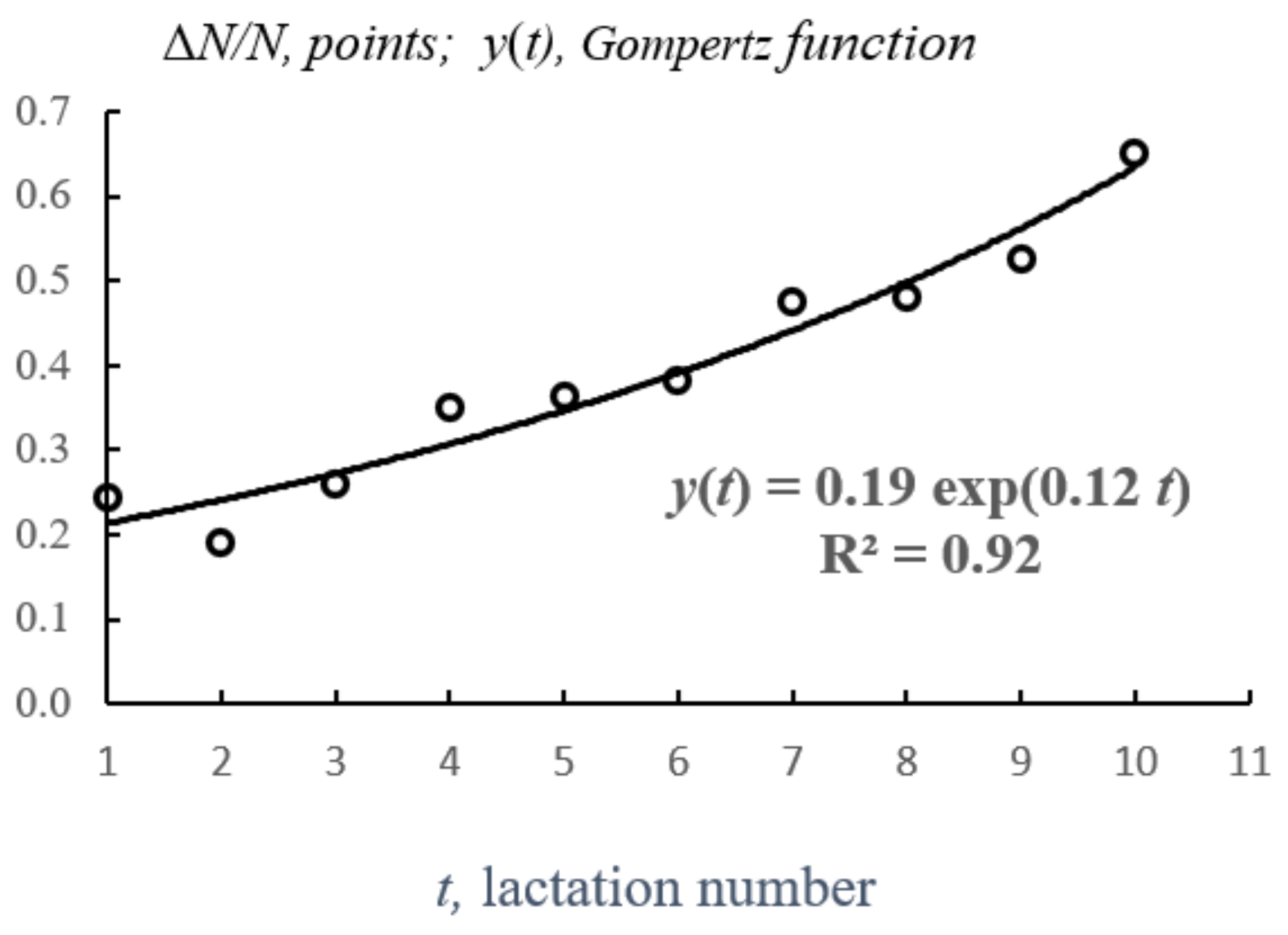
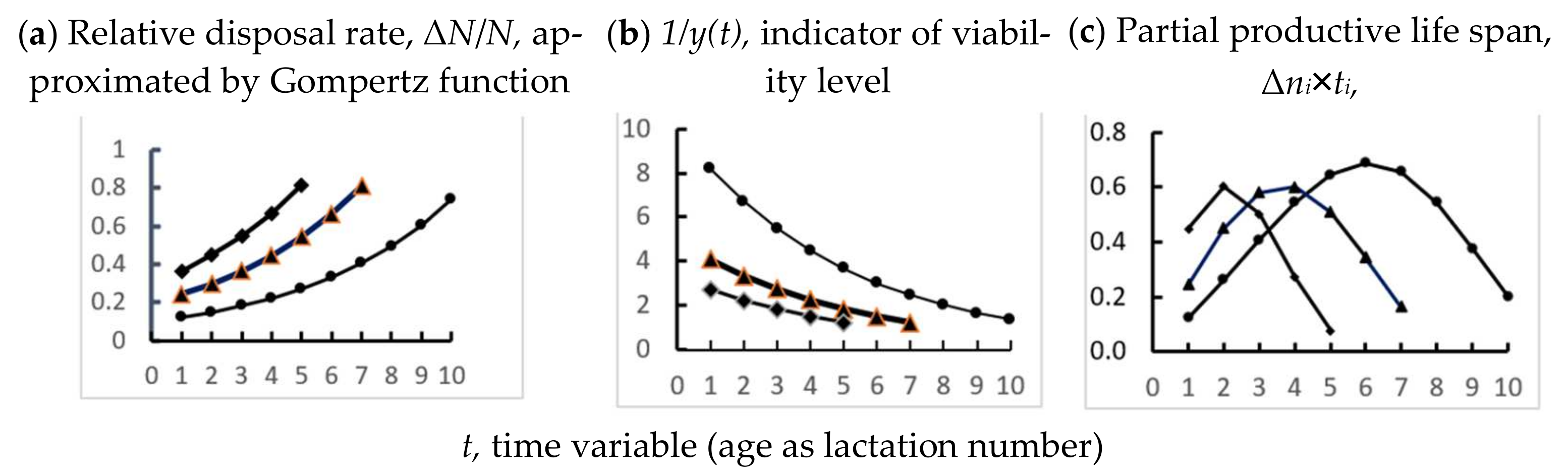
3.1. Study I. Express Method of Using Gompertz Equation to Estimate Viability Indicators for Dairy Cow
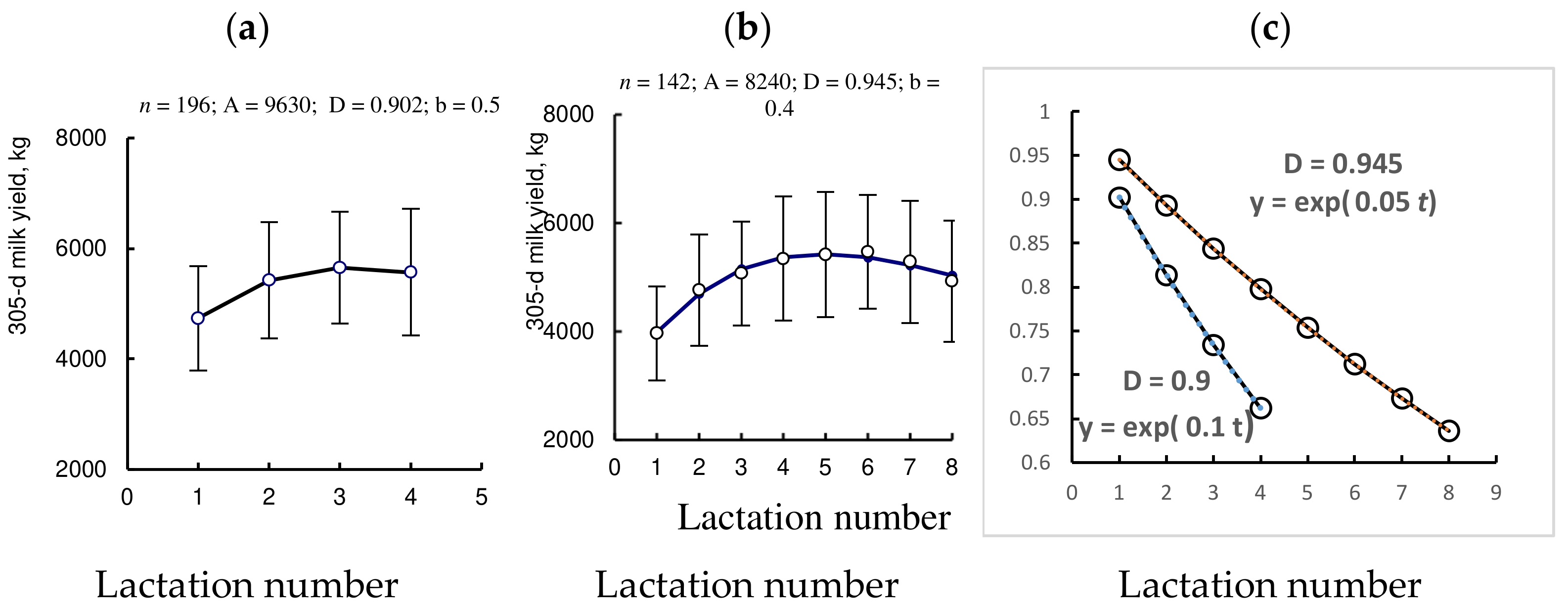
3.2. Study II. Age Dynamics of 305 d Milk Yield in Cows with Various Productive Life Span
t max = 125 D − 110
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauw, W.M.; Kanis, E.; Noordhuizen-Stassen, E.; Grommers, F. Undesirable side effects of selection for high production efficiency in farm animals: A review. Livest. Prod. Sci. 1998, 56, 15–33. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Knegsel, A. Metabolic adaptation during early lactation: Key to cow health, longevity and a sustainable dairy production chain. CAB Rev. Perspect. Agric. Veter. Sci. Nutr. Nat. Resour. 2014, 9, 1–5. [Google Scholar] [CrossRef]
- Walker, M.D.; Duggen, G.; Roulston, N.; Van Slack, A.; Mason, G. Negative affective states and their effects on morbidity, mortality and longevity. Anim. Welf. 2012, 21, 497–509. [Google Scholar] [CrossRef] [Green Version]
- De Vries, A. Economic trade-off between genetic improvement and longevity in dairy cattle. J. Dairy Sci. 2017, 100, 4184–4192. [Google Scholar] [CrossRef] [PubMed]
- McConnel, C.S.; Lombard, J.E.; Wagner, B.A.; Garry, F.B. Evaluation of Factors Associated with Increased Dairy Cow Mortality on United States Dairy Operations. J. Dairy Sci. 2008, 91, 1423–1432. [Google Scholar] [CrossRef]
- Ahlman, T.; Berglund, B.; Rydhmer, L.; Strandberg, E. Culling reasons in organic and conventional dairy herds and genotype by environment interaction for longevity. J. Dairy Sci. 2011, 94, 1568–1575. [Google Scholar] [CrossRef] [Green Version]
- Von Keyserlingk, M.A.G.; Rushen, J.; De Passillé, A.M.B.; Weary, D.M. Invited review: The welfare of dairy cattle—Key concepts and the role of science. J. Dairy Sci. 2009, 92, 4101–4111. [Google Scholar] [CrossRef]
- Pinedo, P.J.; De Vries, A.; Webb, D.W. Dynamics of culling risk with disposal codes reported by Dairy Herd Improvement dairy herds. J. Dairy Sci. 2010, 93, 2250–2261. [Google Scholar] [CrossRef] [Green Version]
- Dechow, C.; Rogers, G.; Clay, J. Heritability and Correlations Among Body Condition Score Loss, Body Condition Score, Production and Reproductive Performance. J. Dairy Sci. 2002, 85, 3062–3070. [Google Scholar] [CrossRef]
- De Vries, A.; Marcondes, M. Review: Overview of factors affecting productive lifespan of dairy cows. Animal 2020, 14, s155–s164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaens, I.; Friggens, N.; Ouweltjes, W.; Scott, H.; Aernouts, B.; Statham, J. Productive life span and resilience rank can be predicted from on-farm first-parity sensor time series but not using a common equation across farms. J. Dairy Sci. 2020, 103, 7155–7171. [Google Scholar] [CrossRef]
- Gompertz, B. On the nature of the function expressive of the law of human mortality and on a new mode of determining life contingencies. Philos. Trans. Roy. Soc. Lond. A. 1825, 115, 513–585. [Google Scholar]
- Vaupel, J.W.; Yasin, A.I. Heterogeneity’s Rules: Some suprising effects of selection on population dynamics. Amer. Statist. 1985, 39, 176–185. [Google Scholar]
- VanRaden, P.M.; Wiggans, G.R. Productive life evaluation: Calculation, accuracy, and economic value. J. Dairy Sci. 1995, 78, 631–638. [Google Scholar] [CrossRef]
- Ducrocq, V.; Casella, G. A Bayesian analysis of mixed survival models. Genet. Sel. Evol. 1996, 28, 505–529. [Google Scholar] [CrossRef]
- Finch, C.E.; Pie, M.C. Naximum life span predictions from the Gompertz mortality model. J. Geront. 1996, 21, B183–B194. [Google Scholar] [CrossRef] [Green Version]
- Novosel’tsev, V.N.; Novosel’tseva, J.A.; Bojko, S.M.; Yashin, A.I. Homeostasis and aging: Slow-fast mathematical model of senescence and death. IFAC Proc. Vol. 2000, 33, 71–76. [Google Scholar]
- Pantanelli, L.; Rossolini, G.; Basso, A.; Piantanelli, A.; Malavolta, M.; Zaia, A. Use of nathenatical models of survivalship in the study of biomaekers of aging: The role of heterogeneity. Mech. Aging Dev. 2001, 122, 1461–1475. [Google Scholar] [CrossRef]
- Roxström, A.; Ducrocq, V.; Strandberg, E. Survival analysis of longevity in dairy cattle on a lactation basis. Genet. Sel. Evol. 2003, 35, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Novosel’tsev, V.N.; Arking, R.; Novosel’tseva, Z.A.; Yashin, A.I. Interdisciplinary modeling of systemic mechanisms by reproduction and aging. Control. Sci. 2004, 4, 27–40. [Google Scholar]
- Novaković, Ž.; Aleksić, S.; Sretenović, L.; Petrović, M.M.; Pantelić, V.; Ostojić-Andrić, D. Longevity of high-yielding cows. Biotechnol. Anim. Husb. 2009, 25, 645–654. [Google Scholar]
- Golubev, A.G. How could the Gompertz-Makeham law evolve? J. Theoret. Biol. 2009, 258, 1–17. [Google Scholar] [CrossRef]
- Odin, V.I. The crisis of gerontology: On the issue of primary health in the XX century. Adv. Gerontol. 2011, 24, 11–23. [Google Scholar]
- Rakyan, V.; Preis, J.; Morgan, H.; Whitelaw, E. The marks, nechanisms and memory of epigenetic states in mammals. Biochem. J. 2001, 356, 1–10. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W.; Walter, J. Epigenetic Reprogramming in Mammalian Development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef] [Green Version]
- Gluckman, P.D.; Hanson, M.A. The developmental origins of the metabolic syndrome. Trends Endocrinol. Metab. 2004, 15, 183–187. [Google Scholar] [CrossRef]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef] [Green Version]
- Hemminki, K.; Lorenzo Bermejo, J.; Forwsi, A. The balance between heritable and environmental etiology of human disease. Nat. Rev. Genet. 2006, 7, 958–965. [Google Scholar]
- Callinan, P.A.; Feinberg, A. The emerging science of epigenomics. Hum. Mol. Genet. 2006, 15, R95–R101. [Google Scholar] [CrossRef]
- Schumacher, A.; Petronis, A. Epigenetics of complex diseases: From general theory to laboratory experiments. Curr. Top. Microbiol. Immunol. 2006, 310, 81–115. [Google Scholar] [PubMed]
- Drake, A.J.; Tang, J.I.; Nyirenda, M.J. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin. Sci. 2007, 113, 219–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolinoy, D.C.; Weidman, J.R.; Jirtle, R.L. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod. Toxicol. 2007, 23, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.; Beedle, A.S. Non-genomic transgenerational inheritance of disease risk. BioEssays 2007, 29, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.F.; Lamb, J. Fetal Origins of Perinatal Morbidity and/or Adult Disease. Semin. Reprod. Med. 2008, 26, 436–445. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.; Cooper, C.; Thornburg, K. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, P.; Buss, C.; Entringer, S.; Swanson, J. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, E.; Larsen, G.; Manikkam, M.; Guerrero-Bosanga, C.; Savenkova, M.I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS ONE 2012, 7, e36129. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.; Curran, S.P. Longevity and the long arm of epigenetics: Acquired parental marks influence lifespan across several generations. Bioessays 2012, 34, 652–654. [Google Scholar] [CrossRef] [Green Version]
- Opsomer, G.; Van Eetvelde, M.; Kamal, M.; Van Soom, A. Epidemiological evidence for metabolic programming in dairy cattle. Reprod. Fertil. Dev. 2016, 29, 52–57. [Google Scholar] [CrossRef]
- Kabacik, S.; Horvath, S.; Cohen, H.; Raj, K. Epigenetic ageing is distinct from senescence-mediated ageing and is not prevented by telomerase expression. Aging 2018, 10, 2800–2815. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odent, M. Primal Health; Century Hutchinson: London, UK, 1986. [Google Scholar]
- Swanson, J.M.; Wadhwa, P.D. Genes, Environments and Human Development. In Proceedings of the Health and Disease (GEHDHD) meeting, Arnold and Mabel Beckman Center of the National Academy of Sciences, Irvine, CA, USA, 7–8 September 2006. [Google Scholar]
- Barker, D.; Godfrey, K.; Gluckman, P.; Harding, J.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- DeCherney, A.H.; Hill, M.J.; Carpinello, O.J. Developmental Origins of Health and Disease: The History of the Barker Hypothesis and Assisted Reproductive Technology. Semin. Reprod. Med. 2018, 36, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Odent, S.; Odent, M. Primal health research in the age of epigenetic clocks. Med. Hypotheses 2019, 133, 109403. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Rogers, J.M. Embryonic and fetal programming of physiological disorders in adulthood. Birth Defects Res. Part C Embryo Today Rev. 2004, 72, 300–312. [Google Scholar] [CrossRef]
- De Boo, H.A.; Harding, J.E. The developmental origins of adult disease (Barker) hypothesis. Aust. New Zldn. J. Obstet. Gynaecol. 2006, 46, 4–14. [Google Scholar] [CrossRef]
- Rutten, C.; Velthuis, A.; Steeneveld, W.; Hogeveen, H. Invited review: Sensors to support health management on dairy farms. J. Dairy Sci. 2013, 96, 1928–1952. [Google Scholar] [CrossRef]
- Steeneveld, W.; Hogeveen, H. Characterization of Dutch dairy farms using sensor systems for cow management. J. Dairy Sci. 2015, 98, 709–771. [Google Scholar] [CrossRef]
- Hudson, C.; Kaler, J.; Down, P. Using big data in cattle practice. Practise 2018, 40, 396–410. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Forsen, T.; Uutela, A.; Osmond, C.; Eriksson, J.G. Size at birth and resilience to effects of poor living conditions in adult life: Longitudinal study. BMJ 2001, 323, 1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemitz, E.L.; Feinberg, A.P. Epigenetics and assisted reproductive technology: A call for investigation. Am. J. Hum. Genet. 2004, 74, 599–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeweerd, J.-M.; Kirschvink, N.; Clegg, P.; Vandenput, S.; Gustin, P.; Saegerman, C. Is evidence-based medicine so evident in veterinary research and practice? History, obstacles and perspectives. Veter. J. 2012, 191, 28–34. [Google Scholar] [CrossRef] [PubMed]
| Years | n | For Successive Lactations, % | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4–5 | 6–7 | 8–9 | 10 and More | ||
| 1985 | 20,528 | 28.4 | 21.6 | 17.0 | 21.1 | 8.4 | 2.8 | 0.7 |
| 1986 | 20,747 | 27.4 | 21.8 | 17.5 | 20.7 | 9.3 | 2.7 | 0.6 |
| 1987 | 20,897 | 28.6 | 20.9 | 17.1 | 21.6 | 8.7 | 2.6 | 0.5 |
| 1988 | 20,596 | 28.2 | 20.8 | 17.2 | 22.3 | 8.4 | 2.6 | 0.5 |
| Mean % | 28.15 | 21.28 | 17.20 | 21.7 | 8.7 | 2.68 | 0.57 | |
| Mean n | 20,692 | 5825 | 4402 | 3559 | 4483 | 1800 | 554 | 118 |
| Analysis of Empirical Data | Numerical Integration of Gompertz Equation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ti | Ni | ΔNi | yi = ΔNi/Ni | Δni = ΔNi/N1 | Δni×i | ti | yi | 1 − yi | Ni |
| 1 | 5825 | 1423 | 0.244 | 0.244 | 0.24 | 1 | 0.244 | 0.7558 | 1500 |
| 2 | 4402 | 843 | 0.192 | 0.145 | 0.29 | 2 | 0.192 | 0.8085 | 1213 |
| 3 | 3559 | 922 | 0.259 | 0.158 | 0.47 | 3 | 0.259 | 0.7410 | 899 |
| 4 | 2637 | 921 | 0.349 | 0.158 | 0.63 | 4 | 0.349 | 0.6506 | 585 |
| 5 | 1716 | 625 | 0.364 | 0.107 | 0.54 | 5 | 0.364 | 0.6359 | 372 |
| 6 | 1091 | 416 | 0.381 | 0.071 | 0.43 | 6 | 0.381 | 0.6188 | 230 |
| 7 | 675 | 320 | 0.474 | 0.055 | 0.38 | 7 | 0.474 | 0.5256 | 121 |
| 8 | 355 | 170 | 0.480 | 0.029 | 0.23 | 8 | 0.480 | 0.5200 | 63 |
| 9 | 185 | 97 | 0.526 | 0.017 | 0.15 | 9 | 0.526 | 0.4735 | 30 |
| 10 | 87 | 57 | 0.650 | 0.010 | 0.10 | 10 | 0.650 | 0.3500 | 10 |
| 11 | 31 | Σ = 3.5 | |||||||
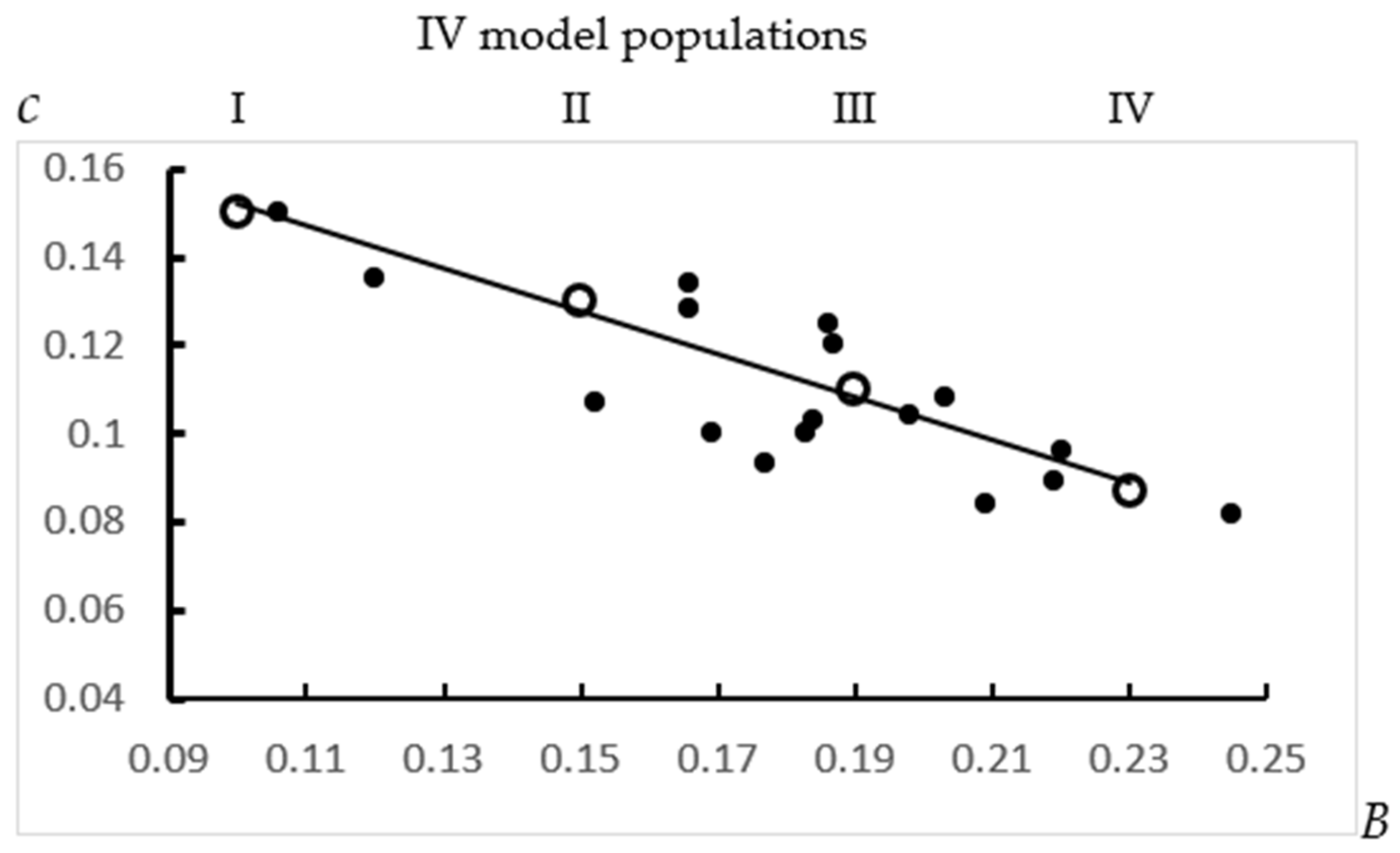
| Nr | Parameters of MP | Parameter B for Subpopulations N1 (Initial Number of Cows in Cohort) | |||
|---|---|---|---|---|---|
| B | c | ||||
| 1 | 0.1 | 0.15 | 0.1 | ||
| 1000 | |||||
| 2 | 0.15 | 0.13 | 0.15 | 0.09 | |
| 600 | 400 | ||||
| 3 | 0.19 | 0.11 | 0.22 | 0.15 | 0.1 |
| 300 | 300 | 400 | |||
| 4 | 0.23 | 0.087 | 0.26 | 0.20 | 0.11 |
| 300 | 300 | 400 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharitonov, E.; Cherepanov, G.; Ostrenko, K. In Silico Predictions on the Productive Life Span and Theory of Its Developmental Origin in Dairy Cows. Animals 2022, 12, 684. https://doi.org/10.3390/ani12060684
Kharitonov E, Cherepanov G, Ostrenko K. In Silico Predictions on the Productive Life Span and Theory of Its Developmental Origin in Dairy Cows. Animals. 2022; 12(6):684. https://doi.org/10.3390/ani12060684
Chicago/Turabian StyleKharitonov, Evgeniy, Gennadiy Cherepanov, and Konstantin Ostrenko. 2022. "In Silico Predictions on the Productive Life Span and Theory of Its Developmental Origin in Dairy Cows" Animals 12, no. 6: 684. https://doi.org/10.3390/ani12060684






